No Difference between the Efficacy of High-Nitrate and Low-Nitrate Vegetable Supplementation on Blood Pressure after 16 Weeks in Individuals with Early-Stage Hypertension: An Exploratory, Double-Blinded, Randomized, Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Design
2.3. Study Participants
2.4. Dietary Interventions
2.5. Outcome Measurements
2.5.1. Office Blood Pressure
2.5.2. Ambulatory Blood Pressure
2.5.3. Central Blood Pressure and Arterial Stiffness
2.5.4. Additional CVD Risk Factors
2.5.5. Compliance and Safety
2.5.6. Statistical Analyses
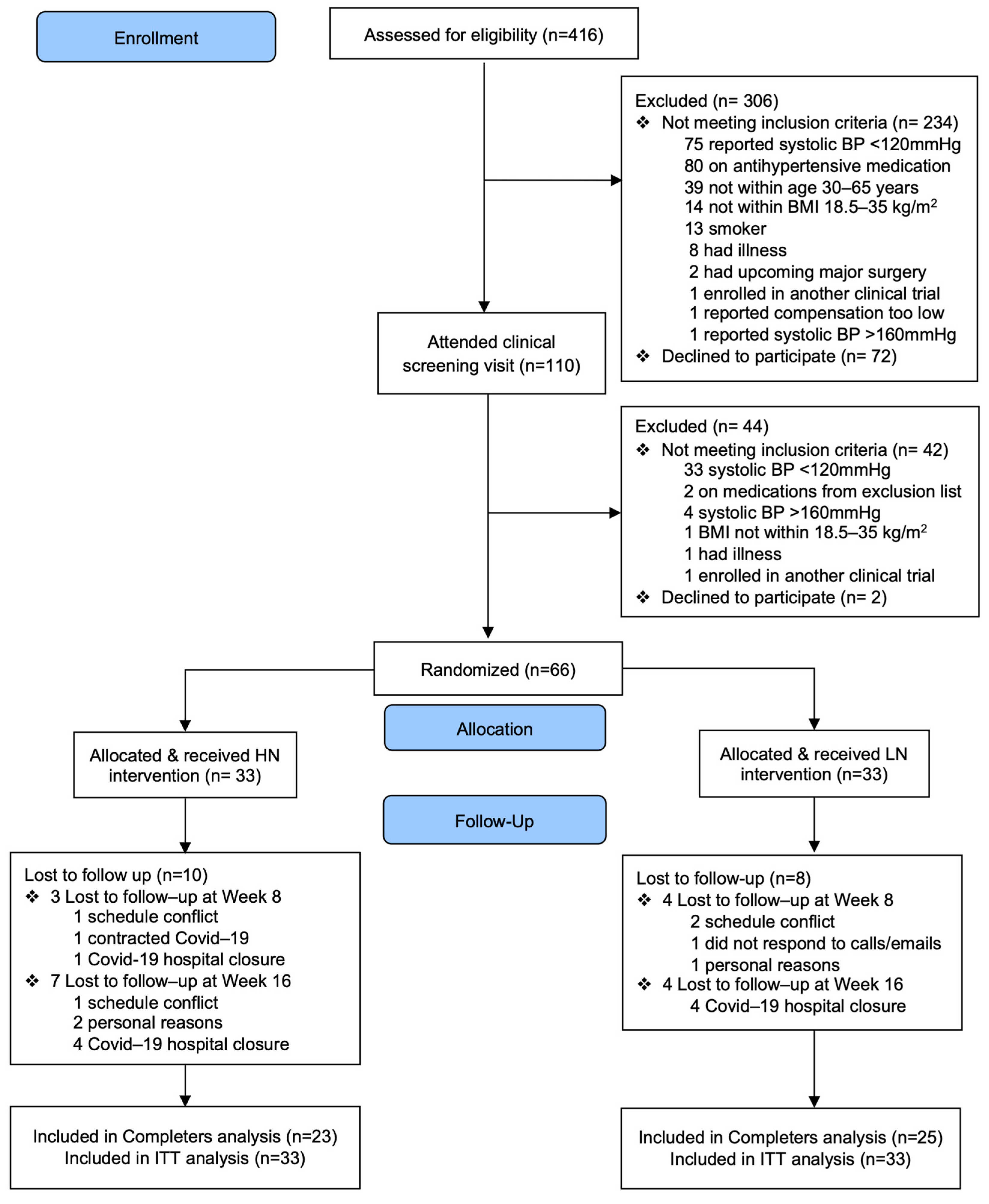
3. Results
3.1. Baseline Clinical Characteristics
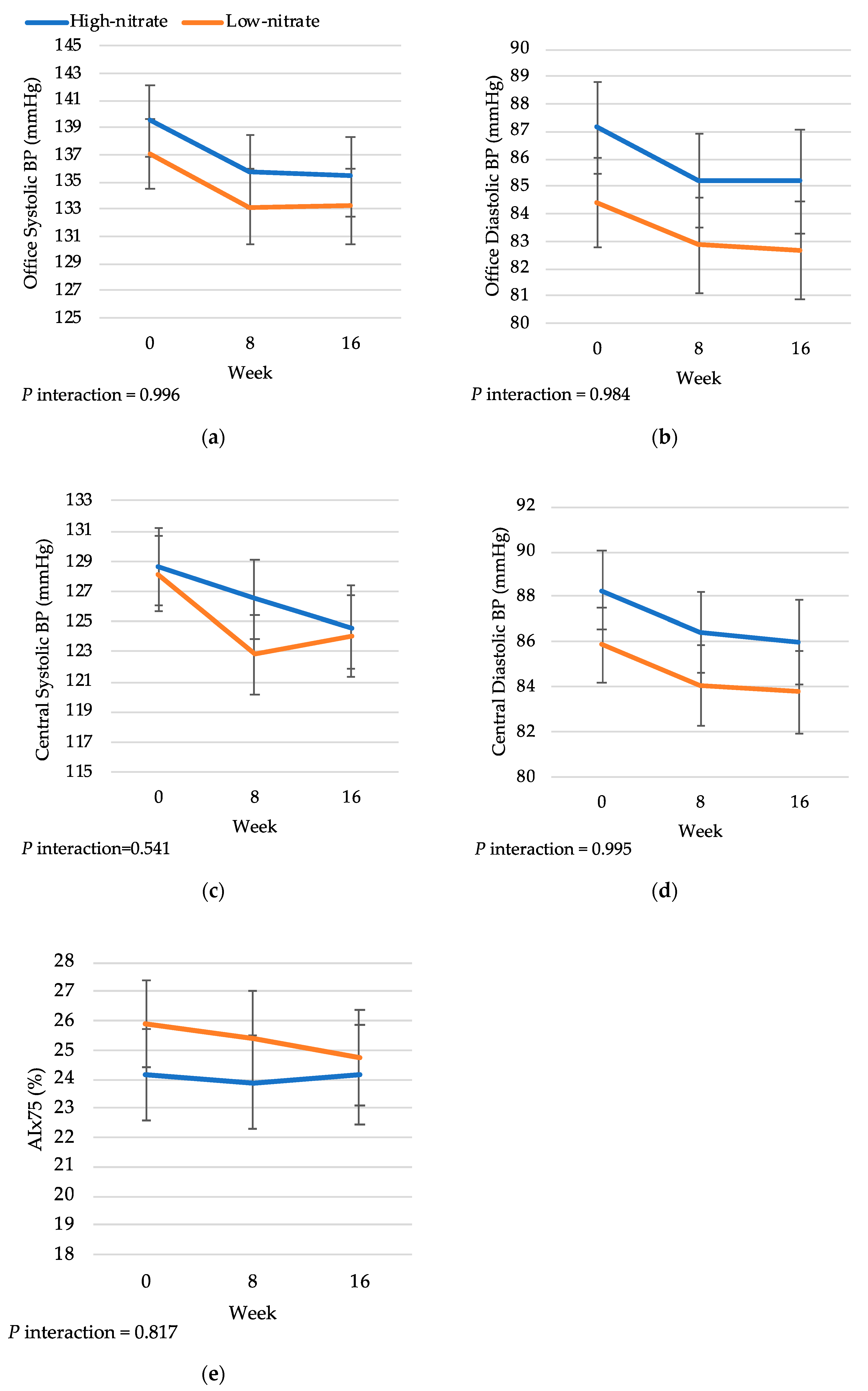
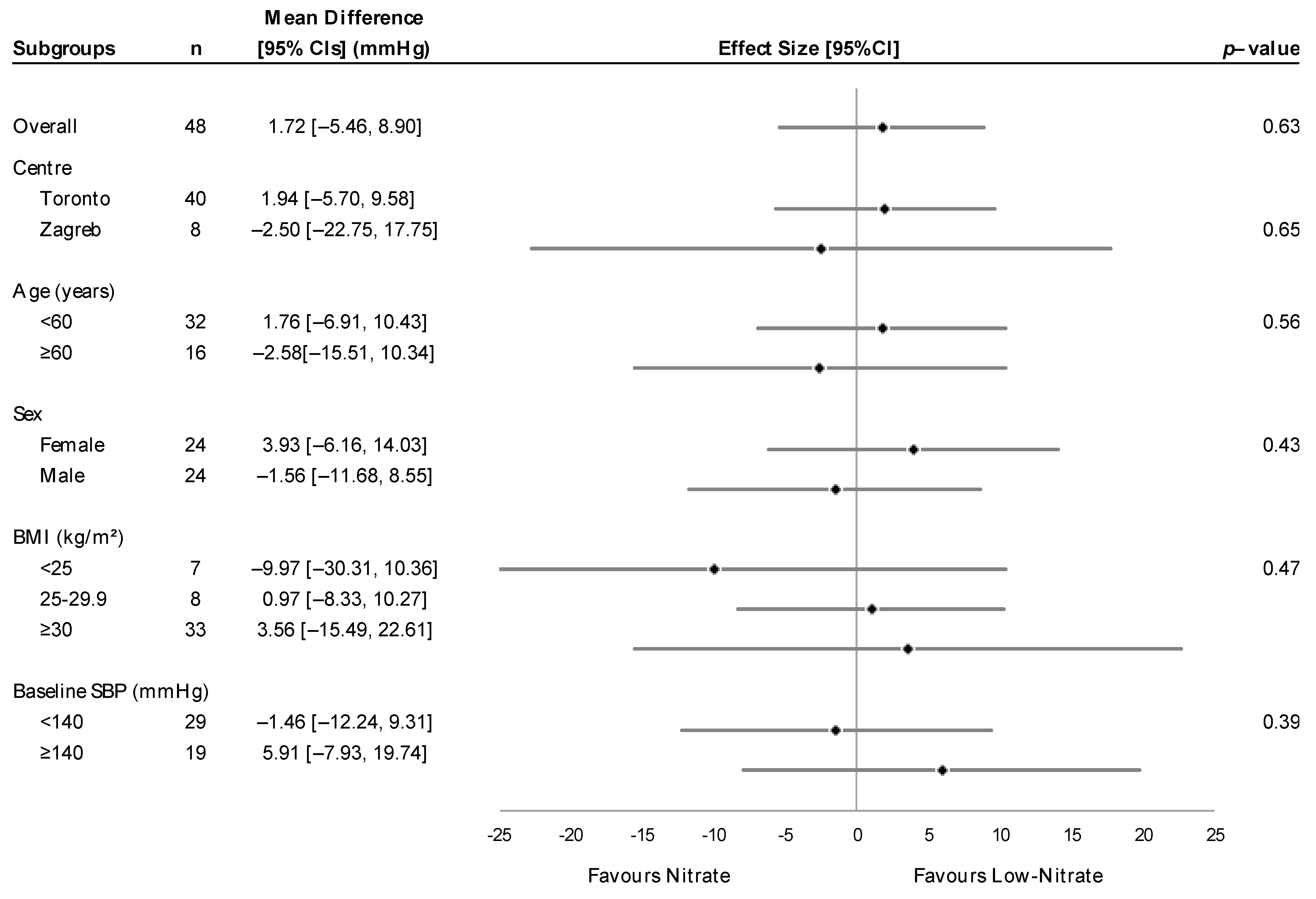
3.2. Treatment Effects on Blood Pressure
3.3. Treatment Effects on Arterial Stiffness
3.4. Treatment Effects on Lipids and C-Reactive Protein
3.5. Adherence to Treatments
3.6. Adverse Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.-C.; Joshipura, K.J.; Jiang, R.; Hu, F.B.; Hunter, D.; Smith-Warner, S.A.; Colditz, G.A.; Rosner, B.; Spiegelman, D.; Willett, W.C. Fruit and vegetable intake and risk of major chronic disease. J. Natl. Cancer Inst. 2004, 96, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef]
- Govoni, M.; Jansson, E.A.; Weitzberg, E.; Lundberg, J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 2008, 19, 333–337. [Google Scholar] [CrossRef]
- Babateen, A.M.; Shannon, O.M.; O’brien, G.M.; Okello, E.; Khan, A.A.; Rubele, S.; Wightman, E.; Smith, E.; McMahon, N.; Olgacer, D.; et al. Acceptability and Feasibility of a 13-Week Pilot Randomised Controlled Trial Testing the Effects of Incremental Doses of Beetroot Juice in Overweight and Obese Older Adults. Nutrients 2021, 13, 769. [Google Scholar] [CrossRef]
- Mills, C.E.; Govoni, V.; Faconti, L.; Casagrande, M.; Morant, S.V.; Crickmore, H.; Iqbal, F.; Maskell, P.; Masani, A.; Nanino, E.; et al. A randomised, factorial trial to reduce arterial stiffness independently of blood pressure: Proof of concept? The VaSera trial testing dietary nitrate and spironolactone. Br. J. Clin. Pharmacol. 2020, 86, 891–902. [Google Scholar] [CrossRef]
- Daiber, A.; Münzel, T. Organic Nitrate Therapy, Nitrate Tolerance, and Nitrate-Induced Endothelial Dysfunction: Emphasis on Redox Biology and Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 899. [Google Scholar] [CrossRef]
- Sundqvist, M.L.; Larsen, F.J.; Carlström, M.; Bottai, M.; Pernow, J.; Hellénius, M.-L.; Weitzberg, E.; Lundberg, J.O. A randomized clinical trial of the effects of leafy green vegetables and inorganic nitrate on blood pressure. Am. J. Clin. Nutr. 2020, 111, 749–756. [Google Scholar] [CrossRef]
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, phase 2, double-blind, placebo-controlled study. Hypertension 2015, 65, 320–327. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults a report of the American College of Cardiology/American Heart Association Task Force on Clinical practice guidelines. Hypertension 2018, 71, E13–E115. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Nevo, E.; Fetics, B.; Pak, P.H.; Yin, F.C.; Maughan, W.L.; Kass, D.A. Estimation of Central Aortic Pressure Waveform by Mathematical Transformation of Radial Tonometry Pressure. Circulation 1997, 95, 1827–1836. [Google Scholar] [CrossRef]
- Pauca, A.L.; O’Rourke, M.F.; Kon, N.D. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 2001, 38, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, I.B.; MacCallum, H.; Flint, L.; Cockcroft, J.R.; Newby, D.E.; Webb, D.J. The influence of heart rate on augmentation index and central arterial pressure in humans. J. Physiol. 2000, 525, 263–270. [Google Scholar] [CrossRef]
- Wilkinson, I.B.; Mohammad, N.H.; Tyrrell, S.; Hall, I.R.; Webb, D.J.; Paul, V.E.; Levy, T.; Cockcroft, J.R. Heart rate dependency of pulse pressure amplification and arterial stiffness. Am. J. Hypertens. 2002, 15, 24–30. [Google Scholar] [CrossRef]
- Yasmin; Brown, M.J. Similarities and differences between augmentation index and pulse wave velocity in the assessment of arterial stiffness. QJM An. Int. J. Med. 1999, 92, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Hung, C.L.; Sun, F.J.; Huang, P.H.; Cheng, Y.F.; Yeh, H.I. Augmentation Index Predicts the Sweat Volume in Young Runners. J. Sports Sci. Med. 2021, 20, 448. [Google Scholar] [CrossRef]
- Cleeman, J.I. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). J. Am. Med. Assoc. 2001, 285, 2486–2497. [Google Scholar]
- Anderson, T.J.; Grégoire, J.; Hegele, R.A.; Couture, P.; Mancini, G.B.J.; McPherson, R.; Francis, G.A.; Poirier, P.; Lau, D.C.; Grover, S.; et al. 2012 Update of the Canadian Cardiovascular Society Guidelines for the Diagnosis and Treatment of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can. J. Cardiol. 2013, 29, 151–167. [Google Scholar] [CrossRef]
- Chakraborty, H.; Gu, H. A Mixed Model Approach for Intent-to-Treat Analysis in Longitudinal Clinical Trials with Missing Values; RTI Press: Research Triangle Park, NC, USA, 2009. [Google Scholar] [CrossRef]
- Pearson, G.J.; Thanassoulis, G.; Anderson, T.J.; Barry, A.R.; Couture, P.; Dayan, N.; Francis, G.A.; Genest, J.; Grégoire, J.; Grover, S.A.; et al. 2021 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in Adults. Can. J. Cardiol. 2021, 37, 1129–1150. [Google Scholar] [CrossRef]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., III; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Janner, J.H.; Godtfredsen, N.S.; Ladelund, S.; Vestbo, J.; Prescott, E. High aortic augmentation index predicts mortality and cardiovascular events in men from a general population, but not in women. Eur. J. Prev. Cardiol. 2012, 20, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Blekkenhorst, L.C.; Lewis, J.R.; Prince, R.L.; Devine, A.; Bondonno, N.P.; Bondonno, C.P.; Wood, L.G.; Puddey, I.B.; Ward, N.C.; Croft, K.D.; et al. Nitrate-rich vegetables do not lower blood pressure in individuals with mildly elevated blood pressure: A 4-wk randomized controlled crossover trial. Am. J. Clin. Nutr. 2018, 107, 894–908. [Google Scholar] [CrossRef]
- Rogerson, D.; Aguilar Mora, F.A.; Young, J.S.; Klonizakis, M. No effect of nitrate-rich beetroot juice on microvascular function and blood pressure in younger and older individuals: A randomised, placebo-controlled double-blind pilot study. Eur. J. Clin. Nutr. 2022, 76, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, S.; Gan, J.M.; Rathod, K.S.; Khambata, R.S.; Ghosh, S.M.; Hartley, A.; Van Eijl, S.; Sagi-Kiss, V.; Chowdhury, T.A.; Curtis, M.; et al. Dietary nitrate improves vascular function in patients with hypercholesterolemia: A randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2016, 103, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, M.; Winyard, P.G.; Aizawa, K.; Anning, C.; Shore, A.; Benjamin, N. Effect of dietary nitrate on blood pressure, endothelial function, and insulin sensitivity in type 2 diabetes. Free Radic. Biol. Med. 2013, 60, 89–97. [Google Scholar] [CrossRef]
- Shepherd, A.I.; Gilchrist, M.; Winyard, P.G.; Jones, A.M.; Hallmann, E.; Kazimierczak, R.; Rembialkowska, E.; Benjamin, N.; Shore, A.C.; Wilkerson, D.P. Effects of dietary nitrate supplementation on the oxygen cost of exercise and walking performance in individuals with type 2 diabetes: A randomized, double-blind, placebo-controlled crossover trial. Free. Radic. Biol. Med. 2015, 86, 200–208. [Google Scholar] [CrossRef]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef]
- Faconti, L.; Mills, C.E.; Govoni, V.; Gu, H.; Morant, S.; Jiang, B.; Cruickshank, J.K.; Webb, A.J. Cardiac effects of 6 months’ dietary nitrate and spironolactone in patients with hypertension and with/at risk of type 2 diabetes, in the factorial design, double-blind, randomized controlled VaSera trial. Br. J. Clin. Pharmacol. 2018, 85, 169–180. [Google Scholar] [CrossRef]
- Ashor, A.W.; Jajja, A.; Sutyarjoko, A.; Brandt, K.; Qadir, O.; Lara, J.; Siervo, M. Effects of beetroot juice supplementation on microvascular blood flow in older overweight and obese subjects: A pilot randomised controlled study. J. Hum. Hypertens. 2014, 29, 511–513. [Google Scholar] [CrossRef]
- Jajja, A.; Sutyarjoko, A.; Lara, J.; Rennie, K.; Brandt, K.; Qadir, O.; Siervo, M. Beetroot supplementation lowers daily systolic blood pressure in older, overweight subjects. Nutr. Res. 2014, 34, 868–875. [Google Scholar] [CrossRef]
- Lara, J.; Ogbonmwan, I.; Oggioni, C.; Zheng, D.; Qadir, O.; Ashor, A.; Brandt, K.; Mathers, J.C.; Siervo, M. Effects of handgrip exercise or inorganic nitrate supplementation on 24-h ambulatory blood pressure and peripheral arterial function in overweight and obese middle age and older adults: A pilot RCT. Maturitas 2015, 82, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.I.; Wilkerson, D.P.; Fulford, J.; Winyard, P.G.; Benjamin, N.; Shore, A.C.; Gilchrist, M. Effect of nitrate supplementation on hepatic blood flow and glucose homeostasis: A double-blind, placebo-controlled, randomized control trial. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G356–G364. [Google Scholar] [CrossRef] [PubMed]
- Lyons, D.; Roy, S.; Patel, M.; Benjamin, N.; Swift, C.G. Impaired Nitric Oxide-Mediated Vasodilatation and Total Body Nitric Oxide Production in Healthy Old Age. Clin. Sci. 1997, 93, 519–525. [Google Scholar] [CrossRef]
- Minamino, T.; Komuro, I. Vascular Cell Senescence. Circ. Res. 2007, 100, 15–26. [Google Scholar] [CrossRef]
- Percival, R.S.; Challacombe, S.J.; Marsh, P.D. Age-related microbiological changes in the salivary and plaque microflora of healthy adults. J. Med. Microbiol. 1991, 35, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Akita, S.; Sacks, F.M.; Svetkey, L.P.; Conlin, P.R.; Kimura, G. Effects of the Dietary Approaches to Stop Hypertension (DASH) Diet on the Pressure-Natriuresis Relationship. Hypertension 2003, 42, 8–13. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (dash) diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Cherukuri, L.; Birudaraju, D.; Kinninger, A.; Chaganti, B.T.; Shekar, C.; Hamal, S.; Shaikh, K.; Flores, F.; Roy, S.K.; Sotka, W.; et al. Effect of a plant-based bioequivalent inorganic nitrate (NO3−) complex with vitamins, antioxidants and phytophenol rich food extracts in hypertensive individuals-A randomized, double-blind, placebo-controlled study. Clin. Nutr. ESPEN 2020, 40, 327–335. [Google Scholar] [CrossRef]
- Carlström, M.; Liu, M.; Yang, T.; Zollbrecht, C.; Huang, L.; Peleli, M.; Borniquel, S.; Kishikawa, H.; Hezel, M.; Persson, A.E.G.; et al. Cross-talk Between Nitrate-Nitrite-NO and NO Synthase Pathways in Control of Vascular NO Homeostasis. Antioxid. Redox Signal 2015, 23, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Nishi, S.K.; Jovanovski, E.; Zurbau, A.; Komishon, A.; Mejia, S.B.; Khan, T.A.; Sievenpiper, J.L.; Milicic, D.; Jenkins, A.; et al. Repeated administration of inorganic nitrate on blood pressure and arterial stiffness: A systematic review and meta-analysis of randomized controlled trials. J. Hypertens. 2020, 38, 2122–2140. [Google Scholar] [CrossRef] [PubMed]
- Cro, S.; Morris, T.P.; Kahan, B.C.; Cornelius, V.R.; Carpenter, J.R. A four-step strategy for handling missing outcome data in randomised trials affected by a pandemic. BMC Med. Res. Methodol. 2020, 20, 208. [Google Scholar] [CrossRef] [PubMed]
| Study Intervention | High Nitrate | Low Nitrate |
|---|---|---|
| Weight (g) | 30.0 | 30.0 |
| Water (g) | 1.9 | 2.1 |
| Calories (kcal) | 86.5 | 95.1 |
| Carbohydrate (g) | 18.7 | 19.2 |
| Fiber (g) | 6.4 | 5.6 |
| Fat (g) | 0.8 | 1 |
| Protein (g) | 5.4 | 4.7 |
| Nitrate (mg) | 404 | 48 |
| Potassium (mg) | 810 | 660 |
| Sodium (mg) | 228 | 57 |
| Magnesium (mg) | 108 | 42 |
| Calcium (mg) | 216 | 105 |
| Vitamin C (mg) | 75.7 | 104.7 |
| Characteristics | High-Nitrate (n = 33) | Low-Nitrate (n = 33) | p Value |
|---|---|---|---|
| Age, years | 49.9 (11.8) | 53.1 (0.7) | 0.23 |
| Male, No. (%) | 20 (60.6) | 19 (57.6) | |
| Female, No. (%) | 13 (39.4) | 14 (42.4) | 0.80 |
| Body weight, kg | 79.5 (14.2) | 81.9 (13.4) | 0.48 |
| BMI, kg/m2 | 27.7 (3.3) | 28.1 (3.2) | 0.59 |
| Body fat, % | 29.1 (7.5) | 30.2 (8.1) | 0.56 |
| Lipids Total Cholesterol, mmol/L | 4.9 (1.0) | 5.3 (1.1) | 0.18 |
| HDL Cholesterol, mmol/L | 1.3 (0.3) | 1.3 (0.3) | 0.59 |
| LDL Cholesterol | 3.0 (1.0) | 3.3 (1.0) | 0.17 |
| Non-HDL Cholesterol, mmol/L | 3.6 (1.0) | 4.0 (1.1) | 0.24 |
| Triglycerides, mmol/L | 1.4 (0.7) | 1.4 (0.6) | 0.91 |
| High-sensitivity C-reactive protein, mg/L | 1.5 (1.1) | 2.5 (3.0) | 0.08 |
| AST, U/L | 20.5 (6.4) | 21.0 (7.1) | 0.77 |
| Creatinine, µmol/L | 80.4 (12.1) | 71.8 (15.5) | 0.02 * |
| Blood Pressure Office Systolic Diastolic | 139.6 (10.0) 88.7 (8.7) | 137.7 (11.0) 85.1 (8.6) | 0.46 0.10 |
| 24-hour ABPM a | |||
| Systolic | 130.4 (11.4) | 130.6 (9.5) | 0.95 |
| Diastolic | 81.1 (8.2) | 82.4 (7.6) | 0.53 |
| Wake ABPM b | |||
| Systolic | 134.5 (11.6) | 135.2 (12.6) | 0.82 |
| Diastolic | 84.9 (9.1) | 85.5 (8.9) | 0.82 |
| Sleep ABPM c | |||
| Systolic | 120.6 (12.7) | 119.2 (11.8) | 0.66 |
| Diastolic | 72.5 (8.3) | 72.8 (8.5) | 0.88 |
| Central | |||
| Systolic | 129.0 (9.8) | 128.8 (12.8) | 0.95 |
| Diastolic | 90.0 (8.7) | 86.6 (9.1) | 0.13 |
| Heart Rate, bpm | 66.6 (9.2) | 67.9 (9.5) | 0.58 |
| Arterial Stiffness | |||
| AIx75, % | 22.3 (11.2) | 25.1 (10.1) | |
| Cf-PWV, m/s d | 7.22 (3.14) | 7.70 (2.86) | 0.55 |
| Duration of Elevated BP, months e | 39.5 (82.4) | 25.8 (88.2) | 0.52 |
| Concurrent Medications, No. (%) | |||
| Antihyperglycemic agents | 4 (12.1) | 3 (9.1) | 0.69 |
| Cholesterol-lowering agents | 4 (12.1) | 4 (12.1) | 1.00 |
| Hypothyroid medication | 2 (6.1) | 1 (3.0) | 0.55 |
| Antidepressants | 3 (9.1) | 1 (3.0) | 0.30 |
| Proton pump inhibitor | 2 (6.1) | 0 (0.0) | 0.15 |
| Steroid/bronchodilator | 1 (3.0) | 1 (3.0) | 1.00 |
| Outcome | High-Nitrate Within Group | Low-Nitrate Within Group | Between Groups | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 16 | Δ | p | Week 0 | Week 16 | Δ | p | Difference | p | ||
| Blood Pressure (mmHg) | |||||||||||
| Office | |||||||||||
| Systolic | 66 | 139.6 ± 1.74 | 138.53 ± 2.85 | −1.30 ± 2.92 | 0.66 | 137.7 ± 1.92 | 134.62 ± 2.65 | −4.39 ± 2.19 | 0.05 | 3.91 ± 3.52 | 0.27 |
| Diastolic | 66 | 88.68 ± 1.52 | 88.39 ± 1.65 | −0.94 ± 1.69 | 0.58 | 85.13 ± 1.50 | 86.18 ± 2.04 | −1.33 ± 1.53 | 0.39 | 2.21 ± 2.09 | 0.30 |
| 24-Hour Ambulatory | |||||||||||
| Systolic | 66 | 129.61 ± 2.12 | 135.61 ± 2.47 | 1.84 ± 1.86 | 0.33 | 131.51 ± 2.26 | 130.62 ± 3.89 | −2.98 ± 2.92 | 0.33 | 4.98 ± 3.49 | 0.18 |
| Diastolic | 66 | 81.00 ± 1.49 | 84.30 ± 2.36 | 0.31 ± 1.51 | 0.84 | 82.97 ± 1.53 | 82.91 ± 1.95 | −1.07 ± 1.84 | 0.56 | 1.39 ± 2.28 | 0.54 |
| Wake Ambulatory | |||||||||||
| Systolic | 66 | 134.52 ± 2.08 | 139.98 ± 2.55 | 1.18 ± 2.13 | 0.58 | 135.23 ± 2.28 | 137.24 ± 2.28 | −1.08 ± 2.21 | 0.63 | 2.74 ± 3.09 | 0.39 |
| Diastolic | 66 | 84.71 ± 1.80 | 86.56 ± 1.81 | −0.75 ± 1.64 | 0.65 | 85.78 ± 1.70 | 88.10 ± 1.82 | 0.78 ± 1.93 | 0.69 | −1.53 ± 2.14 | 0.47 |
| Sleep Ambulatory | |||||||||||
| Systolic | 66 | 120.55 ± 2.41 | 125.80 ± 3.35 | 3.80 ± 2.71 | 0.17 | 120.13 ± 2.49 | 119.98 ± 3.79 | −2.50 ± 3.14 | 0.43 | 5.82 ± 3.92 | 0.14 |
| Diastolic | 66 | 72.42 ± 1.52 | 74.51 ± 3.84 | 1.25 ± 2.48 | 0.62 | 73.12 ± 1.91 | 73.33 ± 3.30 | −0.36 ± 3.26 | 0.91 | 1.18 ± 3.34 | 0.73 |
| Central | |||||||||||
| Systolic | 66 | 128.97 ± 1.71 | 126.90 ± 2.92 | −3.84 ± 2.64 | 0.15 | 128.80 ± 2.23 | 125.67 ± 2.78 | −4.17 ± 2.33 | 0.08 | 1.23 ± 3.56 | 0.73 |
| Diastolic | 66 | 89.97 ± 1.52 | 87.92 ± 2.71 | −2.76 ± 2.34 | 0.25 | 86.64 ± 1.58 | 86.59 ± 1.58 | −2.48 ± 1.31 | 0.06 | 1.34 ± 2.37 | 0.58 |
| Arterial Stiffness | |||||||||||
| Cf-PWV, m/s | 66 | 7.17 ± 0.60 | 7.90 ± 0.81 | 0.05 ± 0.75 | 0.95 | 7.17 ± 0.60 | 7.96 ± 0.72 | −0.04 ± 0.79 | 0.96 | −0.061 ± 0.73 | 0.93 |
| AIx75, % | 66 | 22.28 ± 1.95 | 24.64 ± 1.75 | −0.16 ± 1.62 | 0.92 | 25.05 ± 1.76 | 24.90 ± 1.59 | −0.38 ± 1.22 | 0.76 | −0.26 ± 1.62 | 0.87 |
| Lipids (mmol/L) | |||||||||||
| Total Cholesterol | 66 | 4.83 ± 0.19 | 5.24 ± 0.20 | 0.27 ± 0.18 | 0.15 | 5.29 ± 0.20 | 4.91 ± 0.16 | −0.10 ± 0.15 | 0.48 | 0.34 ± 0.23 | 0.16 |
| LDL-Cholesterol | 66 | 2.91 ± 0.17 | 3.17 ± 0.16 | 0.16 ± 0.14 | 0.30 | 3.27 ± 0.20 | 3.04 ± 0.13 | −0.0017 ± 0.12 | 0.99 | 0.13 ± 0.18 | 0.48 |
| HDL-Cholesterol | 66 | 1.27 ± 0.05 | 1.23 ± 0.05 | −0.03 ± 0.03 | 0.26 | 1.30 ± 0.05 | 1.26 ± 0.04 | −0.01 ± 0.03 | 0.63 | −0.03 ± 0.04 | 0.42 |
| Non-HDL Cholesterol | 66 | 3.60 ± 0.18 | 3.92 ± 0.21 | 0.21 ± 0.18 | 0.26 | 3.95 ± 0.21 | 3.63 ± 0.20 | −0.10 ± 0.15 | 0.51 | 0.29 ± 0.19 | 0.14 |
| Triglycerides | 66 | 1.42 ± 0.13 | 1.73 ± 0.21 | 0.28 ± 0.21 | 0.19 | 1.42 ± 0.11 | 1.36 ± 0.18 | −0.04 ± 0.14 | 0.76 | 0.38 ± 0.22 | 0.10 |
| Inflammation | |||||||||||
| hs-CRP, mg/L | 63 | 1.58 ± 0.24 | 1.68 ± 0.30 | 0.32 ± 0.28 | 0.27 | 1.71 ± 0.27 | 1.16 ± 0.26 | −0.29 ± 0.22 | 0.20 | 0.52 ± 0.40 | 0.21 |
| Measures of Adherence | n | High Nitrate | Low Nitrate | p |
|---|---|---|---|---|
| Powder consumed (%) | 48 | 87.5 ± 3.7 | 92.5 ± 3.6 | 0.33 |
| Body weight (kg) | 48 | 75.1 ± 2.5 | 80.6 ± 2.6 | 0.14 |
| Started antihypertensive medication, n (%) | 66 | 4 (12.1) | 1 (3.0) | 0.16 |
| 24 h urinary sodium excretion | ||||
| mmol/d | 39 | 171 ± 22 | 128 ± 15 | 0.10 |
| mg/d | 39 | 3937 ± 505 | 2934 ± 340 | 0.10 |
| 24 h urinary potassium | ||||
| mmol/d | 36 | 73 ± 7 | 72 ± 10 | 0.99 |
| mg/d | 36 | 2834 ± 277 | 2830 ± 386 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Jovanovski, E.; Zurbau, A.; Sievenpiper, J.; Milicic, D.; El-Sohemy, A.; Vuksan, V. No Difference between the Efficacy of High-Nitrate and Low-Nitrate Vegetable Supplementation on Blood Pressure after 16 Weeks in Individuals with Early-Stage Hypertension: An Exploratory, Double-Blinded, Randomized, Controlled Trial. Nutrients 2024, 16, 3018. https://doi.org/10.3390/nu16173018
Li D, Jovanovski E, Zurbau A, Sievenpiper J, Milicic D, El-Sohemy A, Vuksan V. No Difference between the Efficacy of High-Nitrate and Low-Nitrate Vegetable Supplementation on Blood Pressure after 16 Weeks in Individuals with Early-Stage Hypertension: An Exploratory, Double-Blinded, Randomized, Controlled Trial. Nutrients. 2024; 16(17):3018. https://doi.org/10.3390/nu16173018
Chicago/Turabian StyleLi, Dandan, Elena Jovanovski, Andreea Zurbau, John Sievenpiper, Davor Milicic, Ahmed El-Sohemy, and Vladimir Vuksan. 2024. "No Difference between the Efficacy of High-Nitrate and Low-Nitrate Vegetable Supplementation on Blood Pressure after 16 Weeks in Individuals with Early-Stage Hypertension: An Exploratory, Double-Blinded, Randomized, Controlled Trial" Nutrients 16, no. 17: 3018. https://doi.org/10.3390/nu16173018
APA StyleLi, D., Jovanovski, E., Zurbau, A., Sievenpiper, J., Milicic, D., El-Sohemy, A., & Vuksan, V. (2024). No Difference between the Efficacy of High-Nitrate and Low-Nitrate Vegetable Supplementation on Blood Pressure after 16 Weeks in Individuals with Early-Stage Hypertension: An Exploratory, Double-Blinded, Randomized, Controlled Trial. Nutrients, 16(17), 3018. https://doi.org/10.3390/nu16173018









