Effects of Purified Vitexin and Iso-Vitexin from Mung Bean Seed Coat on Antihyperglycemic Activity and Gut Microbiota in Overweight Individuals’ Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Preparation of Vitexin and Iso-Vitexin from Mung Bean Seed Coat
2.4. Quantitative Determination of Phenolic Compounds in Mung Bean Seed Coat Extract (MBCE)
2.4.1. Quantitative Analysis of MBC Phenolic Compounds
2.4.2. Separation of Vitexin and Iso-Vitexin from MBCE
2.4.3. HPLC Analysis
2.5. Synergistic Reaction Mixture Preparation
2.6. Determination of Antioxidant Activity
2.6.1. The Determination of Antioxidant Activity Using the DPPH Method
2.6.2. The Determination of Antioxidant Activity Using the ABTS•+ Method
2.6.3. The Determination of Antioxidant Activity Using the FRAP Method
2.7. Determination of Antihyperglycemic Activity
2.7.1. Alpha-Amylase Inhibition Assay
2.7.2. Alpha-Glucosidase Inhibition Assay
2.7.3. Determination of Synergistic Activity
2.7.4. HepG2 Cell Cultures for Glucose Uptake
2.7.5. Cytotoxicity Assay
2.7.6. Glucose Uptake in IR-HepG2 Cells
2.8. Fecal Sample Collection
2.9. Simulation of Human Gut Model
2.10. DNA Extraction, 16s rRNA Gene Sequencing, and Analysis
2.11. Statistical Analysis
3. Results
3.1. Quantitative and Qualitative Characters of Phenolic Compounds in Mung Bean Seed Coat Extract (MBCE)
3.2. Antioxidant Activity
3.3. Enzyme Inhibitory Activity and Synergistic Activity of Vitexin and Iso-Vitexin
3.4. Cytotoxicity of HepG2 Cells
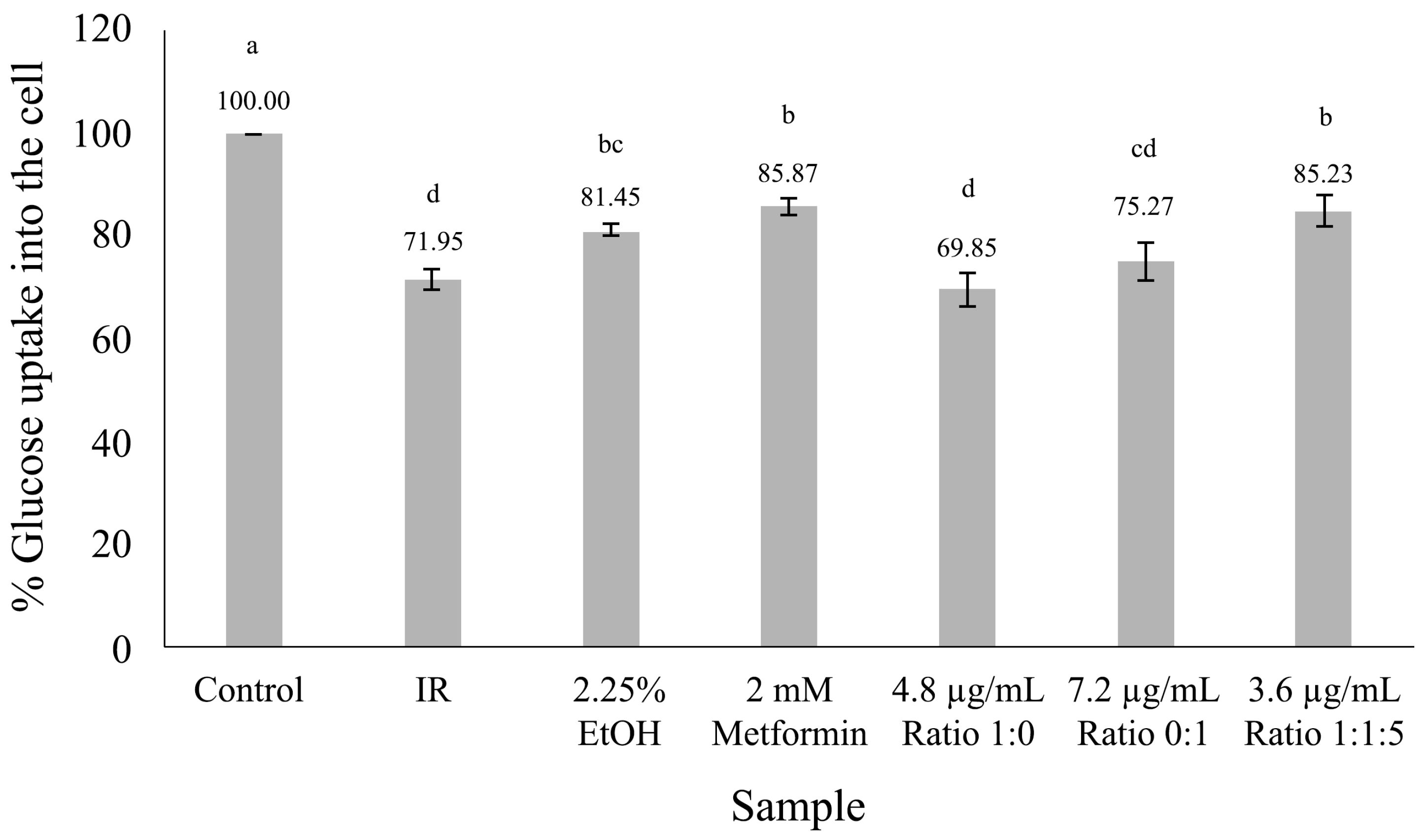
3.5. Glucose Uptake in HepG2 Cell
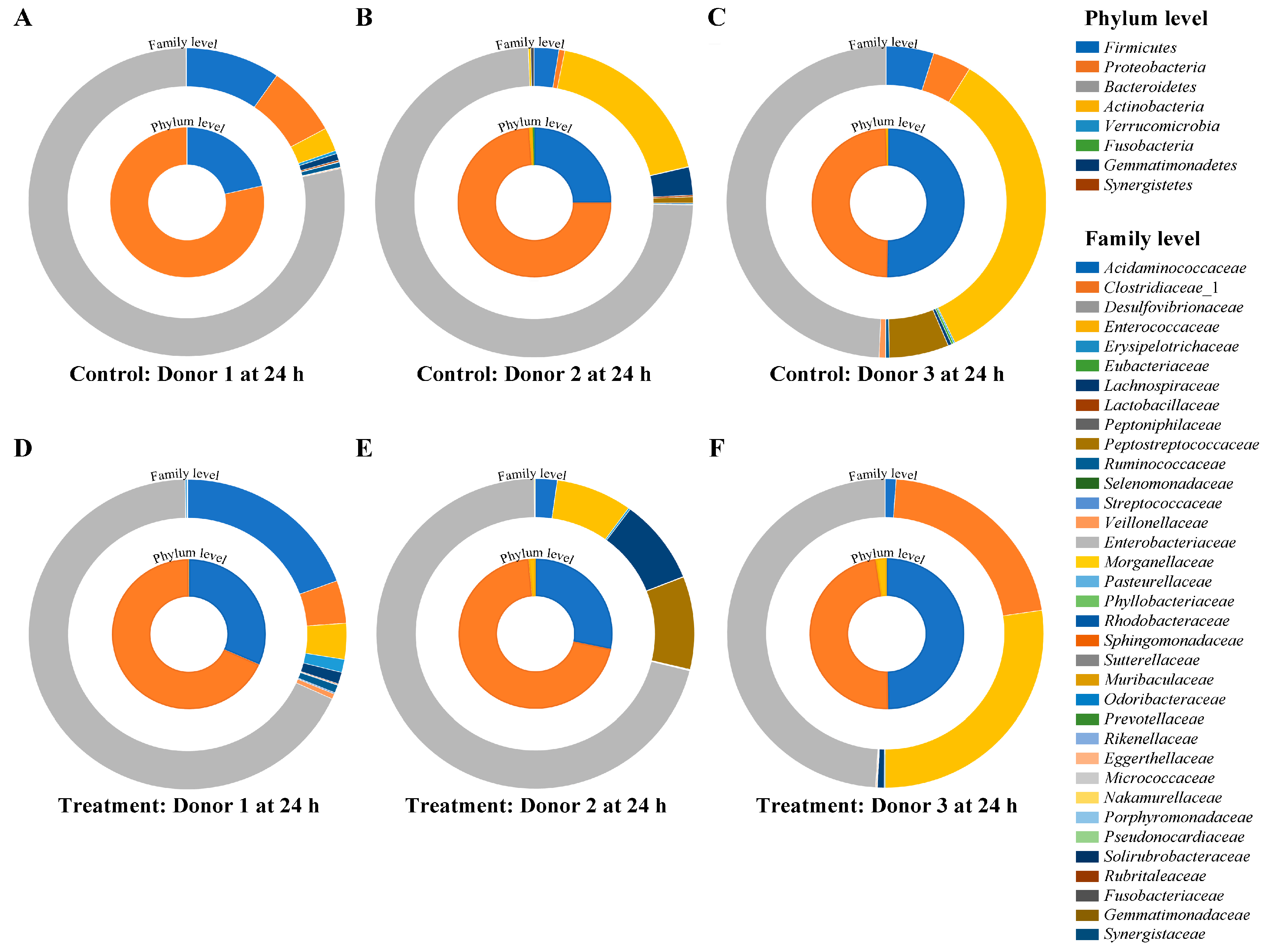
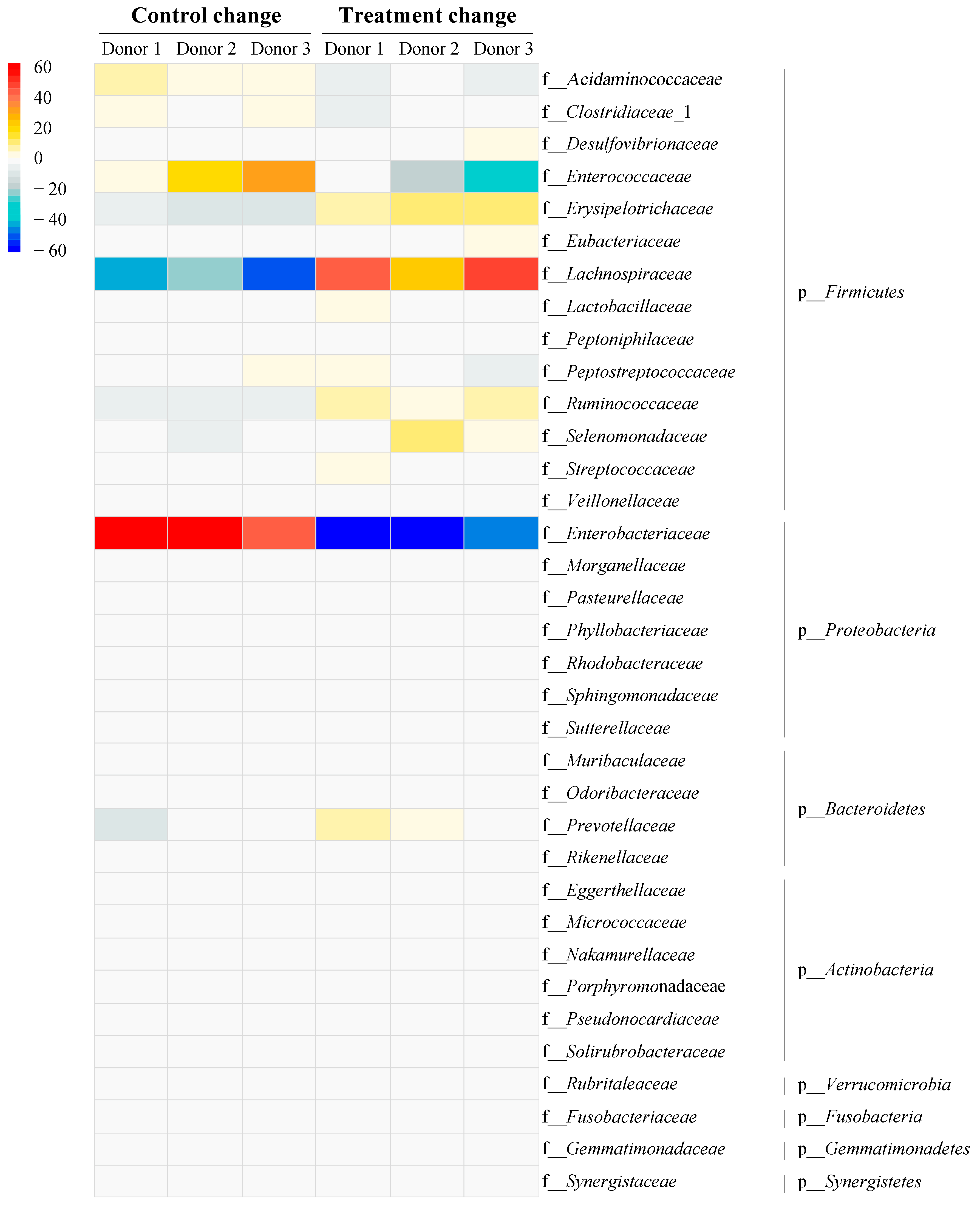
3.6. Impact of Vitexin/Iso-Vitexin in Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Public Health, T. Type-2 Diabetes Mellitus in Thailand in 2019. 2019. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7177402/ (accessed on 4 January 2020).
- Chatterjee, S.; Riewpaiboon, A.; Piyauthakit, P.; Riewpaiboon, W.; Boupaijit, K. Cost of diabetes and its complications in Thailand: A complete picture of economic burden. Health Soc. Care Community 2011, 19, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. 2014, 7, 587–591. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Cardona, F.; Tinahones, F.; Queipo-Ortuño, M.I. Impact of the gut microbiota on the development of obesity and type 2 diabetes mellitus. Front. Microbiol. 2014, 5, 190. [Google Scholar] [CrossRef]
- Chen, K.; Gao, Z.; Ding, Q.; Tang, C.; Zhange, H.; Zhai, T.; Xie, W.; Jin, Z.; Zhao, L.; Liu, W. Effect of natural polyphenols in Chinese herbal medicine on obesity and diabetes: Interactions among gut microbiota, metabolism, and immunity. Front. Nutr. 2022, 9, 962720. [Google Scholar] [CrossRef]
- Sami, W.; Ansari, T.; Butt, N.S.; Abdul Hamid, M.R. Effect of diet on type 2 diabetes mellitus: A review. Int. J. Health Sci. 2017, 11, 65–71. [Google Scholar]
- Bacanli, M.; Dilsiz, S.A.; Basaran, N.; Başaran, A.A. Chapter Five—Effects of phytochemicals against diabetes. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 209–238. [Google Scholar]
- Grosso, G.; Stepaniak, U.; Micek, A.; Kozela, M.; Stefler, D.; Bobak, M.; Pajak, A. Dietary polyphenol intake and risk of type 2 diabetes in the Polish arm of the Health, Alcohol and Psychosocial factors in Eastern Europe (HAPIEE) study. Br. J. Nutr. 2017, 118, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Gan, R.Y.; Zhou, T.; Xu, D.P.; Li, H.B. Optimization of Ultrasound-Assisted Extraction of Antioxidants from the Mung Bean Coat. Molecules 2017, 22, 638. [Google Scholar] [CrossRef]
- Pavasutti, V.; Sinthuvanich, C.; Tayana, N.; Kongkiatpaiboon, S.; Sae-tan, S. Mung bean seed coat water extract restores insulin sensitivity via upregulation of antioxidant defense system and downregulation of inflammation in insulin-resistant HepG2 cells. NFS J. 2023, 32, 100145. [Google Scholar] [CrossRef]
- Maneewan, S.; Tangpromphan, P.; Jaree, A. Separation of Vitexin and Iso-vitexin from Mung Bean Seed Coats Using a Three-Zone Simulated Moving Bed (SMB). Waste Biomass Valorization 2021, 12, 6601–6618. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A critical review on phytochemical profile and health promoting effects of mung bean (Vigna radiata). Food Sci. Hum. Wellness 2018, 7, 11–33. [Google Scholar] [CrossRef]
- Luo, J.; Cai, W.; Wu, T.; Xu, B. Phytochemical distribution in hull and cotyledon of adzuki bean (Vigna angularis L.) and mung bean (Vigna radiate L.), and their contribution to antioxidant, anti-inflammatory and anti-diabetic activities. Food Chem. 2016, 201, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Azubuike-Osu, O.S.; Ohanenye, I.C.; Jacob, C.; Ejike, C.E.C.C.; Udenigwe, C.C. Beneficial Role of Vitexin and Isovitexin Flavonoids in the Vascular Endothelium and Cardiovascular System. Curr. Nutraceuticals 2021, 2, 127–134. [Google Scholar] [CrossRef]
- Agah, S.; Kim, H.; Mertens-Talcott, S.U.; Awika, J.M. Complementary cereals and legumes for health: Synergistic interaction of sorghum flavones and cowpea flavonols against LPS-induced inflammation in colonic myofibroblasts. Mol. Nutr. Food Res. 2017, 61, 1600625. [Google Scholar] [CrossRef]
- Zhang, L.; Virgous, C.; Si, H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]
- He, M.; Min, J.W.; Kong, W.L.; He, X.H.; Li, J.X.; Peng, B.W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef]
- Domínguez-Avila, J.A.; Villa-Rodriguez, J.A.; Montiel-Herrera, M.; Pacheco-Ordaz, R.; Roopchand, D.E.; Venema, K.; González-Aguilar, G.A. Phenolic Compounds Promote Diversity of Gut Microbiota and Maintain Colonic Health. Dig. Dis. Sci. 2020, 66, 3270–3289. [Google Scholar] [CrossRef]
- Hajimehdipoor, H.; Shahrestani, R.; Shekarchi, M. Investigating the synergistic antioxidant effects of some flavonoid and phenolic compounds. Res. J. Pharmacogn. 2014, 1, 35–40. [Google Scholar]
- Wuttisin, N.; Boonsook, W. Total Phenolic, Flavonoid Contents and Antioxidant Activity of Siraitia grosvenorii Fruits Extracts. Food Appl. Biosci. J. 2019, 7, 131–141. [Google Scholar]
- Gourineni, V.P.; Verghese, M.; Boateng, J. Anticancer effects of prebiotics Synergy1® and soybean extracts: Possible synergistic mechanisms in Caco-2 cells. J. Cancer Res. 2010, 6, 220–233. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, A.; Rice-Evan, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [PubMed]
- Etsassala, N.G.E.R.; Badmus, J.A.; Marnewick, J.L.; Iwuoha, E.I.; Nchu, F.; Hussein, A.A. Alpha-Glucosidase and Alpha-Amylase Inhibitory Activities, Molecular Docking, and Antioxidant Capacities of Salvia aurita Constituents. Antioxidants 2020, 9, 1149. [Google Scholar] [CrossRef]
- Asghari, B.; Salehi, P.; Sonboli, A.; Ebrahimi, S.N. Flavonoids from Salvia chloroleuca with α-Amylsae and α-Glucosidase Inhibitory Effect. Iran. J. Pharm. Res. 2015, 14, 609–615. [Google Scholar]
- Rumjuankiat, K.; Perez, R.H.; Pilasombut, K.; Keaawsompong, S.; Zendo, T.; Sonomoto, K.; Nitisinprasert, S. Purification and characterization of a novel plantaricin, KL-1Y, from Lactobacillus plantarum KL-1. World J. Microbiol. Biotechnol. 2015, 31, 983–994. [Google Scholar] [CrossRef]
- Choi, J.S.; Islam, M.N.; Ali, M.Y.; Kim, Y.M.; Kungm, H.A. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food Chem. Toxicol. 2014, 64, 27–33. [Google Scholar] [CrossRef]
- Zou, C.; Wang, Y.; Shen, Z. 2-NBDG as a fluorescent indicator for direct glucose uptake measurement. J. Biochem. Biophys. Methods 2005, 64, 207–215. [Google Scholar] [CrossRef]
- Onumpai, C.; Kolidam, S.; Bonnin, E.; Rastall, R.A. Microbial Utilization and Selectivity of Pectin Fractions with Various Structures. Appl. Environ. Microbiol. 2011, 77, 5747–5754. [Google Scholar] [CrossRef]
- Pusuntisumpun, N.; Tunsagool, P.; Nitisinprasent, S.; Nakphaichit, M. Impacts of combining Limosilactobacillus reuteri KUB-AC5 and Limosilactobacillus fermentum KUB-D18 on overweight gut microbiota using a simulated human colon model. Int. J. Food Sci. Technol. 2024, 59, 1898–1910. [Google Scholar] [CrossRef]
- Oboh, G.; Issac, A.T.; Akinyemi, A.J.; Ajani, R.A. Inhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside induced lipid peroxidation in rats’ pancreas by phenolic extracts of avocado pear leaves and fruit. Int. J. Biomed. Sci. 2014, 10, 208–216. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols-Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Charoensiddhi, S.; Chanput, W.P.; Sae-Tan, S. Gut Microbiota Modulation, Anti-Diabetic and Anti-Inflammatory Properties of Polyphenol Extract from Mung Bean Seed Coat (Vigna radiata L.). Nutrients 2022, 14, 2275. [Google Scholar] [CrossRef]
- Supasatyankul, B.; Saisriyoot, M.; Klinkesorn, U.; Rattanaporn, K.; Sae-tan, S. Extraction of Phenolic and Flavonoid Compounds from Mung Bean (Vigna radiata L.) Seed Coat by Pressurized Liquid Extraction. Molecules 2022, 27, 2085. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, S.Y. Can antioxidants be effective therapeutics for type 2 diabetes? Yeungnam Univ. J. Med. 2021, 38, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Montonen, J.; Knekt, P.; Järvinen, A.; Reunanen, A. Dietary Antioxidant Intake and Risk of Type 2 Diabetes. Diabetes Care 2004, 27, 362–366. [Google Scholar] [CrossRef]
- Kumar, S.; Sandhir, R.; Ojha, S. Evaluation of antioxidant activity and total phenol in different varieties of Lantana camara leaves. BMC Res. Notes 2014, 7, 560. [Google Scholar] [CrossRef]
- Abdulai, I.L.; Kwofie, S.K.; Gbewonyo, W.S.; Boison, D.; Puplampum, J.B.; Adinortey, M.B. Multitargeted Effects of Vitexin and Isovitexin on Diabetes Mellitus and Its Complications. Sci. World J. 2021, 2021, 6641128. [Google Scholar] [CrossRef]
- Olszowy-Tomczyk, M. Synergistic, antagonistic and additive antioxidant effects in the binary mixtures. Phytochem. Rev. 2020, 19, 63–103. [Google Scholar] [CrossRef]
- Mohamed, E.A.H.; Siddiqui, M.J.A.; Ang, L.F.; Sadikun, A.; Chan, S.H.; Tan, S.C.; Asmawi, M.Z.; Yam, M.F. Potent α-glucosidase and α-amylase inhibitory activities of standardized 50% ethanolic extracts and sinensetin from Orthosiphon stamineus Benth as anti-diabetic mechanism. BMC Complement. Altern. Med. 2012, 12, 176. [Google Scholar] [CrossRef]
- Eom, S.H.; Lee, S.H.; Yoon, N.Y.; Jung, W.K.; Jeon, Y.J.; Kim, S.K.; Lee, M.S.; Lim, Y.M. α-Glucosidase- and α-amylase-inhibitory activities of phlorotannins from Eisenia bicyclis. J. Sci. Food Agric. 2012, 92, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Marmouzi, I.; Kharbach, M.; Jemli, M.E.; Bouyahya, A.; Cherrah, Y.; Bouklouze, A.; Heyden, Y.V.; Faouzi, M.E.A. Antidiabetic, dermatoprotective, antioxidant and chemical functionalities in Zizyphus lotus leaves and fruits. Ind. Crops Prod. 2019, 132, 134–139. [Google Scholar] [CrossRef]
- Oboh, G. Antioxidant and antimicrobial properties of ethanolic extract of Ocimum gratissimum leaves. J. Pharmacol. Toxicol. 2006, 1, 47–53. [Google Scholar] [CrossRef]
- Ni, M.; Hu, X.; Gong, D.; Zhang, G. Inhibitory mechanism of vitexin on α-glucosidase and its synergy with acarbose. Food Hydrocoll. 2020, 105, 105824. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; Ulrih, N.P.; Sengupta, P.K.; Xiao, J. Plasma protein binding of dietary polyphenols to human serum albumin: A high performance affinity chromatography approach. Food Chem. 2019, 270, 257–263. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, Z.; Tang, Y.; Chen, P.; Liu, R. The effects of glycosylation on the bioactivity of flavonoids. J. Agric. Food Chem. 2019, 67, 10539–10551. [Google Scholar]
- Reaven, G.M. Pathophysiology of insulin resistance in human disease. Physiol. Rev. 1995, 75, 473–486. [Google Scholar] [CrossRef]
- Hou, J.C.; Williams, D.; Vicogene, J.; Pessin, J.E. The Glucose Transporter 2 Undergoes Plasma Membrane Endocytosis and Lysosomal Degradation in a Secretagogue-Dependent Manner. Endocrinology 2009, 150, 4056–4064. [Google Scholar] [CrossRef] [PubMed]
- Santoleri, D.; Titchenell, P.M. Resolving the Paradox of Hepatic Insulin Resistance. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 447–456. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Doble, M. Synergistic effect of phytochemicals in combination with hypoglycemic drugs on glucose uptake in myotubes. Phytomedicine 2009, 16, 1119–1126. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, L.; Teng, H.; Song, H.; Wu, X.; Xu, M. Phenolic compounds ameliorate the glucose uptake in HepG2 cells’ insulin resistance via activating AMPK: Anti-diabetic effect of phenolic compounds in HepG2 cells. J. Funct. Foods 2015, 19, 487–494. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Chen, H.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Chan, L.C. Manilkara zapota (L.) P. Royen Leaf Water Extract Induces Apoptosis in Human Hepatocellular Carcinoma (HepG2) Cells via ERK1/2/Akt1/JNK1 Signaling Pathways. Evid.-Based Complement. Altern. Med. 2018, 2018, 7826576. [Google Scholar] [CrossRef] [PubMed]
- Umeki, S.; Hisamoto, N.; Hara, Y. Study on background factors associated with impaired glucose tolerance and/or diabetes mellitus. Eur. J. Endocrinol. 1989, 120, 729–734. [Google Scholar] [CrossRef]
- Mokuda, O.; Tanaka, H.; Hayashi, T.; Ooka, H.; Okazaki, R.; Sakamoto, Y. Ethanol Stimulates Glycogenolysis and Inhibits both Glycogenesis via Gluconeogenesis and from Exogenous Glucose in Perfused Rat Liver. Ann. Nutr. Metab. 2004, 48, 276–280. [Google Scholar] [CrossRef]
- Feng, L.; Song, Y.F.; Guan, Q.B.; Liu, H.J.; Ban, B.; Dong, H.X.; Hou, X.L.; Lee, K.O.; Gao, L.; Zhao, J.J. Long-term ethanol exposure inhibits glucose transporter 4 expression via an AMPK-dependent pathway in adipocytes. Acta Pharmacol. Sin. 2010, 31, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Lederberg, J.; McCray, A.T. ‘Ome Sweet ‘Omics—A Genealogical Treasury of Words. Scientist 2001, 15, 8. [Google Scholar]
- Bielka, W.; Przezak, A.; Pawlik, A. The Role of the Gut Microbiota in the Pathogenesis of Diabetes. Int. J. Mol. Sci. 2022, 23, 480. [Google Scholar] [CrossRef]
- Kootte, R.S.; Vrieze, A.; Holleman, F.; Dallinga-Thie, G.M.; Vos, W.M.; Groen, A.K.; Hoekstra, J.B.L.; Stroes, E.S.; Nieuwdorp, M. The therapeutic potential of manipulating gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Obes. Metab. 2012, 14, 112–120. [Google Scholar] [CrossRef]
- Relman, D.A.; Falkow, S. The meaning and impact of the human genome sequence for microbiology. Trends Microbiol. 2001, 9, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef]
- Somnuk, S.; Komindr, S.; Monkhai, S.; Poolsawat, T.; Nakphaichit, M.; Wanikorn, B. Metabolic and inflammatory profiles, gut microbiota and lifestyle factors in overweight and normal weight young thai adults. PLoS ONE 2023, 18, e0288286. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Stephens, J.W.; Harris, D.A. Gut microbiota influence in type 2 diabetes mellitus (T2DM). Gut Pathog. 2021, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Pushpanathan, P.; Srikanth, P.; Seshadri, K.G.; Selvarajan, S.; Pitanim, R.S.; Kumar, T.D.; Janarthanan, R. Gut Microbiota in Type 2 Diabetes Individuals and Correlation with Monocyte Chemoattractant Protein1 and Interferon Gamma from Patients Attending a Tertiary Care Centre in Chennai, India. Indian J. Endocrinol. Metab. 2016, 20, 523–530. [Google Scholar]
- Horrocks, V.; King, O.G.; Marques, I.M.; McDonald, J.A.K. Role of the gut microbiota in nutrient competition and protection against intestinal pathogen colonization. Microbiology 2023, 169, 001377. [Google Scholar] [CrossRef]
- Lebreton, F.; Willems, R.; Gilmore, M. Enterococcus Diversity, Origins in Nature, and Gut Colonization. 2014. Available online: https://www.ncbi.nlm.nih.gov/books/NBK190427 (accessed on 19 April 2024).
- Karlsson, C.L.J.; Onnerfält, J.; Xu, J.; Molin, G.; Ahrné, S.; Thorngren-Jerneck, K. The Microbiota of the Gut in Preschool Children with Normal and Excessive Body Weight. Obesity 2012, 20, 2257–2261. [Google Scholar] [CrossRef]
- Rodriguez, D.M.; Benninghoff, A.D.; Aardema, N.D.J.; Phatak, S.; Hintze, K.J. Basal Diet Determined Long-Term Composition of the Gut Microbiome and Mouse Phenotype to a Greater Extent than Fecal Microbiome Transfer from Lean or Obese Human Donors. Nutrients 2019, 11, 1630. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Wang, W.; Zhang, B. Research on the intestinal flora of the 239 patients in the elderly non-intestinal diseases. Chongqing Med. 2012, 41, 2400–2401. [Google Scholar]
- Ma, Q.; Li, Y.; Li, P.; Wang, M.; Wang, J.; Tang, Z.; Wang, T.; Luo, L.; Wang, C.; Wang, T.; et al. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed. Pharmacother. 2019, 117, 109138. [Google Scholar] [CrossRef] [PubMed]
- Biddle, A.; Stewart, L.; Blanchaed, J.; Leschine, S. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Andrade, B.G.N.; Bressani, F.A.; Cuadrat, R.R.C.; Tizioto, P.C.; de Oliveira, P.S.N.; Mourão, G.B.; Coutinho, L.L.; Reecy, J.M.; Koltes, J.E.; Walsh, P.; et al. The structure of microbial populations in Nelore GIT reveals inter-dependency of methanogens in feces and rumen. J. Anim. Sci. Biotechnol. 2020, 11, 6. [Google Scholar] [CrossRef]
- O’Connor, K.; Morrissette, M.; Strandwitz, P.; Ghiglieri, M.; Caboni, M.; Liu, H.; Khoo, C.; D’Onofrio, A.; Lewis, K. Cranberry extracts promote growth of Bacteroidaceae and decrease abundance of Enterobacteriaceae in a human gut simulator model. PLoS ONE 2019, 14, e0224836. [Google Scholar] [CrossRef]
- Ecevit, K.; Barros, A.A.; Silva, J.M.; Reis, R.L. Preventing Microbial Infections with Natural Phenolic Compounds. Future Pharmacol. 2022, 2, 460–498. [Google Scholar] [CrossRef]
- Marina, M.-O.; Alain, O.; Sandrine, P.C. Functional characterisation of gut microbiota and metabolism in Type 2 diabetes indicates that Clostridiales and Enterococcus could play a key role in the disease. bioRxiv 2019, 836114. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, S.J.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Aguirre, M.; de Souza, C.B.; Venema, K. The Gut Microbiota from Lean and Obese Subjects Contribute Differently to the Fermentation of Arabinogalactan and Inulin. PLoS ONE 2016, 11, e0159236. [Google Scholar] [CrossRef]


| Sample | Ratio | IC50 (µg/mL) | FRAP (mg Trolox/g MBCE) | |
|---|---|---|---|---|
| DPPH | ABTS | |||
| Vitexin/iso-vitexin | 0:1 | 4.91 ± 0.71 g | 1.27 ± 0.20 f | 41.04 ± 0.04 a |
| 1:0 | 10.22 ± 1.84 f | 9.55 ± 1.51 e | 37.81 ± 0.04 a | |
| 1:1 | 73.22 ± 7.31 a | 71.76 ± 10.13 a | 39.06 ± 0.03 a | |
| 1:1.5 | 63.64 ± 12.34 b | 54.67 ± 1.80 b | 18.36 ± 0.04 b | |
| 1:2 | 42.44 ± 4.45 d | 41.80 ± 1.56 c | 15.32 ± 0.05 bc | |
| 1:2.5 | 36.12 ± 0.18 de | 48.16 ± 1.96 bc | 12.52 ± 0.05 cd | |
| 1:3 | 30.19 ± 0.42 c | 31.24 ± 0.52 d | 10.38 ± 0.05 de | |
| 1.5:1 | 78.31 ± 2.70 a | 53.06 ± 1.02 b | 9.33 ± 0.01 e | |
| 2:1 | 74.14 ± 0.01 a | 42.10 ± 2.98 c | 14.64 ± 0.02 c | |
| 2.5:1 | 52.48 ± 6.44 c | 29.31 ± 12.64 d | 12.29 ± 0.01 cd | |
| 3:1 | 52.16 ± 7.30 c | 31.83 ± 0.88 d | 9.96 ± 0.04 e | |
| Trolox | - | 30.00 ± 0.05 e | 44.00 ± 0.03 c | - |
| Sample | Ratio (Vitexin/Iso-Vitexin) | %Alpha-Amylase Inhibition | FIA Index |
|---|---|---|---|
| MBCE | 0:1 | 63.07 ± 0.40 g | 1.00 |
| 1:0 | 71.31 ± 0.22 a | 1.00 | |
| 1:1 | 63.94 ± 0.88 g | 1.26 | |
| 1:1.5 | 68.45 ± 1.30 fed | 2.13 | |
| 1:2 | 62.67 ± 0.37 gh | 2.43 | |
| 1:2.5 | 60.79 ± 0.59 h | 1.61 | |
| 1:3 | 69.10 ± 0.17 bcd | 2.01 | |
| 1.5:1 | 68.56 ± 1.84 fed | 2.44 | |
| 2:1 | 67.83 ± 0.84 fed | 2.41 | |
| 2.5:1 | 66.49 ± 0.39 f | 2.15 | |
| 3:1 | 68.50 ± 0.89 fed | 2.44 | |
| Commercial standard | 0:1 | 62.53 ± 0.80 gh | 1.00 |
| 1:0 | 70.84 ± 0.32 a | 1.00 | |
| 1:1 | 63.81 ± 1.32 g | 1.92 | |
| 1:1.5 | 68.30 ± 1.17 fed | 2.06 | |
| 1:2 | 63.06 ± 1.94 g | 1.88 | |
| 1:2.5 | 68.30 ± 1.58 fed | 2.06 | |
| 1:3 | 69.30 ± 0.40 bcd | 2.09 | |
| 1.5:1 | 68.70 ± 1.76 fed | 2.07 | |
| 2:1 | 68.23 ± 1.47 fed | 2.05 | |
| 2.5:1 | 66.75 ± 0.62 f | 1.93 | |
| 3:1 | 69.23 ± 0.66 bcd | 2.08 | |
| Acarbose | - | 70.78 ± 0.83 ab | - |
| Sample | Ratio (Vitexin/Iso-Vitexin) | %Alpha-Glucosidase Inhibition | FIA Index |
|---|---|---|---|
| MBCE | 0:1 | 52.30 ± 1.88 fg | 1.00 |
| 1:0 | 60.83 ± 0.84 bc | 1.00 | |
| 1:1 | 35.45 ± 1.70 i | 1.91 | |
| 1:1.5 | 59.83 ± 1.14 bc | 2.05 | |
| 1:2 | 54.48 ± 1.14 def | 1.97 | |
| 1:2.5 | 45.29 ± 1.06 h | 1.82 | |
| 1:3 | 56.46 ± 1.43 ef | 2.06 | |
| 1.5:1 | 44.42 ± 1.49 h | 1.33 | |
| 2:1 | 32.17 ± 0.76 i | 0.96 | |
| 2.5:1 | 56.45 ± 1.68 ef | 2.09 | |
| 3:1 | 57.99 ± 1.98 cd | 1.73 | |
| Commercial standard | 0:1 | 53.16 ± 1.75 fg | 1.00 |
| 1:0 | 61.49 ± 0.84 bc | 1.00 | |
| 1:1 | 36.32 ± 0.87 i | 1.09 | |
| 1:1.5 | 63.89 ± 1.32 bc | 1.92 | |
| 1:2 | 51.20 ± 1.33 g | 2.13 | |
| 1:2.5 | 45.08 ± 1.27 h | 1.36 | |
| 1:3 | 56.89 ± 0.89 ef | 1.71 | |
| 1.5:1 | 45.51 ± 1.99 h | 1.37 | |
| 2:1 | 36.55 ± 1.54 i | 1.10 | |
| 2.5:1 | 56.89 ± 1.05 efg | 2.25 | |
| 3:1 | 56.02 ± 1.33 efg | 1.69 | |
| Acarbose | - | 71.55 ± 1.09 a | - |
| Genus | Control | Treatment | ||||
|---|---|---|---|---|---|---|
| Donor 1 | Donor 2 | Donor 3 | Donor 1 | Donor 2 | Donor 3 | |
| Adlercreutzia | −0.0332 | −0.0061 | −0.0052 | 0.0401 | 0.0036 | 0.0094 |
| Terrisporobacter | −0.0302 | −0.0056 | −0.0690 | 0.04240 | 0.0010 | 0.0673 |
| Promicromonospora | −0.5617 | −0.2125 | −1.5741 | 1.2150 | 0.3586 | 1.9098 |
| Pseudonocardia | −0.7932 | −1.5335 | −1.4444 | 2.1024 | 3.2836 | 1.6846 |
| Anaerostipes | −1.9218 | −0.1260 | −0.2782 | 3.0414 | 0.2375 | 0.2779 |
| Akkermansia | −0.0042 | −0.0097 | −0.0197 | 0.0070 | 0.0272 | 0.0328 |
| Alistipes | −0.0286 | −0.0466 | −0.8382 | 0.0316 | 0.0646 | 0.8999 |
| Parabacteroides | −16.3417 | −15.4376 | −26.1212 | 13.5192 | 12.4244 | 23.1782 |
| Ruminococcus 2 | −1.4958 | −14.1600 | −18.5971 | 2.2853 | 10.2125 | 16.5730 |
| Roseburia | −7.3000 | −0.1716 | −1.4926 | 5.0034 | 0.2746 | 1.4147 |
| Megasphaera | −0.5343 | −1.5168 | −3.0740 | 1.0407 | 1.7551 | 3.6659 |
| Weissella | −0.0064 | −0.0020 | −0.0249 | 0.0190 | 0.0056 | 0.0407 |
| Neglecta | −6.7556 | 0.0000 | −0.06885 | 5.9144 | 0.0000 | 0.0965 |
| Romboutsia | −1.0271 | −3.0153 | −1.3982 | 1.5801 | 2.2438 | 1.1298 |
| Enterocloster | −3.8984 | −1.5035 | −3.2172 | 5.3252 | 2.6369 | 3.1128 |
| Peptacetobacter | 0.0000 | −6.5689 | −0.7458 | 0.0000 | 12.1658 | 1.2034 |
| Collinsella | −11.2280 | −2.7956 | −0.0431 | 6.8622 | 1.3445 | 0.0448 |
| Paraclostridium | −0.0916 | −0.2868 | −0.8999 | 0.2199 | 0.7557 | 1.1106 |
| Duncaniella | −1.7761 | −0.0102 | −1.2393 | 1.4411 | 0.0195 | 1.0158 |
| Streptococcus | −0.8264 | −0.1352 | −0.0379 | 1.0430 | 0.1934 | 0.0423 |
| Gillisia | −1.9951 | −1.2536 | −0.0099 | 2.1649 | 0.6211 | 1.0158 |
| Lawsonibacter | 6.6491 | 0.0000 | 2.1445 | −6.5535 | 0.0000 | −2.1697 |
| Proteus | 2.4677 | 17.6293 | 33.3981 | −2.4674 | −17.4997 | −33.3996 |
| Butyricicoccus | 2.2504 | 1.2140 | 21.3012 | −1.8365 | −0.9277 | −20.3182 |
| Bifidobacterium | 0.0260 | 0.0025 | 0.0000 | −0.0225 | −0.0015 | −0.0005 |
| Fusobacterium | 0.4532 | 2.0020 | 2.0617 | 0.2838 | −1.9637 | −1.9650 |
| Flavonifractor | 0.0016 | 0.5628 | 0.0000 | −0.0011 | −0.5103 | 0.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yutharaksanukul, P.; Tangpromphan, P.; Tunsagool, P.; Sae-tan, S.; Nitisinprasert, S.; Somnuk, S.; Nakphaichit, M.; Pusuntisumpun, N.; Wanikorn, B. Effects of Purified Vitexin and Iso-Vitexin from Mung Bean Seed Coat on Antihyperglycemic Activity and Gut Microbiota in Overweight Individuals’ Modulation. Nutrients 2024, 16, 3017. https://doi.org/10.3390/nu16173017
Yutharaksanukul P, Tangpromphan P, Tunsagool P, Sae-tan S, Nitisinprasert S, Somnuk S, Nakphaichit M, Pusuntisumpun N, Wanikorn B. Effects of Purified Vitexin and Iso-Vitexin from Mung Bean Seed Coat on Antihyperglycemic Activity and Gut Microbiota in Overweight Individuals’ Modulation. Nutrients. 2024; 16(17):3017. https://doi.org/10.3390/nu16173017
Chicago/Turabian StyleYutharaksanukul, Pornlada, Preuk Tangpromphan, Paiboon Tunsagool, Sudathip Sae-tan, Sunee Nitisinprasert, Surasawadee Somnuk, Massalin Nakphaichit, Nut Pusuntisumpun, and Bandhita Wanikorn. 2024. "Effects of Purified Vitexin and Iso-Vitexin from Mung Bean Seed Coat on Antihyperglycemic Activity and Gut Microbiota in Overweight Individuals’ Modulation" Nutrients 16, no. 17: 3017. https://doi.org/10.3390/nu16173017
APA StyleYutharaksanukul, P., Tangpromphan, P., Tunsagool, P., Sae-tan, S., Nitisinprasert, S., Somnuk, S., Nakphaichit, M., Pusuntisumpun, N., & Wanikorn, B. (2024). Effects of Purified Vitexin and Iso-Vitexin from Mung Bean Seed Coat on Antihyperglycemic Activity and Gut Microbiota in Overweight Individuals’ Modulation. Nutrients, 16(17), 3017. https://doi.org/10.3390/nu16173017







