Serum Erythritol and Risk of Overall and Cause-Specific Mortality in a Cohort of Men
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Serum Erythritol Measurement
2.3. Mortality Endpoints
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Billaux, M.S.; Flourie, B.; Jacquemin, C.; Messing, B. Sugar alcohols. In Handbook of Sweeteners; Marie, S., Piggott, J.R., Eds.; Springer: Boston, MA, USA; pp. 72–103. [CrossRef]
- Regnat, K.; Mach, R.L.; Mach-Aigner, A.R. Erythritol as sweetener-wherefrom and whereto? Appl. Microbiol. Biotechnol. 2018, 102, 587–595. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Castle, L.; Degen, G.; Engel, K.-H.; Fowler, P.J.; Fernandez, M.J.F.; Fürst, P.; Gundert-Remy, U.; et al. Re-evaluation of erythritol (E 968) as a food additive. EFSA J. 2023, 21, e8430. [Google Scholar]
- Roberts, A. The safety and regulatory process for low calorie sweeteners in the United States. Physiol. Behav. 2016, 164, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A. Sweeteners permitted in the European Union: Safety aspects. Scand. J. Food Nutr. 2006, 50, 104–116. [Google Scholar] [CrossRef]
- Mishra, A.; Ahmed, K.; Froghi, S.; Dasgupta, P. Systematic review of the relationship between artificial sweetener consumption and cancer in humans: Analysis of 599,741 participants. Int. J. Clin. Pract. 2015, 69, 1418–1426. [Google Scholar] [CrossRef]
- Debras, C.; Chazelas, E.; Sellem, L.; Porcher, R.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, F.S.; Agaësse, C.; De Sa, A.; Lutchia, R.; et al. Artificial sweeteners and risk of cardiovascular diseases: Results from the prospective NutriNet-Santé cohort. BMJ 2022, 378, e071204. [Google Scholar] [CrossRef] [PubMed]
- Debras, C.; Chazelas, E.; Srour, B.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, F.S.; Agaësse, C.; De Sa, A.; Lutchia, R.; Gigandet, S.; et al. Artificial sweeteners and cancer risk: Results from the NutriNet-Santé population-based cohort study. PLoS Med. 2022, 19, e1003950. [Google Scholar] [CrossRef]
- Fagherazzi, G.; Gusto, G.; Affret, A.; Mancini, F.R.; Dow, C.; Balkau, B.; Clavel-Chapelon, F.; Bonnet, F.; Boutron-Ruault, M.-C. Chronic Consumption of Artificial Sweetener in Packets or Tablets and Type 2 Diabetes Risk: Evidence from the E3N-European Prospective Investigation into Cancer and Nutrition Study. Ann. Nutr. Metab. 2017, 70, 51–58. [Google Scholar] [CrossRef]
- Witkowski, M.; Wilcox, J.; Province, V.; Wang, Z.; Nemet, I.; Tang, W.W.; Hazen, S.L. Ingestion of the Non-Nutritive Sweetener Erythritol, but Not Glucose, Enhances Platelet Reactivity and Thrombosis Potential in Healthy Volunteers. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 2136–2141. [Google Scholar] [CrossRef]
- Witkowski, M.; Nemet, I.; Alamri, H.; Wilcox, J.; Gupta, N.; Nimer, N.; Haghikia, A.; Li, X.S.; Wu, Y.; Saha, P.P.; et al. The artificial sweetener erythritol and cardiovascular event risk. Nat. Med. 2023, 29, 710–718. [Google Scholar] [CrossRef]
- Oaks, Z.; Patel, A.; Huang, N.; Choudhary, G.; Winans, T.; Faludi, T.; Krakko, D.; Duarte, M.; Lewis, J.; Beckford, M.; et al. Cytosolic aldose metabolism contributes to progression from cirrhosis to hepatocarcinogenesis. Nat. Metab. 2023, 5, 41–60. [Google Scholar] [CrossRef] [PubMed]
- The ATBC Cancer Prevention Study Group. The alpha-tocopherol, beta-carotene lung cancer prevention study: Design, methods, participant characteristics, and compliance. Ann. Epidemiol. 1994, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kattermann, R.; Jaworek, D.; Möller, G. Multicentre study of a new enzymatic method of cholesterol determination. J. Clin. Chem. Clin. Biochem. 1984, 22, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Milne, D.B.; Botnen, J. Retinal, alpha-tocopherol, lycopene, and alpha and beta-carotene simultaneously determined in plasma by isocratic liquid chromatography. Clin. Chem. 1986, 32, 874–876. [Google Scholar] [CrossRef]
- Mondul, A.M.; Sampson, J.N.; Moore, S.C.; Weinstein, S.J.; Evans, A.M.; Karoly, E.D.; Virtamo, J.; Albanes, D. Metabolomic profile of response to supplementation with β-carotene in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am. J. Clin. Nutr. 2013, 98, 488–493. [Google Scholar] [CrossRef]
- Mondul, A.M.; Moore, S.C.; Weinstein, S.J.; Evans, A.M.; Karoly, E.D.; Männistö, S.; Sampson, J.N.; Albanes, D. Serum metabolomic response to long-term supplementation with all-rac-alpha-tocopheryl acetate in a randomized controlled trial. J. Nutr. Metab. 2016, 2016, 6158436. [Google Scholar] [CrossRef]
- Huang, J.; Weinstein, S.; Mack, W.; Hodis, H.; Albanes, D. Serum Metabolomic Response to Low- and High-Dose Vitamin E Supplementation in Two Randomized Controlled Trials. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1329–1334. [Google Scholar] [CrossRef]
- Mondul, A.M.; Moore, S.; Weinstein, S.; Mannisto, S.; Sampson, J.; Albanes, D. 1-stearoylglycerol is associated with risk of prostate cancer: Results from serum metabolomic profiling. Metabolomics 2014, 10, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.M.; Moore, S.C.; Weinstein, S.J.; Karoly, E.D.; Sampson, J.N.; Albanes, D. Metabolomic analysis of prostate cancer risk in a prospective cohort: The alpha-tocolpherol, beta-carotene cancer prevention (ATBC) study. Int. J. Cancer 2015, 137, 2124–2132. [Google Scholar] [CrossRef]
- Huang, J.; Mondul, A.M.; Weinstein, S.J.; Derkach, A.; Moore, S.C.; Sampson, J.N.; Albanes, D. Prospective serum metabolomic profiling of lethal prostate cancer. Int. J. Cancer 2019, 145, 3231–3243. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, B.; Weinstein, S.J.; Albanes, D.; Mondul, A.M. Metabolomic profile of prostate cancer-specific survival among 1812 Finnish men. BMC Med. 2022, 20, 362. [Google Scholar] [CrossRef] [PubMed]
- Stolzenberg-Solomon, R.; Derkach, A.; Moore, S.; Weinstein, S.J.; Albanes, D.; Sampson, J. Associations between metabolites and pancreatic cancer risk in a large prospective epidemiological study. Gut 2020, 69, 2008–2015. [Google Scholar] [CrossRef]
- Huang, J.; Weinstein, S.J.; Kitahara, C.M.; Karoly, E.D.; Sampson, J.N.; Albanes, D. A prospective study of serum metabolites and glioma risk. Oncotarget 2017, 8, 70366–70377. [Google Scholar] [CrossRef]
- Lim, J.E.; Huang, J.; Weinstein, S.J.; Parisi, D.; Männistö, S.; Albanes, D. Serum metabolomic profile of hair dye use. Sci. Rep. 2023, 13, 3776. [Google Scholar] [CrossRef]
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef] [PubMed]
- Lina, B.A.; Bos-Kuijpers, M.H.; Til, H.P.; Bar, A. Chronic toxicity and carcinogenicity study of erythritol in rats. Regul. Toxicol. Pharmacol. 1996, 24, S264–S279. [Google Scholar] [CrossRef] [PubMed]
- Flint, N.; Hamburg, N.M.; Holbrook, M.; Dorsey, P.G.; LeLeiko, R.M.; Berger, A.; de Cock, P.; Bosscher, D.; Vita, J.A. Effects of erythritol on endothelial function in patients with type 2 diabetes mellitus: A pilot study. Acta Diabetol. 2014, 51, 513–516. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Toews, I.; Lohner, S.; Küllenberg de Gaudry, D.; Sommer, H.; Meerpohl, J.J. Association between intake of non-sugar sweeteners and health outcomes: Systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ 2019, 364, k4718. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Jiang, Y.W.; Chen, J.X.; Xia, P.F.; Pan, A. Association of Consumption of Sugar-Sweetened Beverages or Artificially Sweetened Beverages with Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2021, 12, 374–383. [Google Scholar] [CrossRef]
- Mazi, T.A.; Stanhope, K.L. Elevated Erythritol: A Marker of Metabolic Dysregulation or Contributor to the Pathogenesis of Cardiometabolic Disease? Nutrients 2023, 15, 4011. [Google Scholar] [CrossRef]
- Hootman, K.C.; Trezzi, J.-P.; Kraemer, L.; Burwell, L.S.; Dong, X.; Guertin, K.A.; Jaeger, C.; Stover, P.J.; Hiller, K.; Cassano, P.A. Erythritol is a pentose-phosphate pathway metabolite and associated with adiposity gain in young adults. Proc. Natl. Acad. Sci. USA 2017, 114, E4233–E4240. [Google Scholar] [CrossRef] [PubMed]
- Schlicker, L.; Szebenyi, D.M.E.; Ortiz, S.R.; Heinz, A.; Hiller, K.; Field, M.S. Unexpected roles for ADH1 and SORD in catalyzing the final step of erythritol biosynthesis. J. Biol. Chem. 2019, 294, 16095–16108. [Google Scholar] [CrossRef] [PubMed]
- Bordier, V.; Teysseire, F.; Senner, F.; Schlotterbeck, G.; Drewe, J.; Beglinger, C.; Wölnerhanssen, B.K.; Meyer-Gerspach, A.C. Absorption and Metabolism of the Natural Sweeteners Erythritol and Xylitol in Humans: A Dose-Ranging Study. Int. J. Mol. Sci. 2022, 23, 9867. [Google Scholar] [CrossRef] [PubMed]
- van Gerwen, M.; Colicino, E.; Guan, H.; Dolios, G.; Nadkarni, G.N.; Vermeulen, R.C.H.; Wolff, M.S.; Arora, M.; Genden, E.M.; Petrick, L.M. Per- and polyfluoroalkyl substances (PFAS) exposure and thyroid cancer risk. EBioMedicine 2023, 97, 104831. [Google Scholar] [CrossRef]
- Lee-Sarwar, K.A.; Fischer-Rasmussen, K.; Bønnelykke, K.; Bisgaard, H.; Chawes, B.; Kelly, R.S.; Lasky-Su, J.; Zeiger, R.S.; O’connor, G.T.; Bacharier, L.B.; et al. Omega-3 Fatty Acids Interact with DPP10 Region Genotype in Association with Childhood Atopy. Nutrients 2023, 15, 2416. [Google Scholar] [CrossRef]
- Yang, J.J.; Shu, X.-O.; Herrington, D.M.; Moore, S.C.; A Meyer, K.; Ose, J.; Menni, C.; Palmer, N.D.; Eliassen, H.; Harada, S.; et al. Circulating trimethylamine N-oxide in association with diet and cardiometabolic biomarkers: An international pooled analysis. Am. J. Clin. Nutr. 2021, 113, 1145–1156. [Google Scholar] [CrossRef]
| Characteristics | All Participants (N = 4468) | Deaths (N = 3377) 1 | Non-Deaths (N = 1091) | p-Values |
|---|---|---|---|---|
| Serum erythritol 2 | 1.02 ± 0.35 | 1.04 ± 0.36 | 0.98 ± 0.32 | <0.0001 |
| Age (years) | 57.8 ± 5.1 | 58.8 ± 5.1 | 54.9 ± 3.8 | <0.0001 |
| Years of follow-up | 19.1 ± 7.0 | 16.7 ± 6.3 | 26.8 ± 0.9 | <0.0001 |
| Height (cm) 3 | 173.7 ± 6.2 | 173.5 ± 6.1 | 174.3 ± 6.3 | <0.0001 |
| Weight (kg) 3 | 79.3 ± 12.6 | 78.9 ± 12.8 | 80.3 ± 11.9 | 0.0007 |
| Body mass index (kg/m2) 3 | 26.2 ± 3.7 | 26.2 ± 3.7 | 26.4 ± 3.4 | 0.06 |
| Systolic blood pressure (mmHg) 3 | 141.5 ± 18.7 | 142.6 ± 19.0 | 137.9 ± 17.3 | <0.0001 |
| Diastolic blood pressure (mmHg) 3 | 87.2 ± 10.5 | 87.4 ± 10.7 | 86.9 ± 10.0 | 0.16 |
| Cigarettes smoked per day | 19.6 ± 8.5 | 19.9 ± 8.5 | 18.6 ± 8.5 | <0.0001 |
| Years of smoking | 35.9 ± 8.7 | 37.2 ± 8.6 | 32.0 ± 8.1 | <0.0001 |
| Serum biochemistry | ||||
| Retinol (µg/L) | 591 ± 127 | 588 ± 127 | 601 ± 125 | <0.0001 |
| Total cholesterol (mmol/L) | 6.2 ± 1.2 | 6.2 ± 1.2 | 6.2 ± 1.1 | 0.87 |
| HDL cholesterol (mmol/L) | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.50 |
| α-Tocopherol (mg/L) | 11.9 ± 3.3 | 11.9 ± 3.3 | 12.2 ± 3.0 | 0.005 |
| β-Carotene (µg/L) | 226 ± 201 | 216 ± 198 | 256 ± 206 | <0.0001 |
| Alcohol consumption (ethanol g/day) | 15.7 ± 19.8 | 16.0 ± 20.5 | 14.6 ± 17.5 | 0.03 |
| Trial α-tocopherol supplementation (%) | 48.3 | 48.2 | 48.4 | 0.93 |
| Trial β-carotene supplementation (%) | 50.6 | 51.0 | 49.6 | 0.43 |
| Diabetes mellitus (%) | 3.2 | 3.7 | 1.5 | 0.0002 |
| Light leisure time activity (%) | 39.2 | 40.6 | 34.7 | <0.0001 |
| Mortality Outcome | # Events | Q1 HR | Q2 HR (95% CI) | Q3 HR (95% CI) | Q4 HR (95% CI) | P for Trend | HR (95% CI) (Continuous) | p-Value |
|---|---|---|---|---|---|---|---|---|
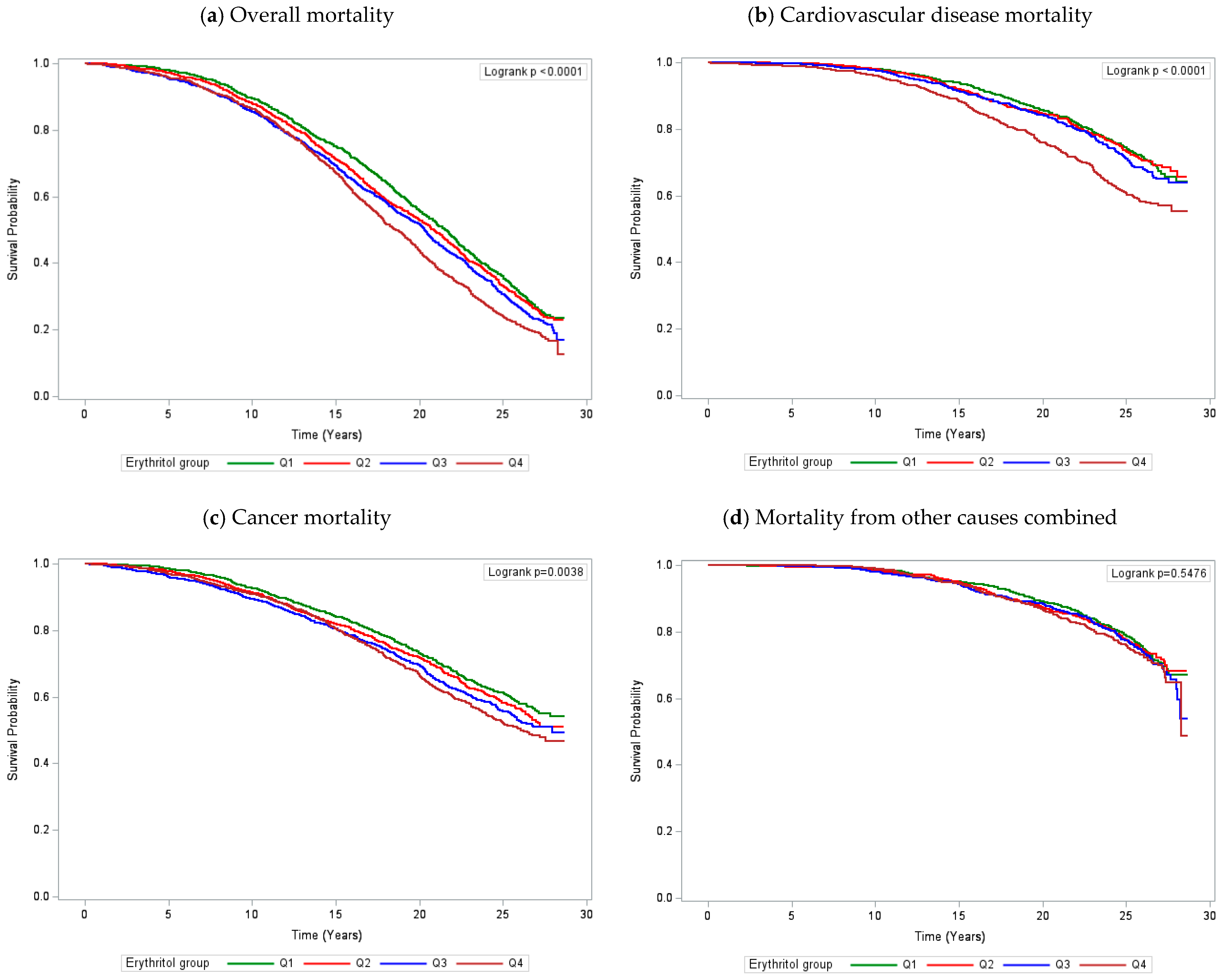
| All causes | 3375 | 1.00 | 1.02 (0.93–1.13) | 1.06 (0.97–1.17) | 1.15 (1.04–1.27) | 0.003 | 1.50 (1.17–1.92) | 0.001 |
| Cardiovascular disease | 983 | 1.00 | 0.93 (0.77–1.12) | 0.97 (0.81–1.17) | 1.21 (1.02–1.45) | 0.02 | 1.86 (1.18–2.94) | 0.008 |
| Heart disease | 168 | 1.00 | 0.99 (0.62–1.58) | 1.05 (0.66–1.66) | 1.33 (0.86–2.06) | 0.17 | 3.03 (1.00–9.17) | 0.05 |
| Stroke | 189 | 1.00 | 0.69 (0.45–1.08) | 1.02 (0.68–1.52) | 1.20 (0.81–1.79) | 0.15 | 2.06 (0.72–5.90) | 0.18 |
| Cancer | 1657 | 1.00 | 1.08 (0.94–1.24) | 1.14 (0.99–1.31) | 1.17 (1.02–1.35) | 0.02 | 1.54 (1.09–2.19) | 0.02 |
| Other causes combined | 735 | 1.00 | 1.03 (0.84–1.27) | 1.05 (0.86–1.29) | 1.02 (0.83–1.26) | 0.80 | 1.07 (0.63–1.81) | 0.80 |
| Subgroups 1 | No. of Deaths | HR (95% CI) for Q4 versus Q1 2 | HR (95% CI) (Continuous) 2,3 | P for Interaction 2,3 |
|---|---|---|---|---|
| Age (years) | 0.003 | |||
| <55 | 803 | 0.95 (0.77–1.16) | 0.93 (0.57–1.52) | |
| ≥55 | 2572 | 1.36 (1.21–1.52) | 2.27 (1.72–3.00) | |
| BMI (kg/m2) | 0.08 | |||
| <26.0 | 1710 | 1.02 (0.89–1.17) | 1.21 (0.86–1.72) | |
| ≥26.0 | 1666 | 1.30 (1.13–1.50) | 1.85 (1.31–2.60) | |
| Number of cigarettes smoked daily | 0.66 | |||
| <20 | 1335 | 1.12 (0.96–1.30) | 1.52 (1.02–2.26) | |
| ≥20 | 2040 | 1.17 (1.03–1.32) | 1.50 (1.10–2.04) | |
| Years of smoking | 0.25 | |||
| <37 | 1413 | 1.05 (0.91–1.22) | 1.23 (0.85–1.80) | |
| ≥37 | 1962 | 1.23 (1.08–1.41) | 1.69 (1.22–2.34) | |
| Systolic blood pressure (mmHg) | 0.35 | |||
| <140 | 1525 | 1.14 (0.99–1.32) | 1.35 (0.93–1.96) | |
| ≥140 | 1850 | 1.15 (1.01–1.32) | 1.60 (1.15–2.22) | |
| Diastolic blood pressure (mmHg) | 0.01 | |||
| <88 | 1650 | 0.97 (0.84–1.12) | 1.06 (0.74–1.52) | |
| ≥88 | 1725 | 1.36 (1.19–1.56) | 2.07 (1.48–2.90) | |
| Serum total cholesterol (mmol/L) | 0.84 | |||
| <6.17 | 1681 | 1.16 (1.01–1.33) | 1.56 (1.10–2.20) | |
| ≥6.17 | 1694 | 1.13 (0.99–1.30) | 1.39 (0.98–1.97) | |
| Diabetes mellitus | 0.60 | |||
| No | 3250 | 1.16 (1.05–1.28) | 1.52 (1.19–1.96) | |
| Yes | 125 | 0.95 (0.52–1.71) | 0.98 (0.26–3.69) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.; Hong, H.G.; Huang, J.; Stolzenberg-Solomon, R.; Mondul, A.M.; Weinstein, S.J.; Albanes, D. Serum Erythritol and Risk of Overall and Cause-Specific Mortality in a Cohort of Men. Nutrients 2024, 16, 3099. https://doi.org/10.3390/nu16183099
Lim J, Hong HG, Huang J, Stolzenberg-Solomon R, Mondul AM, Weinstein SJ, Albanes D. Serum Erythritol and Risk of Overall and Cause-Specific Mortality in a Cohort of Men. Nutrients. 2024; 16(18):3099. https://doi.org/10.3390/nu16183099
Chicago/Turabian StyleLim, Jungeun, Hyokyoung G. Hong, Jiaqi Huang, Rachael Stolzenberg-Solomon, Alison M. Mondul, Stephanie J. Weinstein, and Demetrius Albanes. 2024. "Serum Erythritol and Risk of Overall and Cause-Specific Mortality in a Cohort of Men" Nutrients 16, no. 18: 3099. https://doi.org/10.3390/nu16183099
APA StyleLim, J., Hong, H. G., Huang, J., Stolzenberg-Solomon, R., Mondul, A. M., Weinstein, S. J., & Albanes, D. (2024). Serum Erythritol and Risk of Overall and Cause-Specific Mortality in a Cohort of Men. Nutrients, 16(18), 3099. https://doi.org/10.3390/nu16183099





