Oral Protein Supplements Might Improve Nutritional Status and Quality of Life in Elderly Patients after Standard Pancreatic Resection
Abstract
1. Introduction
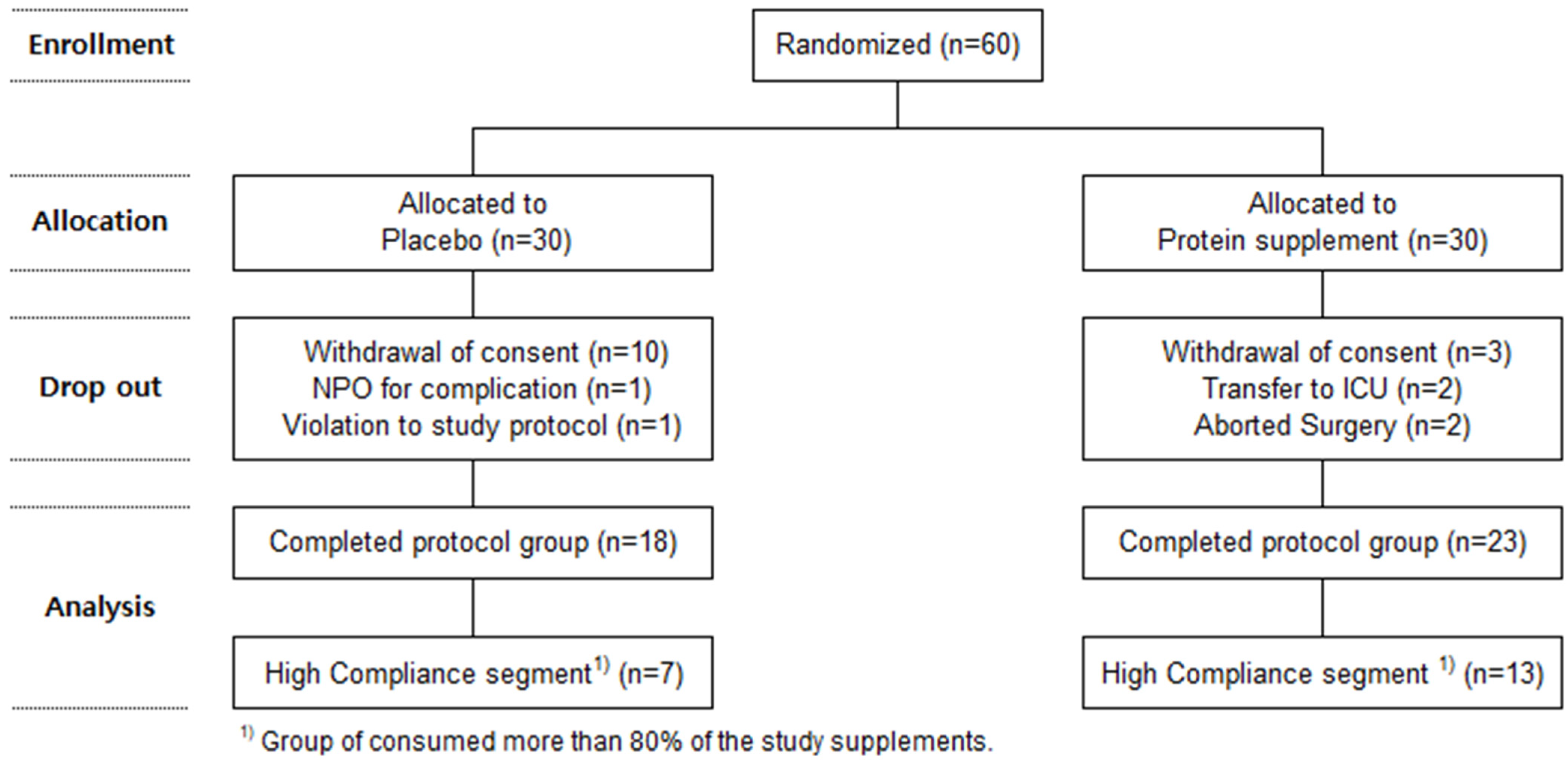
2. Materials and Methods
2.1. Research Subjects
2.2. Research Method
2.3. Methods of Data Collection and Evaluation
2.3.1. Evaluation of Nutritional Status
2.3.2. Evaluation of Intake
2.3.3. Evaluation of Quality of Life
2.3.4. Evaluation of Indicators Related to Sarcopenia
- Evaluation of Walking Speed
- Evaluation of Muscle Mass
2.4. Method of Statistical Analysis
3. Results
3.1. Evaluation of Nutritional Status (Figure 2)
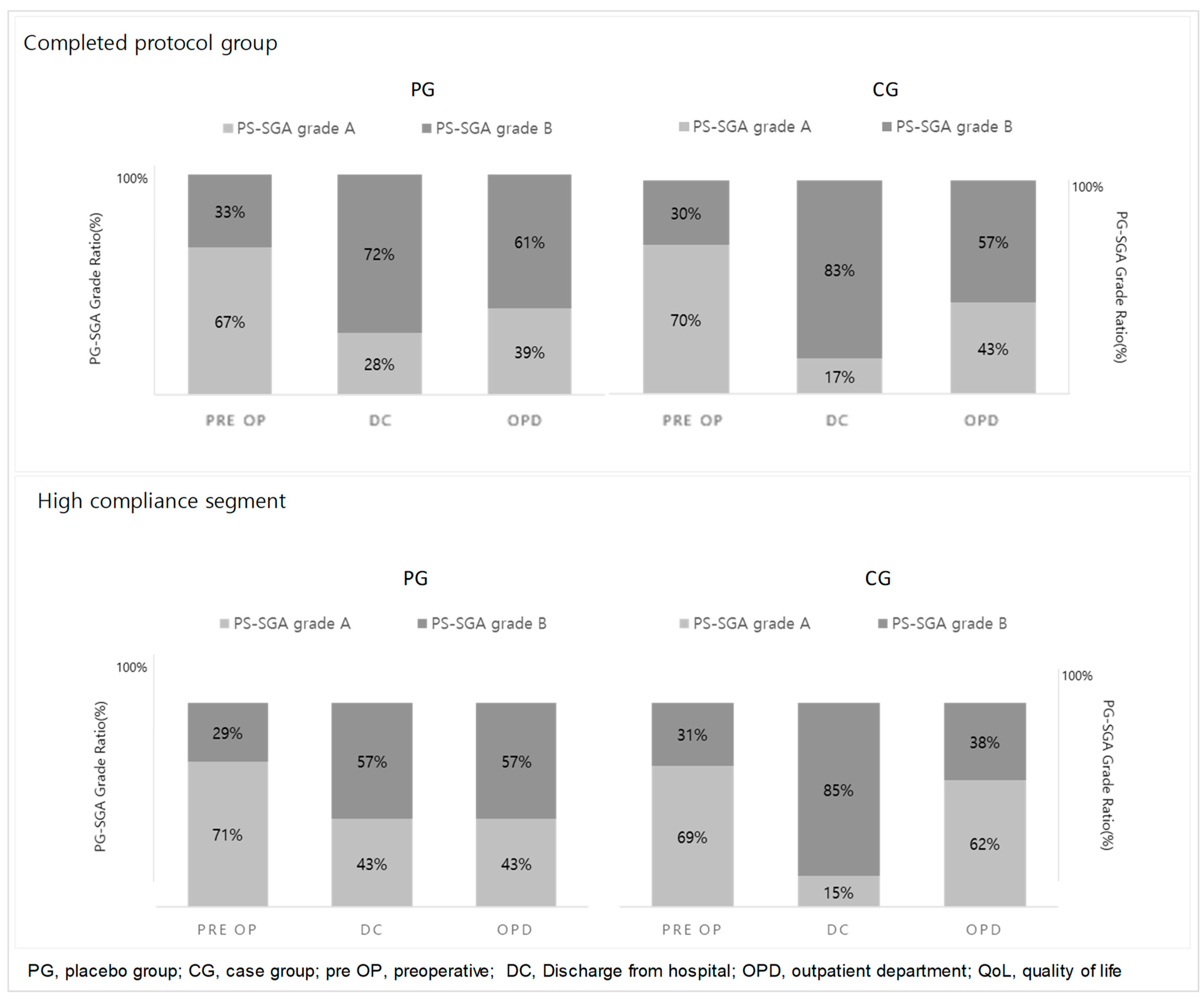
3.2. Analysis and Comparison of Nutrient Intake (Table 2)
| Variables (1) | Completed Protocol Group | High Compliance Segment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit | PG (n = 18) | CG (n = 23) | p-Value (2) | PG (n = 7) | CG (n = 13) | p-Value (2) | |||||||||
| Energy (kcal/day) | pre OP | 1549.3 | ± | 99.4 | 1678.2 | ± | 88.0 | 1481.4 | ± | 166.4 | 1811.7 | ± | 122.1 | ||
| DC | 770.6 | ± | 99.4 | 879.2 | ± | 88.0 | 0.699 | 917.4 | ± | 166.4 | 1024.1 | ± | 122.1 | 0.276 | |
| OPD | 1406.8 | ± | 99.4 | 1530.9 | ± | 88.0 | 0.819 | 1566.6 | ± | 166.4 | 1735.9 | ± | 122.1 | 0.705 | |
| Carbohydrate (g/day) | pre OP | 206.2 | ± | 12.6 | 224.0 | ± | 11.1 | 190.0 | ± | 17.5 | 243.3 | ± | 12.8 | ||
| DC | 90.9 | ± | 12.6 | 88.4 | ± | 11.1 | 0.319 | 99.2 | ± | 17.5 | 99.8 | ± | 12.8 | 0.077 | |
| OPD | 209.6 | ± | 12.6 | 214.1 | ± | 11.1 | 0.514 | 225.4 | ± | 17.5 | 249.6 | ± | 12.8 | 0.322 | |
| Protein (g/day) | pre OP | 72.9 | ± | 6.0 | 77.3 | ± | 5.3 | 70.7 | ± | 10.2 | 85.0 | ± | 7.5 | ||
| DC | 34.1 | ± | 6.0 | 52.1 | ± | 5.3 | 0.090 | 45.1 | ± | 10.2 | 61.9 | ± | 7.5 | 0.629 | |
| OPD | 56.7 | ± | 6.0 | 77.3 | ± | 5.3 | 0.049 | 67.6 | ± | 10.2 | 87.9 | ± | 7.5 | 0.475 | |
| Fat (g/day) | pre OP | 48.9 | ± | 4.6 | 52.4 | ± | 4.1 | 49.1 | ± | 8.0 | 56.5 | ± | 5.8 | ||
| DC | 29.3 | ± | 4.6 | 34.6 | ± | 4.1 | 0.968 | 37.0 | ± | 8.0 | 41.0 | ± | 5.8 | 0.658 | |
| OPD | 37.3 | ± | 4.6 | 42.9 | ± | 4.1 | 0.973 | 43.9 | ± | 8.0 | 48.3 | ± | 5.8 | 0.548 | |
3.3. Quality of Life (Table 3)
| Variables (1) | Completed Protocol Group | High Compliance Segment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit | PG (n = 18) | CG (n = 23) | p-Value (2) | PG (n = 7) | CG (n = 13) | p-Value (2) | |||||||||
| QoL (score) | pre OP | 9.8 | ± | 0.6 | 9.4 | ± | 0.5 | 9.8 | ± | 1.0 | 9.6 | ± | 0.7 | ||
| DC | 7.4 | ± | 0.6 | 8.7 | ± | 0.5 | 0.016 | 7.8 | ± | 0.9 | 9.1 | ± | 0.7 | 0.066 | |
| OPD | 8.3 | ± | 0.5 | 9.1 | ± | 0.5 | 0.042 | 7.9 | ± | 0.9 | 10.0 | ± | 0.6 | 0.017 | |
| 10-m walking speed (s) | pre OP | 10.5 | ± | 0.6 | 9.7 | ± | 0.6 | 9.9 | ± | 1.0 | 9.5 | ± | 0.7 | ||
| DC | 11.6 | ± | 0.6 | 10.4 | ± | 0.6 | 0.240 | 11.9 | ± | 1.0 | 10.2 | ± | 0.7 | 0.035 | |
| 1st OPD | 9.2 | ± | 0.6 | 9.4 | ± | 0.6 | 0.628 | 8.8 | ± | 1.0 | 9.7 | ± | 0.7 | 0.651 | |
| Body Composition | |||||||||||||||
| BCM (kg) | pre OP | 30.7 | ± | 1.4 | 32.5 | ± | 1.3 | 29.4 | ± | 2.3 | 33.6 | ± | 1.7 | ||
| DC | 31.2 | ± | 1.4 | 32.4 | ± | 1.3 | 0.446 | 29.7 | ± | 2.3 | 33.4 | ± | 1.7 | 0.738 | |
| 1st OPD | 29.7 | ± | 1.4 | 31.5 | ± | 1.3 | 0.928 | 28.7 | ± | 2.3 | 33.1 | ± | 1.7 | 0.891 | |
| PA (°) | pre OP | 5.5 | ± | 0.2 | 6.1 | ± | 0.2 | 5.6 | ± | 0.4 | 6.5 | ± | 0.3 | ||
| DC | 5.3 | ± | 0.2 | 5.8 | ± | 0.2 | 0.747 | 5.3 | ± | 0.4 | 6.0 | ± | 0.3 | 0.667 | |
| 1st OPD | 4.7 | ± | 0.2 | 5.5 | ± | 0.2 | 0.365 | 4.8 | ± | 0.4 | 5.7 | ± | 0.3 | 0.999 | |
| ASM (kg) | pre OP | 20.0 | ± | 1.2 | 21.3 | ± | 1.1 | 18.9 | ± | 1.9 | 22.0 | ± | 1.4 | ||
| DC | 20.5 | ± | 1.2 | 21.3 | ± | 1.1 | 0.413 | 19.1 | ± | 1.9 | 22.0 | ± | 1.4 | 0.875 | |
| 1st OPD | 20.2 | ± | 1.2 | 21.5 | ± | 1.0 | 0.823 | 19.1 | ± | 1.9 | 22.7 | ± | 1.4 | 0.578 | |
| SMI (kg/m2) | pre OP | 7.6 | ± | 0.3 | 8.0 | ± | 0.2 | 7.6 | ± | 0.5 | 8.1 | ± | 0.3 | ||
| DC | 7.8 | ± | 0.3 | 8.0 | ± | 0.2 | 0.249 | 7.7 | ± | 0.5 | 8.1 | ± | 0.3 | 0.715 | |
| 1st OPD | 7.7 | ± | 0.3 | 8.1 | ± | 0.2 | 0.615 | 7.7 | ± | 0.5 | 8.4 | ± | 0.3 | 0.700 | |
3.4. Evaluation of 10 m Walking Speed (Table 3)
3.5. Evaluation of Muscle Mass
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Powell-Brett, S.; de Liguori Carino, N.; Roberts, K. Understanding pancreatic exocrine insufficiency and replacement therapy in pancreatic cancer. Eur. J. Surg. Oncol. 2021, 47, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Pathanki, A.M.; Attard, J.A.; Bradley, E.; Powell-Brett, S.; Dasari, B.V.M.; Isaac, J.R.; Roberts, K.J.; Chatzizacharias, N.A. Pancreatic exocrine insufficiency after pancreaticoduodenectomy: Current evidence and management. World J. Gastrointest. Pathophysiol. 2020, 11, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, H.; Akezaki, Y.; Tominaga, R.; Okamoto, M.; Kikuuchi, M.; Hamada, M.; Mikuriya, Y.; Ohta, K.; Sugihara, S. Changes in Physical Function and Effects on QOL in Patients after Pancreatic Cancer Surgery. Healthcare 2021, 9, 882. [Google Scholar] [CrossRef] [PubMed]
- Poulia, K.A.; Antoniadou, D.; Sarantis, P.; Karamouzis, M.V. Pancreatic Cancer Prognosis, Malnutrition Risk, and Quality of Life: A Cross-Sectional Study. Nutrients 2022, 14, 442. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Nelson, D.R.; Zhao, Y.; Cui, Z.; Johnston, J.A. Relationship between muscle mass and muscle strength, and the impact of comorbidities: A population-based, cross-sectional study of older adults in the United States. BMC Geriatr. 2013, 13, 74. [Google Scholar] [CrossRef]
- Kamarajah, S.K.; Bundred, J.; Tan, B.H.L. Body composition assessment and sarcopenia in patients with gastric cancer: A systematic review and meta-analysis. Gastric Cancer 2019, 22, 10–22. [Google Scholar] [CrossRef]
- Ishii, N.; Iwata, Y.; Nishikawa, H.; Enomoto, H.; Aizawa, N.; Ishii, A.; Miyamoto, Y.; Yuri, Y.; Hasegawa, K.; Nakano, C.; et al. Effect of pretreatment psoas muscle mass on survival for patients with unresectable pancreatic cancer undergoing systemic chemotherapy. Oncol. Lett. 2017, 14, 6059–6065. [Google Scholar] [CrossRef]
- Choi, M.H.; Yoon, S.B.; Lee, K.; Song, M.; Lee, I.S.; Lee, M.A.; Hong, T.H.; Choi, M.G. Preoperative sarcopenia and post-operative accelerated muscle loss negatively impact survival after resection of pancreatic cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 326–334. [Google Scholar] [CrossRef]
- Yang, L.; He, Y.; Li, X. Sarcopenia Predicts Relevant Clinical Outcomes in Biliary Tract Cancer Patients: A Systematic Review and Meta-Analysis. Nutr. Cancer 2022, 74, 3274–3283. [Google Scholar] [CrossRef]
- Choi, M.H.; Yoon, S.B. Sarcopenia in pancreatic cancer: Effect on patient outcomes. World J. Gastrointest. Oncol. 2022, 14, 2302–2312. [Google Scholar] [CrossRef]
- Wijma, A.G.; Hogenbirk, R.N.M.; Driessens, H.; Kluifhooft, D.A.; Jellema-Betten, E.S.; Tjalsma-de Vries, M.; Liem, M.S.L.; Nieuwenhuijs, V.B.; Manusama, E.M.; Hoogwater, F.J.H.; et al. Nutritional support in pancreatic cancer patients and its effect on nutritional status: An observational regional HPB network study investigating current practice. Support. Care Cancer 2024, 32, 487. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, R.; Chapman, I.M. Undernutrition and anorexia in the older person. Gastroenterol. Clin. N. Am. 2009, 38, 393–409. [Google Scholar] [CrossRef]
- Reber, E.; Gomes, F.; Vasiloglou, M.F.; Schuetz, P.; Stanga, Z. Nutritional Risk Screening and Assessment. J. Clin. Med. 2019, 8, 1065. [Google Scholar] [CrossRef]
- Tangvik, R.J.; Tell, G.S.; Eisman, J.A.; Guttormsen, A.B.; Henriksen, A.; Nilsen, R.M.; Øyen, J.; Ranhoff, A.H. The nutritional strategy: Four questions predict morbidity, mortality and health care costs. Clin. Nutr. 2014, 33, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Mourão, F.; Amado, D.; Ravasco, P.; Vidal, P.M.; Camilo, M.E. Nutritional risk and status assessment in surgical patients: A challenge amidst plenty. Nutr. Hosp. 2004, 19, 83–88. [Google Scholar]
- La Torre, M.; Ziparo, V.; Nigri, G.; Cavallini, M.; Balducci, G.; Ramacciato, G. Malnutrition and pancreatic surgery: Prevalence and outcomes. J. Surg. Oncol. 2013, 107, 702–708. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.; Sobotka, L.; et al. ESPEN practical guideline: Clinical nutrition and hydration in geriatrics. Clin. Nutr. 2022, 41, 958–989. [Google Scholar] [CrossRef] [PubMed]
- Silander, E.; Nyman, J.; Bove, M.; Johansson, L.; Larsson, S.; Hammerlid, E. Impact of prophylactic percutaneous endoscopic gastrostomy on malnutrition and quality of life in patients with head and neck cancer: A randomized study. Head Neck 2012, 34, 1–9. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.M.; Jeung, H.C.; Lee, I.J.; Park, J.S.; Song, M.; Lee, D.K.; Lee, S.M. The Effect of Nutrition Intervention with Oral Nutritional Supplements on Pancreatic and Bile Duct Cancer Patients Undergoing Chemotherapy. Nutrients 2019, 11, 1145. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Choi, J.E.; Hwang, H.S. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2018, 108, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Junior, H.J.; Calvani, R.; Azzolino, D.; Picca, A.; Tosato, M.; Landi, F.; Cesari, M.; Marzetti, E. Protein Intake and Sarcopenia in Older Adults: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 8718. [Google Scholar] [CrossRef]
- Ten Haaf, D.S.M.; Eijsvogels, T.M.H.; Bongers, C.; Horstman, A.M.H.; Timmers, S.; de Groot, L.; Hopman, M.T.E. Protein supplementation improves lean body mass in physically active older adults: A randomized placebo-controlled trial. J. Cachexia Sarcopenia Muscle 2019, 10, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Kim, N.; Choi, Y.J.; Lee, Y.; Yun, J.; Park, S.J.; Park, H.S.; Chung, Y.S.; Park, Y.K. Leucine-Enriched Protein Supplementation Increases Lean Body Mass in Healthy Korean Adults Aged 50 Years and Older: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2020, 12, 1816. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Arnau, F.M.; Fonfría-Vivas, R.; Buigues, C.; Castillo, Y.; Molina, P.; Hoogland, A.J.; van Doesburg, F.; Pruimboom, L.; Fernández-Garrido, J.; Cauli, O. Effects of Leucine Administration in Sarcopenia: A Randomized and Placebo-controlled Clinical Trial. Nutrients 2020, 12, 932. [Google Scholar] [CrossRef]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef]
- Englund, D.A.; Kirn, D.R.; Koochek, A.; Zhu, H.; Travison, T.G.; Reid, K.F.; von Berens, Å.; Melin, M.; Cederholm, T.; Gustafsson, T.; et al. Nutritional Supplementation with Physical Activity Improves Muscle Composition in Mobility-Limited Older Adults, The VIVE2 Study: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 95–101. [Google Scholar] [CrossRef]
- Bechshøft, R.L.; Reitelseder, S.; Højfeldt, G.; Castro-Mejía, J.L.; Khakimov, B.; Ahmad, H.F.; Kjær, M.; Engelsen, S.B.; Johansen, S.M.; Rasmussen, M.A.; et al. Counteracting Age-related Loss of Skeletal Muscle Mass: A clinical and ethnological trial on the role of protein supplementation and training load (CALM Intervention Study): Study protocol for a randomized controlled trial. Trials 2016, 17, 397. [Google Scholar] [CrossRef]
- Grönstedt, H.; Vikström, S.; Cederholm, T.; Franzén, E.; Seiger, Å.; Wimo, A.; Faxén-Irving, G.; Boström, A.M. A study protocol of Older Person’s Exercise and Nutrition Study (OPEN)—A sit-to-stand activity combined with oral protein supplement—Effects on physical function and independence: A cluster randomized clinical trial. BMC Geriatr. 2018, 18, 138. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Calvani, R.; Tosato, M.; Landi, F.; Picca, A.; Marzetti, E. Protein intake and physical function in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 81, 101731. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Jang, J.Y.; Kim, E.J.; Kang, M.J.; Kwon, W.; Chang, Y.R.; Han, I.W.; Kim, S.W. Effects of pancreatectomy on nutritional state, pancreatic function and quality of life. Br. J. Surg. 2013, 100, 1064–1070. [Google Scholar] [CrossRef]
- Shin, Y.C.; Han, Y.; Kim, E.; Kwon, W.; Kim, H.; Jang, J.Y. Effects of pancreatectomy on nutritional state, pancreatic function, and quality of life over 5 years of follow up. J. Hepatobiliary Pancreat. Sci. 2022, 29, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.Y.; Lin, Y.H.; Liu, Y.W.; Liu, Y.Y.; Li, W.F.; Kuo, M.C.; Huang, S.W.; Yeh, C.H.; Lin, Y.C.; Yin, S.M. The impact of preoperative nutritional status on postoperative outcomes: An insight from Geriatric Nutritional Risk Index in elderly pancreaticoduodenectomy patients. BMC Surg. 2024, 24, 100. [Google Scholar] [CrossRef]
- Kapoor, N.; Naufahu, J.; Tewfik, S.; Bhatnagar, S.; Garg, R.; Tewfik, I. A Prospective Randomized Controlled Trial to Study the Impact of a Nutrition-Sensitive Intervention on Adult Women with Cancer Cachexia Undergoing Palliative Care in India. Integr. Cancer Ther. 2017, 16, 74–84. [Google Scholar] [CrossRef]
- Carey, S.; Storey, D.; Biankin, A.V.; Martin, D.; Young, J.; Allman-Farinelli, M. Long term nutritional status and quality of life following major upper gastrointestinal surgery—A cross-sectional study. Clin. Nutr. 2011, 30, 774–779. [Google Scholar] [CrossRef]
- Houston, D.K.; Tooze, J.A.; Garcia, K.; Visser, M.; Rubin, S.; Harris, T.B.; Newman, A.B.; Kritchevsky, S.B. Protein Intake and Mobility Limitation in Community-Dwelling Older Adults: The Health ABC Study. J. Am. Geriatr. Soc. 2017, 65, 1705–1711. [Google Scholar] [CrossRef]
- Rogeri, P.S.; Zanella, R., Jr.; Martins, G.L.; Garcia, M.D.A.; Leite, G.; Lugaresi, R.; Gasparini, S.O.; Sperandio, G.A.; Ferreira, L.H.B.; Souza-Junior, T.P.; et al. Strategies to Prevent Sarcopenia in the Aging Process: Role of Protein Intake and Exercise. Nutrients 2021, 14, 52. [Google Scholar] [CrossRef]
- Pereira, M.M.E.; Queiroz, M.; de Albuquerque, N.M.C.; Rodrigues, J.; Wiegert, E.V.M.; Calixto-Lima, L.; de Oliveira, L.C. The Prognostic Role of Phase Angle in Advanced Cancer Patients: A Systematic Review. Nutr. Clin. Pract. 2018, 33, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lis, C.G.; Dahlk, S.L.; Vashi, P.G.; Grutsch, J.F.; Lammersfeld, C.A. Bioelectrical impedance phase angle as a prognostic indicator in advanced pancreatic cancer. Br. J. Nutr. 2004, 92, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Gade, J.; Beck, A.M.; Bitz, C.; Christensen, B.; Klausen, T.W.; Vinther, A.; Astrup, A. Protein-enriched, milk-based supplement to counteract sarcopenia in acutely ill geriatric patients offered resistance exercise training during and after hospitalisation: Study protocol for a randomised, double-blind, multicentre trial. BMJ Open 2018, 8, e019210. [Google Scholar] [CrossRef] [PubMed]
- Onvani, S.; Haghighatdoost, F.; Surkan, P.J.; Larijani, B.; Azadbakht, L. Adherence to the Healthy Eating Index and Alternative Healthy Eating Index dietary patterns and mortality from all causes, cardiovascular disease and cancer: A meta-analysis of observational studies. J. Hum. Nutr. Diet. 2017, 30, 216–226. [Google Scholar] [CrossRef] [PubMed]
| Variables (1) | Completed Protocol Group | High Compliance Segment | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 41) | Placebo (n = 18) | Protein Supplement (n = 23) | p-Value (2) | All (n = 20) | Placebo (n = 7) | Protein Supplement (n = 13) | p-Value (2) | |||||||||||||
| Gender (male/female) | 21/20 | 11/7 | 10/13 | 0.767 | 7/13 | 4/3 | 9/4 | 1.000 | ||||||||||||
| Age (yr) | 72.6 | ± | 0.8 | 73.2 | ± | 1.3 | 72.1 | ± | 1.0 | 0.496 | 73.7 | ± | 1.1 | 75.0 | ± | 1.7 | 73.0 | ± | 1.4 | 0.380 |
| Ht (cm) | 161.4 | ± | 1.5 | 161.3 | ± | 1.8 | 161.5 | ± | 2.2 | 0.810 | 161.2 | ± | 2.2 | 157.4 | ± | 2.4 | 163.3 | ± | 3.1 | 0.218 |
| Wt (kg) | 60.6 | ± | 1.6 | 59.7 | ± | 2.0 | 61.4 | ± | 2.3 | 0.987 | 61.8 | ± | 2.5 | 57.8 | ± | 2.8 | 63.9 | ± | 3.4 | 0.249 |
| BMI (kg/m2) | 23.2 | ± | 0.4 | 23.0 | ± | 0.7 | 23.4 | ± | 0.5 | 0.923 | 23.6 | ± | 0.6 | 23.3 | ± | 1.1 | 23.8 | ± | 0.8 | 0.732 |
| Diagnosis | 0.437 | 1.000 | ||||||||||||||||||
| Pancreatic cancer | 18 | 10 | 8 | 8 | 4 | 4 | ||||||||||||||
| Cholangiocacinoma | 14 | 6 | 8 | 7 | 3 | 4 | ||||||||||||||
| Others | 9 | 2 | 7 | 5 | 0 | 5 | ||||||||||||||
| Operation (PPPD/DP) | 31/10 | 14/4 | 17/6 | 0.703 | 14/6 | 5/2 | 9/4 | 1.000 | ||||||||||||
| PG-SGA | ||||||||||||||||||||
| Grade A/B/C | 28/13/0 | 12/6/0 | 16/7/0 | 0.843 | 14/6/0 | 5/2/0 | 9/4/0 | 1.000 | ||||||||||||
| Score | 5.3 | ± | 0.5 | 5.1 | ± | 0.7 | 5.6 | ± | 0.7 | 0.971 | 4.8 | ± | 0.7 | 4.7 | ± | 1.0 | 4.8 | ± | 0.9 | 0.930 |
| Variable | PA | SMI | ||||
|---|---|---|---|---|---|---|
| DC | OPD | DC | OPD | |||
| Total Oral Protein | DC | |R| (1) | 0.29 | 0.47 | −0.02 | 0.43 |
| p-Value | 0.060 | 0.002 | 0.877 | 0.005 | ||
| OPD | |R| | 0.27 | 0.55 | 0.09 | 0.37 | |
| p-Value | 0.082 | 0.000 | 0.561 | 0.019 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, N.R.; Hwang, H.K.; Lee, H.; Kang, C.M. Oral Protein Supplements Might Improve Nutritional Status and Quality of Life in Elderly Patients after Standard Pancreatic Resection. Nutrients 2024, 16, 2988. https://doi.org/10.3390/nu16172988
Lee NR, Hwang HK, Lee H, Kang CM. Oral Protein Supplements Might Improve Nutritional Status and Quality of Life in Elderly Patients after Standard Pancreatic Resection. Nutrients. 2024; 16(17):2988. https://doi.org/10.3390/nu16172988
Chicago/Turabian StyleLee, Na Rae, Ho Kyoung Hwang, Hosun Lee, and Chang Moo Kang. 2024. "Oral Protein Supplements Might Improve Nutritional Status and Quality of Life in Elderly Patients after Standard Pancreatic Resection" Nutrients 16, no. 17: 2988. https://doi.org/10.3390/nu16172988
APA StyleLee, N. R., Hwang, H. K., Lee, H., & Kang, C. M. (2024). Oral Protein Supplements Might Improve Nutritional Status and Quality of Life in Elderly Patients after Standard Pancreatic Resection. Nutrients, 16(17), 2988. https://doi.org/10.3390/nu16172988






