A Natural Astragalus-Based Nutritional Supplement Lengthens Telomeres in a Middle-Aged Population: A Randomized, Double-Blind, Placebo-Controlled Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Studied
2.2. Study Design
2.3. Blood Samples
2.4. Measurement of TL
2.5. Clinical Laboratory Assays
2.6. Statistical Analysis
3. Results
3.1. Compliance and Tolerance
3.2. Changes in Telomere Length
3.2.1. Median Telomere Length
3.2.2. Short Telomere Length
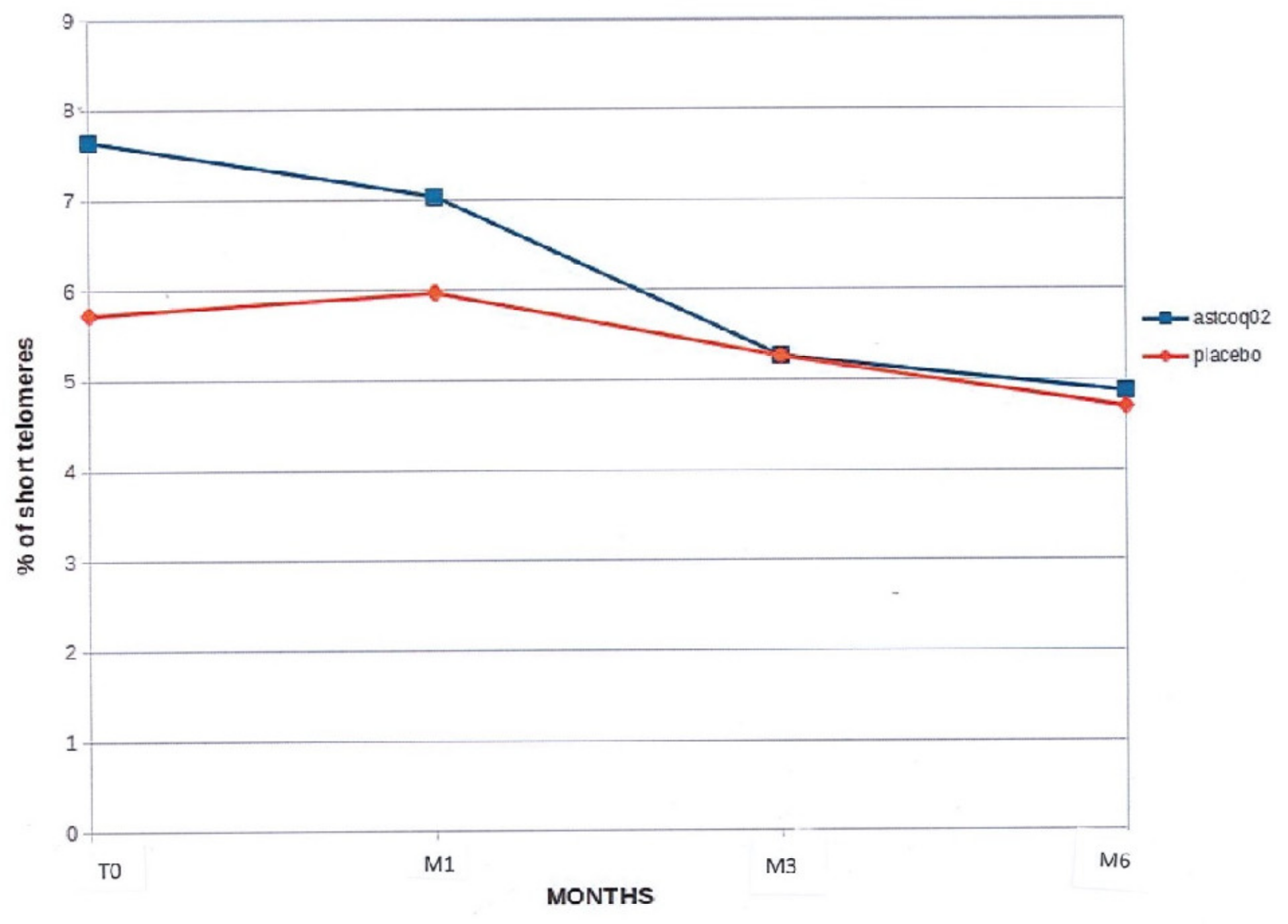
3.2.3. Percentage of Short Telomeres
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blasco, A.B. Telomeres and human disease: Ageing, cancer and beyond. Nat. Rev. Genet. 2005, 6, 611–622. [Google Scholar] [CrossRef]
- Rubtsova, M.; Dontsova, O. Human telomerase RNA: Telomerase component or more? Biomolecules 2020, 10, 873. [Google Scholar] [CrossRef] [PubMed]
- Kamal, S.; Junaid, M.; Ejaz, A.; Bibi, I.; Akash, M.S.H.; Rehman, K. The secrets of telomerase: Retrospective analysis and future prospects. Life Sci. 2020, 257, 118115. [Google Scholar] [CrossRef] [PubMed]
- Blasco, M. Telomere length, stem cells and aging. Nat. Chem. Biol. 2007, 3, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Pusceddu, I.; März, W.; Herrmann, W. Telomere biology and age-related diseases. Clin. Chem. Lab. Med. 2018, 56, 1210–1222. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. Mortality and immortality at the cellular level. A review. Biochemistry 1997, 62, 1180–1190. [Google Scholar] [PubMed]
- Sahin, E.; Depinho, R.A. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 2010, 464, 520–528. [Google Scholar] [CrossRef]
- D’Adda di Fagagna, F.; Reaper, P.M.; Clay-Farrace, L.; Fiegler, H.; Carr, P.; von Zglinicki, T.; Saretzki, G.; Carter, N.P.; Jackson, S.P. A DNA damage checkpoint response in telomere-initiated senescence. Nature 2003, 426, 194–198. [Google Scholar] [CrossRef]
- Chatterjee, S. Telomeres in health and disease. J. Oral. Maxillofac. Pathol. 2017, 21, 87–91. [Google Scholar] [CrossRef]
- Valdes, A.M.; Andrew, T.; Gardner, J.P.; Kimura, M.; Oelsner, E.; Cherkas, L.; Aviv, A.; Spector, T. Obesity, cigarette smoking, and telomere length in women. Lancet 2005, 366, 662–664. [Google Scholar] [CrossRef]
- Ruiz-Narváez, E.A.; Baylin, A.; Azofeifa, J.; Leal, A.; Rosero-Bixby, L. Diet and leukocyte telomere length in a population with extended longevity: The Costa Rican Longevity and Healthy Aging Study (CRELES). Nutrients 2021, 13, 2585. [Google Scholar] [CrossRef]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and telomere length: A general overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef]
- Nittis, T.; Guittat, L.; Stewart, S.A. Alternative lengthening of telomeres (ALT) and chromatin: Is there a connection? Biochimie 2008, 90, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Wolkowitz, O.M.; Epel, E.S.; Mellon, S. When blue turns to grey: Do stress and depression accelerate cell aging? World J. Biol. Psychiatry 2008, 9, 2–5. [Google Scholar] [CrossRef]
- Aubert, G.; Lansdorp, P.M. Telomeres and aging. Physiol. Rev. 2008, 88, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, C.; Ruan, Y.; Guo, Y.; Sun, S.; Shi, Y.; Wu, F. Dynamics of leukocyte telomere length in adults aged 50 and older: A longitudinal population-based cohort study. Geroscience 2021, 43, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.J.; Adres, V. Telomere biology and cardiovascular disease. Circ. Res. 2006, 99, 1167–1180. [Google Scholar] [CrossRef]
- Toupance, S.; Labat, C.; Temmar, M.; Rossignol, P.; Kimura, M.; Aviv, A.; Benetos, A. Short telomeres, but not telomere attrition rates, are associated with carotid atherosclerosis. Hypertension 2017, 70, 420–425. [Google Scholar] [CrossRef]
- McNally, E.J.; Luncsford, P.J.; Armanios, M. Long telomeres and cancer risk: The price of cellular immortality. J. Clin. Investig. 2019, 129, 3474–3481. [Google Scholar] [CrossRef]
- Chatterjee, S.; de Gonzalo-Calvo, D.; Derda, A.A.; Schimmel, K.; Sonnenschein, K.; Bavendiek, U.; Bauersachs, J.; Bär, C.; Thum, T. Leukocyte telomere length correlates with hypertrophic cardiomyopathy severity. Sci. Rep. 2018, 8, 11227. [Google Scholar] [CrossRef]
- Samavat, H.; Luu, H.N.; Beckman, K.B.; Jin, A.; Wang, R.; Koh, W.P.; Yuan, J.M. Leukocyte telomere length, cancer incidence and all-cause mortality among Chinese adults: Singapore Chinese Health Study. Int. J. Cancer 2021, 148, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Samavat, H.; Xun, X.; Jin, A.; Wang, R.; Koh, W.P.; Yuan, J.M. Association between prediagnostic leukocyte telomere length and breast cancer risk: The Singapore Chinese Health Study. Breast Cancer Res. 2019, 21, 50. [Google Scholar] [CrossRef]
- Zheng, X.; Wezel, F.; Azoitei, A.; Meessen, S.; Wang, W.; Najjar, G.; Wang, X.; Kraus, J.M.; Kestler, H.A.; John, A.; et al. Shorter leukocyte telomere length is associated with worse survival of patients with bladder cancer and renal cell carcinoma. Cancers 2021, 13, 3774. [Google Scholar] [CrossRef] [PubMed]
- Monroy-Jaramillo, N.; Dyukova, E.; Walss-Bass, C. Telomere length in psychiatric disorders: Is. it more than an ageing marker? World J. Biol. Psychiatry 2018, 19 (Suppl. S2), S2–S20. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Raschenberger, J.; Heydon, E.E.; Tsimikas, S.; Haun, M.; Mayr, A.; Weger, S.; Witztum, J.L.; Butterworth, A.S.; Willeit, J.; et al. Leucocyte telomere length and risk of type 2 diabetes mellitus: New prospective cohort study and literature-based meta-analysis. PLoS ONE 2014, 9, e112483. [Google Scholar] [CrossRef]
- Aviv, A. Genetics of leukocyte telomere length and its role in atherosclerosis. Mutat. Res. 2012, 730, 68–74. [Google Scholar] [CrossRef]
- Cawthon, R.M.; Smith, K.; O’brien, E.; Sivatchenko, A.; Kerber, R.A. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003, 361, 393–395. [Google Scholar] [CrossRef]
- Turner, K.J.; Vasu, V.; Griffin, D.K. Telomere biology and human phenotype. Cells 2019, 8, 73. [Google Scholar] [CrossRef]
- Helby, J.; Nordestgaard, B.G.; Benfield, T.; Bojesen, S.E. Shorter leukocyte telomere length is associated with higher risk of infections: A prospective study of 75,309 individuals from the general population. Haematologica 2017, 102, 1457–1465. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Fragkiadaki, P.; Docea, A.O.; Alegakis, A.K.; Sarandi, E.; Thanasoula, M.; Spandidos, D.A.; Tsatsakis, A.; Razgonova, M.P.; Calina, D. Discovery of potent telomerase activators: Unfolding new therapeutic and anti-aging perspectives. Mol. Med. Rep. 2019, 20, 3701–3708. [Google Scholar] [CrossRef]
- Harley, C.B.; Liu, W.; Flom, P.L.; Raffaele, J.M. A natural product telomerase activator as part of a health maintenance program: Metabolic and cardiovascular response. Rejuvenation Res. 2013, 16, 386–395. [Google Scholar] [CrossRef]
- de Jesus, B.B.; Schneeberger, K.; Vera, E.; Tejera, A.; Harley, C.B.; Blasco, M.A. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell 2011, 10, 604–621. [Google Scholar] [CrossRef]
- Le Saux, C.J.; Davy, P.; Brampton, C.; Ahuja, S.S.; Fauce, S.; Shivshankar, P.; Nguyen, H.; Ramaseshan, M.; Tressler, R.; Pirot, Z.; et al. A novel telomerase activator suppresses lung damage in a murine model of idiopathic pulmonary fibrosis. PLoS ONE 2013, 8, e58423. [Google Scholar] [CrossRef] [PubMed]
- Salvador, L.; Singaravelu, G.; Harley, C.B.; Flom, P.; Suram, A.; Raffaele, J.M. A natural product telomerase activator lengthens telomeres in humans: A randomized, double-blind, and placebo-controlled study. Rejuvenation Res. 2016, 19, 478–484. [Google Scholar] [CrossRef] [PubMed]
- de Jaeger, C.; Lamberti, C.; Van Leeuwen, V.; Voronska, E.; Kruiskamp, S. Results of a preliminary study on the effects of a compound on telomeres length, biological and physiological parameters. J. Biol. Med. 2021, 5, 8–15. [Google Scholar]
- de Pedro, N.; Díez, M.; García, I.; García, J.; Otero, L.; Fernández, L.; García, B.; González, R.; Rincón, S.; Pérez, D.; et al. Analytical validation of Telomere Analysis Technology® for the high-throughput analysis of multiple telomere-associated variables. Biol. Proced. Online 2020, 22, 2. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, L.; Yang, Y.; Liu, Y. Cycloastragenol: An exciting novel candidate for age-associated diseases. Exp. Ther. Med. 2018, 16, 2175–2182. [Google Scholar] [CrossRef]
- Lekmine, S.; Boussekine, S.; Akkal, S.; Martín-García, A.I.; Boumegoura, A.; Kadi, K.; Djeghim, H.; Mekersi, N.; Bendjedid, S.; Bensouici, C.; et al. Investigation of photoprotective, anti-inflammatory, antioxidant capacities and LC-ESI-MS phenolic profile of Astragalus gombiformis Pomel. Foods 2021, 10, 1937. [Google Scholar] [CrossRef] [PubMed]
- Molgora, B.; Bateman, R.; Sweeney, G.; Finger, D.; Dimler, T.; Effros, R.B.; Valenzuela, H.F. Functional assessment of pharmacological telomerase activators in human T cells. Cells 2013, 2, 57–66. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Barbalace, M.C.; Croce, L.; Malaguti, M.; Campennì, A.; Rotondi, M.; Cannavò, S.; Hrelia, S. Autoimmune Thyroid Disorders: The Mediterranean Diet as a Protective Choice. Nutrients 2023, 15, 3953. [Google Scholar] [CrossRef]
- Baliou, S.; Ioannou, P.; Apetroaei, M.M.; Vakonaki, E.; Fragkiadaki, P.; Kirithras, E.; Tzatzarakis, M.N.; Arsene, A.L.; Docea, A.O.; Tsatsakis, A. The Impact of the Mediterranean Diet on Telomere Biology: Implications for Disease Management-A Narrative Review. Nutrients 2024, 16, 2525. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flores, I.; Benetti, R.; Blasco, M.A. Telomerase regulation and stem cell behaviour. Curr. Opin. Cell Biol. 2006, 18, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Harley, C.B.; Futcher, A.B.; Greider, C.W. Telomeres shorten during ageing of human fibroblasts. Nature 1990, 345, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Giannotti, G.; Doerries, C.; Mocharla, P.S.; Mueller, M.F.; Bahlmann, F.H.; Horvàth, T.; Jiang, H.; Sorrentino, S.A.; Steenken, N.; Manes, C.; et al. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: Relation to endothelial dysfunction. Hypertension 2010, 55, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Haycock, P.C.; Heydon, E.E.; Kaptoge, S.; Butterworth, A.S.; Thompson, A.; Willeit, P. Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2014, 349, g4227. [Google Scholar] [CrossRef]
- D’Mello, M.J.; Ross, S.A.; Briel, M.; Anand, S.S.; Gerstein, H.; Pare, G. Association between shortened leukocyte telomere length and cardiometabolic outcomes: Systematic review and meta-analysis. Circ. Cardiovasc. Genet. 2015, 8, 82–90. [Google Scholar] [CrossRef]
- Xu, X.; Hu, H.; Lin, Y.; Huang, F.; Ji, H.; Li, Y.; Lin, S.; Chen, X.; Duan, S. Differences in leukocyte telomere length between coronary heart disease and normal population: A multipopulation meta-analysis. BioMed Res. Int. 2019, 2019, 5046867. [Google Scholar] [CrossRef]
- Hecker, M.; Fitzner, B.; Jäger, K.; Bühring, J.; Schwartz, M.; Hartmann, A.; Walter, M.; Zettl, U.K. Leukocyte telomere length in patients with multiple sclerosis and its association with clinical phenotypes. Mol. Neurobiol. 2021, 58, 2886–2896. [Google Scholar] [CrossRef]
- Gielen, M.; Hageman, G.J.; Antoniou, E.E.; Nordfjall, K.; Mangino, M.; Balasubramanyam, M.; De Meyer, T.; Hendricks, A.E.; Giltay, E.J.; Hunt, S.C.; et al. Body mass index is negatively associated with telomere length: A collaborative cross-sectional meta-analysis of 87 observational studies. Am. J. Clin. Nutr. 2018, 108, 453–475. [Google Scholar] [CrossRef]
- Nordfjall, K.; Eliasson, M.; Stegmayr, B.; Melander, O.; Nilsson, P.; Roos, G. Telomere length is associated with obesity parameters but with a gender difference. Obesity 2008, 16, 2682–2689. [Google Scholar] [CrossRef]
- Morla, M.; Busquets, X.; Pons, J.; Sauleda, J.; MacNee, W.; Agusti, A.G.N. Telomere shortening in smokers with and without COPD. Eur. Respir. J. 2006, 27, 525–528. [Google Scholar] [CrossRef]
- Lung, F.W.; Chen, N.C.; Shu, B.C. Genetic pathway of major depressive disorder in shortening telomeric length. Psychiatr. Genet. 2007, 17, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Tentolouris, N.; Nzietchueng, R.; Cattan, V.; Poitevin, G.; Lacolley, P.; Papazafiropoulou, A.; Perrea, D.; Katsilambros, N.; Benetos, A. White blood cells telomere length is shorter in males with type 2 diabetes and microalbuminuria. Diabetes Care 2007, 30, 2909–2915. [Google Scholar] [CrossRef] [PubMed]
- Lung, F.W.; Ku, C.S.; Kao, W.T. Telomere length may be associated with hypertension. J. Hum. Hypertens. 2008, 22, 230–232. [Google Scholar] [CrossRef]
- Arsenis, N.C.; You, T.; Ogawa, E.F.; Tinsley, G.M.; Zuo, L. Physical activity and telomere length: Impact of aging and potential mechanisms of action. Oncotarget 2017, 8, 45008–45019. [Google Scholar] [CrossRef]
- Aviv, A.; Levy, D. Telomeres, atherosclerosis, and the hemothelium: The longer view. Annu. Rev. Med. 2012, 63, 293–301. [Google Scholar] [CrossRef]
- Matthews, C.; Gorenne, I.; Scott, S.; Figg, N.; Kirkpatrick, P.; Ritchie, A.; Goddard, M.; Bennett, M. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: Effects of telomerase and oxidative stress. Circ. Res. 2006, 99, 156–164. [Google Scholar] [CrossRef]
- Davies, M.J.; Woolf, N.; Rowels, P.M.; Pepper, J. Morphology of the endothelium over atherosclerotic plaques in human coronary arteries. Br. Heart J. 1988, 60, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Burrig, K.F. The endothelium of advanced arteriosclerotic plaques in humans. Arterioscler. Thromb. 1991, 11, 1678–1689. [Google Scholar] [CrossRef]
- Ogami, M.; Ikura, Y.; Ohsawa, M.; Matsuo, T.; Kayo, S.; Yoshimi, N.; Hai, E.; Shirai, N.; Ehara, S.; Komatsu, R.; et al. Telomere shortening in human coronary artery diseases. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 546–550. [Google Scholar] [CrossRef]
- Minamino, T.; Miyauchi, H.; Yoshida, T.; Ishida, Y.; Yoshida, H.; Komuro, I. Endothelial cell senescence in human atherosclerosis: Role of telomere in endothelial dysfunction. Circulation 2002, 105, 1541–1544. [Google Scholar] [CrossRef] [PubMed]
- Brouilette, S.W.; Whittaker, A.; Stevens, S.E.; van der Harst, P.; Goodall, A.H.; Samani, N.J. Telomere length is shorter in healthy offspring of subjects with coronary artery disease: Support for the telomere hypothesis. Heart 2008, 94, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Goglin, S.E.; Farzaneh-Far, R.; Epel, E.S.; Lin, J.; Blackburn, E.H.; Whooley, M.A. Change in leukocyte telomere length predicts mortality in patients with stable coronary heart disease from the Heart and Soul study. PLoS ONE 2016, 11, e0160748. [Google Scholar]
- Jaskelioff, M.; Muller, F.L.; Paik, J.H.; Thomas, E.; Jiang, S.; Adams, A.C.; Sahin, E.; Kost-Alimova, M.; Protopopov, A.; Cadiñanos, J.; et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 2011, 469, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Brouilette, S.W.; Moore, J.S.; McMahon, A.D.; Thompson, J.R.; Shepherd, J.; Packard, C.J.; Samani, N.J. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: A nested case-control study. Lancet 2007, 369, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Minami, Y.; Takahashi, Y.; Tabuchi, T.; Itoh, T.; Nakamura, M. Effect of intensive lipid-lowering therapy on telomere erosion in endothelial progenitor cells obtained from patients with coronary artery disease. Clin. Sci. 2009, 116, 827–835. [Google Scholar] [CrossRef]
- Werner, C.; Gensch, C.; Pöss, J.; Haendeler, J.; Böhm, M.; Laufs, U. Pioglitazone activates aortic telomerase and prevents stress-induced endothelial apoptosis. Atherosclerosis 2011, 216, 23–34. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Thomas, M.S.; Lemos, B.S.; DiMarco, D.M.; Missimer, A.; Melough, M.; Chun, O.K.; Murillo, A.G.; Alyousef, H.M.; Medina-Vera, I. TA-65, a telomerase activator, improves cardiovascular markers in patients with metabolic syndrome. Curr. Pharm. Des. 2018, 24, 1905–1911. [Google Scholar] [CrossRef]
- Puterman, E.; Lin, J.; Blackburn, E.; O’Donovan, A.; Adler, N.; Epel, E. The power of exercise: Buffering the effect of chronic stress on telomere length. PLoS ONE 2010, 5, e10837. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jaeger, C.; Kruiskamp, S.; Voronska, E.; Lamberti, C.; Baramki, H.; Beaudeux, J.L.; Cherin, P. A Natural Astragalus-Based Nutritional Supplement Lengthens Telomeres in a Middle-Aged Population: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2024, 16, 2963. https://doi.org/10.3390/nu16172963
de Jaeger C, Kruiskamp S, Voronska E, Lamberti C, Baramki H, Beaudeux JL, Cherin P. A Natural Astragalus-Based Nutritional Supplement Lengthens Telomeres in a Middle-Aged Population: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2024; 16(17):2963. https://doi.org/10.3390/nu16172963
Chicago/Turabian Stylede Jaeger, Christophe, Saskia Kruiskamp, Elena Voronska, Carla Lamberti, Hani Baramki, Jean Louis Beaudeux, and Patrick Cherin. 2024. "A Natural Astragalus-Based Nutritional Supplement Lengthens Telomeres in a Middle-Aged Population: A Randomized, Double-Blind, Placebo-Controlled Study" Nutrients 16, no. 17: 2963. https://doi.org/10.3390/nu16172963
APA Stylede Jaeger, C., Kruiskamp, S., Voronska, E., Lamberti, C., Baramki, H., Beaudeux, J. L., & Cherin, P. (2024). A Natural Astragalus-Based Nutritional Supplement Lengthens Telomeres in a Middle-Aged Population: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients, 16(17), 2963. https://doi.org/10.3390/nu16172963





