Consumption of Sylimarin, Pyrroloquinoline Quinone Sodium Salt and Myricetin: Effects on Alcohol Levels and Markers of Oxidative Stress—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
- aged between 18 and 35 years;
- no history of alcohol abuse or other substances;
- white ethnicity;
- non-smoker;
- good health condition, with no autoimmune, endocrine, infectious, cardiac, renal, hepatic or metabolic diseases;
- no pregnancy or lactation conditions.
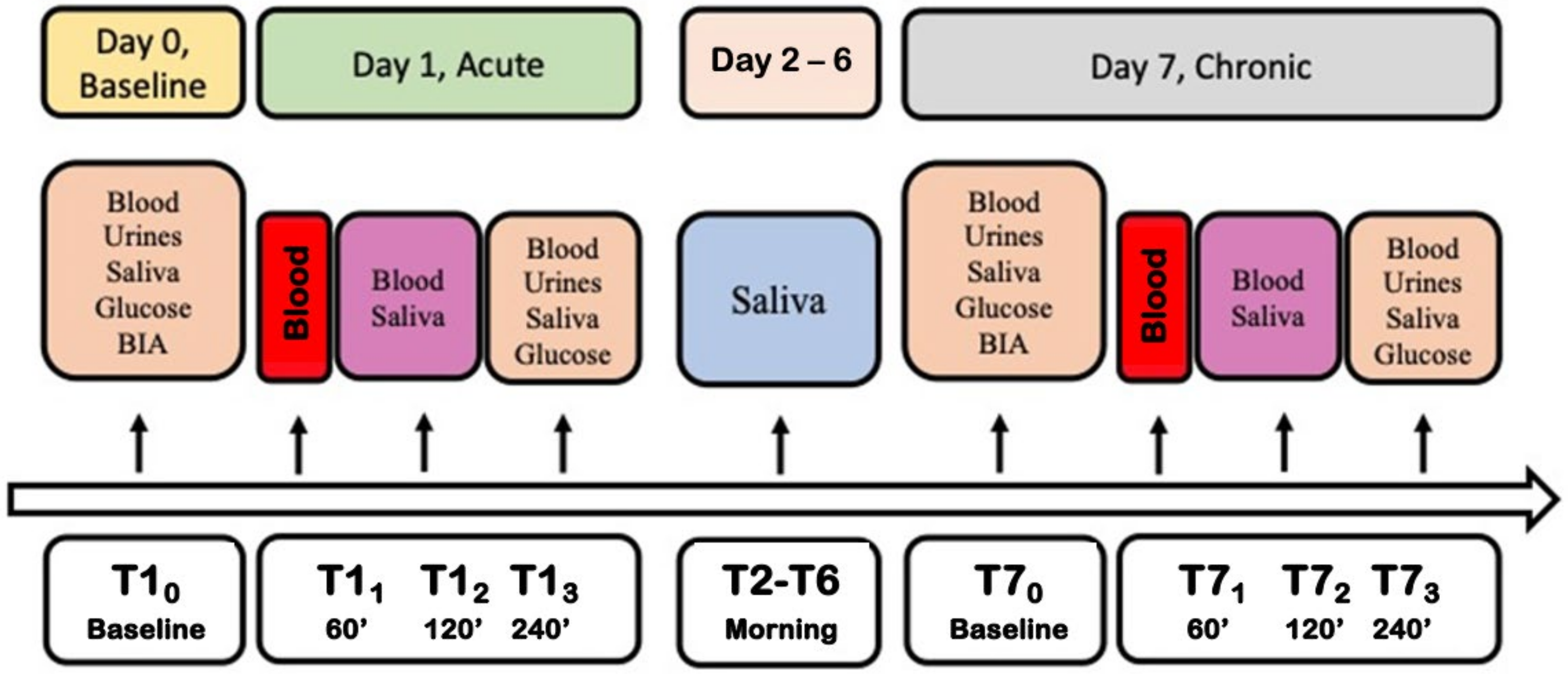
2.2. Experimental Protocol
- (A)
- Placebo (sham control) group.
- (B)
- Si.Pi.Mi group.
- Day 0: baseline before the experiment. Blood, saliva and urine samples were collected (basal measures).
- Day 1: to evaluate the acute wine intake, blood samples were drawn 60, 120 and 240 min after drinking (150 mL of red wine) to establish an ethanol metabolism curve. The placebo or Si.Pi.Mi. were taken after the wine dose. Saliva was collected 120 and 240 min after drinking, while urine was collected after 240 min.
- Day 2–6: for the long-term intake, volunteers drank one wine glass at lunch and another one at dinner (300 mL/die, corresponding to 30 g of ethanol/die approximately). They consumed the wine dose at the beginning of the meal, and then continued to eat a balanced daily menu according to individual energy needs. They took the placebo or Si.Pi.Mi. after the meal, depending on the group. Saliva was collected in the morning.
- Day 7: biological samples were collected in the same way as day 1, up to the end of the study.
2.3. Blood, Saliva and Urine Samples
2.4. Biomarker Analysis
2.4.1. Blood Alcohol Level
2.4.2. Urine Ethyl Glucuronoide (ETG)
2.4.3. Plasma ROS Production
2.4.4. Total Antioxidant Capacity (TAC)
2.4.5. 8-Isoprostane (8-Iso-PGF2α)
2.4.6. NO Metabolites
2.4.7. Co Q10 Coenzyme
2.4.8. Thiols Measurement
2.4.9. Creatinine, Neopterin, and Uric Acid
2.5. Statistic Analysis
3. Results
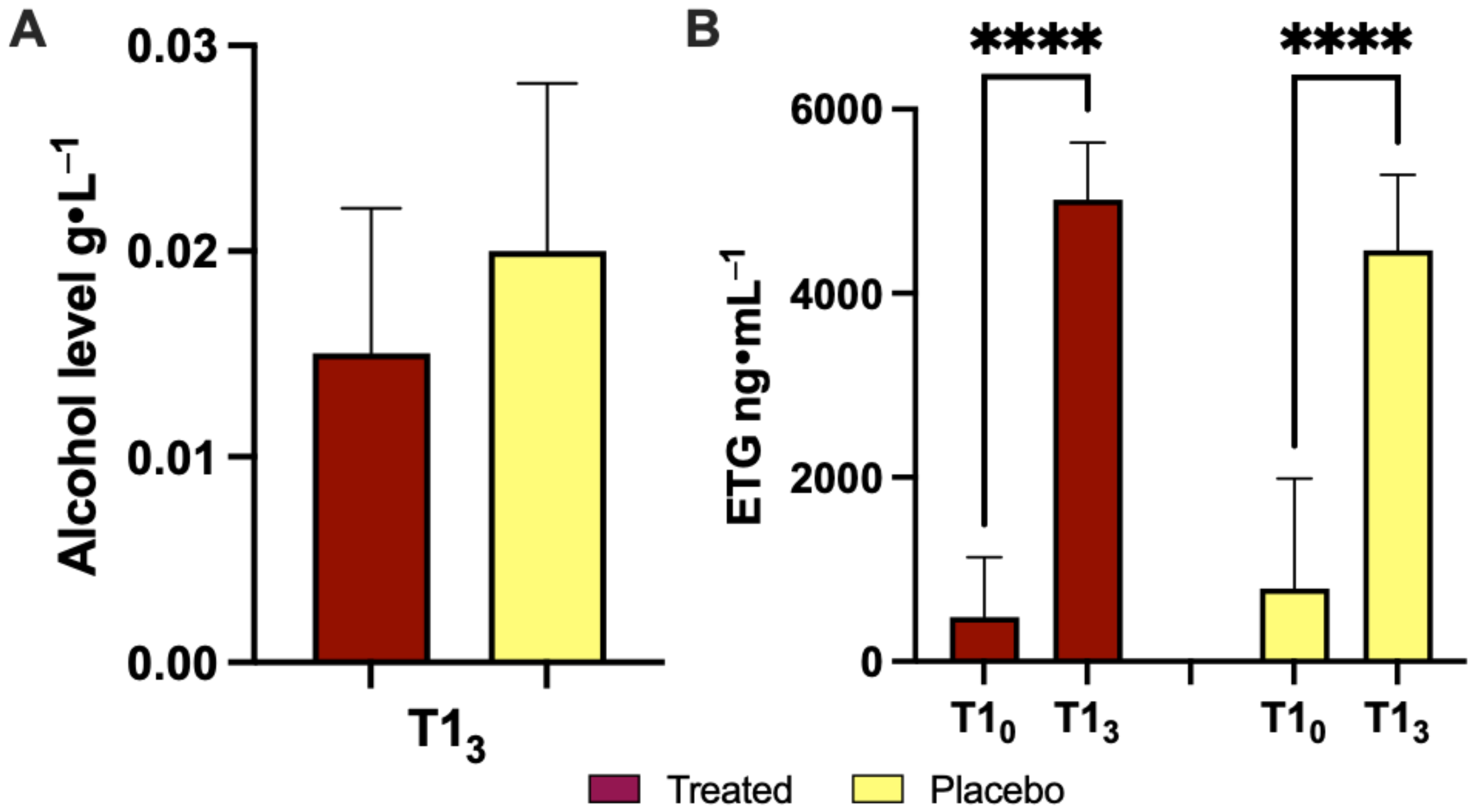
3.1. Effects of Product Intake on Ethanol Concentration during the Acute Phase
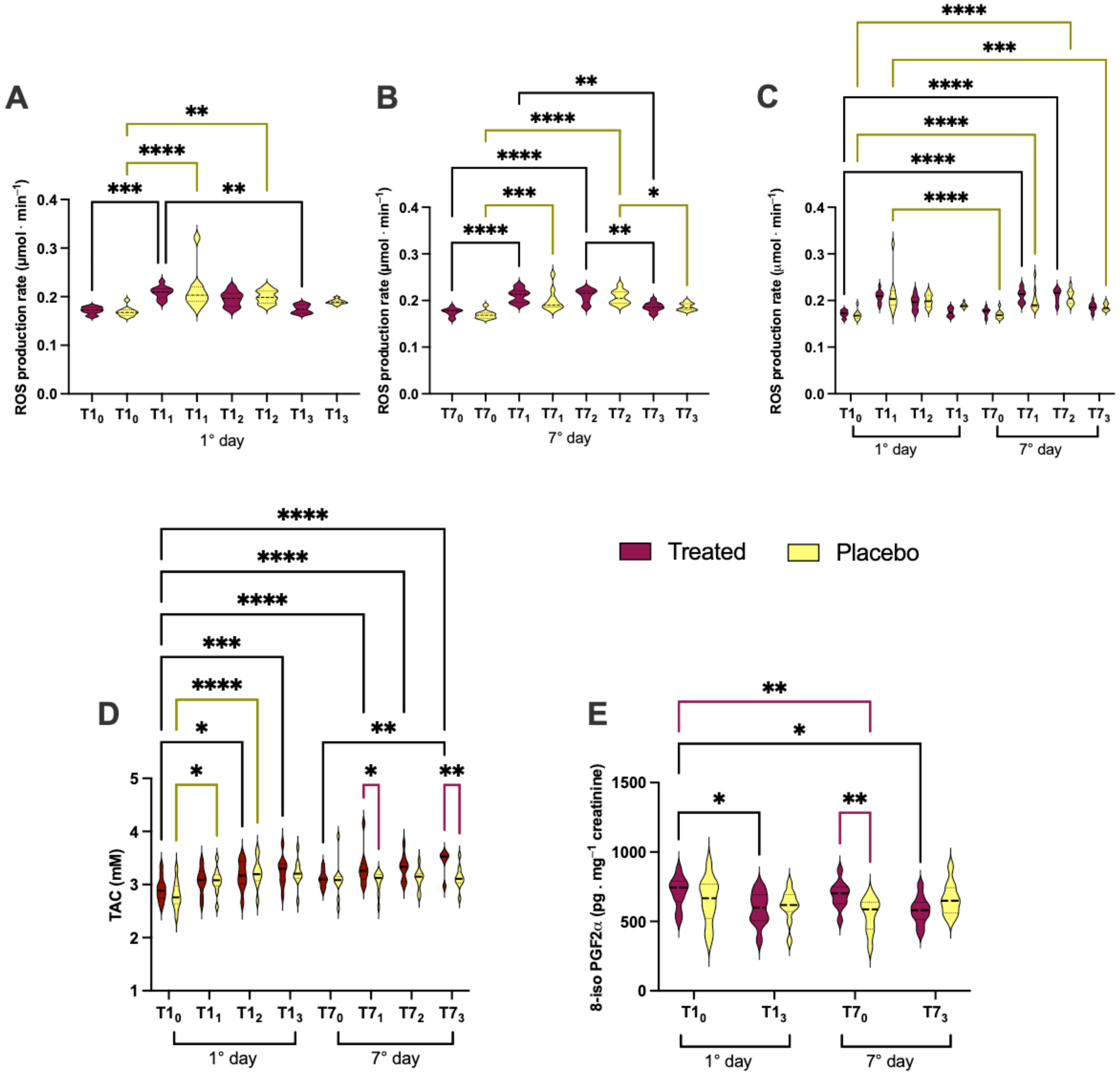
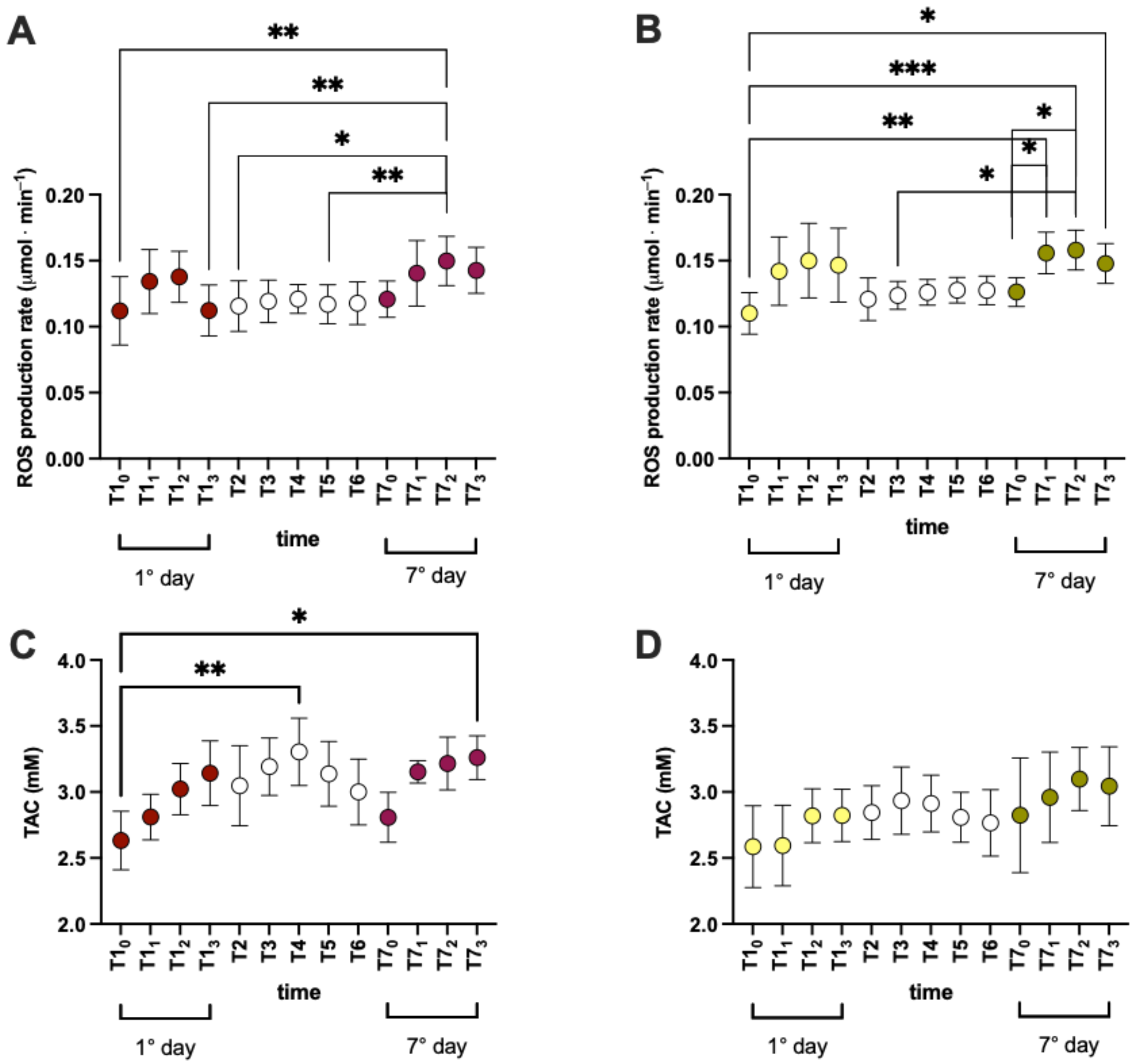
3.2. Effects of Product Intake on Oxidative Stress in Short and Long Phase
- (i)
- in treated group1st day: T10 vs. T11 (0.171 ± 0.007 vs. 0.208 ± 0.012); T11 vs. T13 (0.208 ± 0.012 vs. 0.174 ± 0.008);7th day: T70 vs. T71 (0.175 ± 0.008 vs. 0.210 ± 0.014); T70 vs. T72 (0.175 ± 0.008 vs. 0.210 ± 0.015); T71 vs. T73 (0.210 ± 0.014vs 1.186 ± 0.009); T72 vs. T73 (0.210 ± 0.015vs 1.186 ± 0.009);1st vs. 7th day: T10 vs. T71 (0.171 ± 0.007 vs. 0.210 ± 0.014vs); and T10 vs. T72 (0.171 ± 0.007 vs. 0.210 ± 0.015);
- (ii)
- in placebo group1st day: T10 vs. T11 (0.169 ± 0.009 vs. 0.212 ± 0.040); T11 vs. T12 (0.212 ± 0.040 vs. 0.199 ± 0.013); T70 vs. T71 (0.169 ± 0.008 vs. 0.200 ± 0.023);7th day: T70 vs. T72 (0.169 ± 0.008 vs. 0.207 ± 0.015); T70 vs. T73 (0.169 ± 0.008 vs. 0.186 ± 0.007); T72 vs. T73 (0.207 ± 0.015 vs. 0.186 ± 0.007); T10 vs. T71 (0.169 ± 0.009 vs. 0.200 ± 0.023);1st vs. 7th day: T10 vs. T72 (0.169 ± 0.009 vs. 0.207 ± 0.015); T11 vs. T70 (0.212 ± 0.040 vs. 0.169 ± 0.008); and T71 vs. T73 (0.200 ± 0.023vs 0.186 ± 0.007);
- (iii)
- inter-group (treated vs. placebo): no significant differences.
- (i)
- in the treated group1st day: T10 vs. T12 (2.911 ± 0.227 vs. 3.176 ± 0.269); T10 vs. T13 (2.911 ± 0.227 vs. 3.255 ± 0.265);7th day: T70 vs. T73 (3.109 ± 0.153 vs. 3.420 ± 0.241);1st vs. 7th day: T10 vs. T71 (2.911 ± 0.227 vs. 3.304 ± 0.333); T10 vs. T72 (2.911 ± 0.227 vs. 3.315 ± 0.231); T10 vs. T73 (2.911 ± 0.227 vs. 3.420 ± 0.241);
- (ii)
- in placebo group1st day: T10 vs. T11 (2.799 ± 0.258 vs. 3.068 ± 0.241); and T10 vs. T12 (2.799 ± 0.258 vs. 3.224 ± 0.269);
- (iii)
- inter-group (treated vs. placebo): at T71 (3.109 ± 0.153 vs. 3.083 ± 0.353); and T73 (3.420 ± 0.241 vs. 3.130 ± 0.209).
- (i)
- in the treated group: T10 vs. T13 (793.3 ± 120.6 vs. 617.5± 131.0); and T10 vs. T73 (793.3 ± 120.6 vs. 579.9 ± 100.4);
- (ii)
- in placebo group no significant differences;
- (iii)
- inter-group (treated vs. placebo): at T70 (793.3 ± 120.6 vs. 651.7 ± 165.6), and T10 vs. T70 (793.3 ± 120.6 vs. 545.7 ± 122.0).
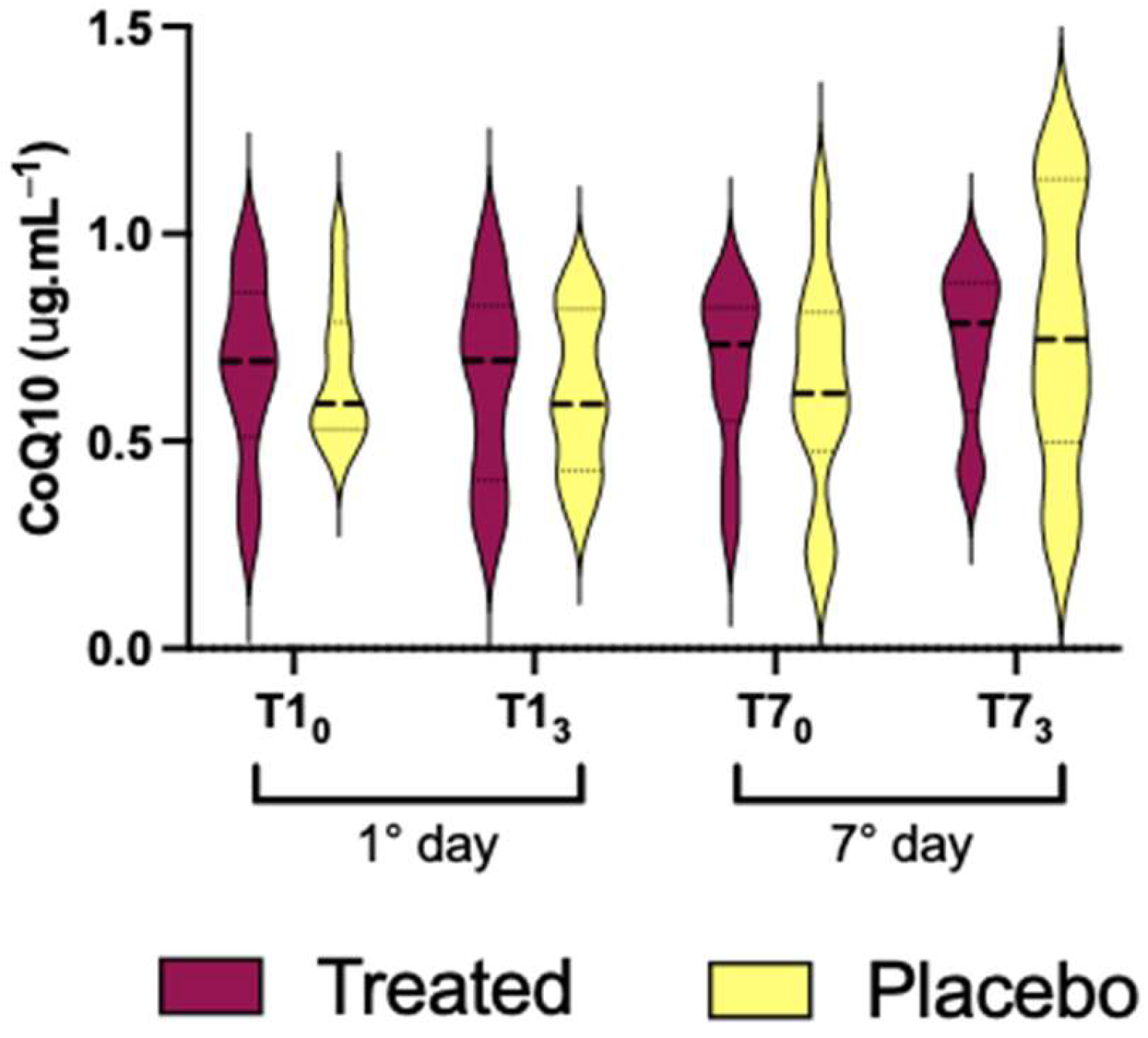
3.3. CoQ10 Coenzyme
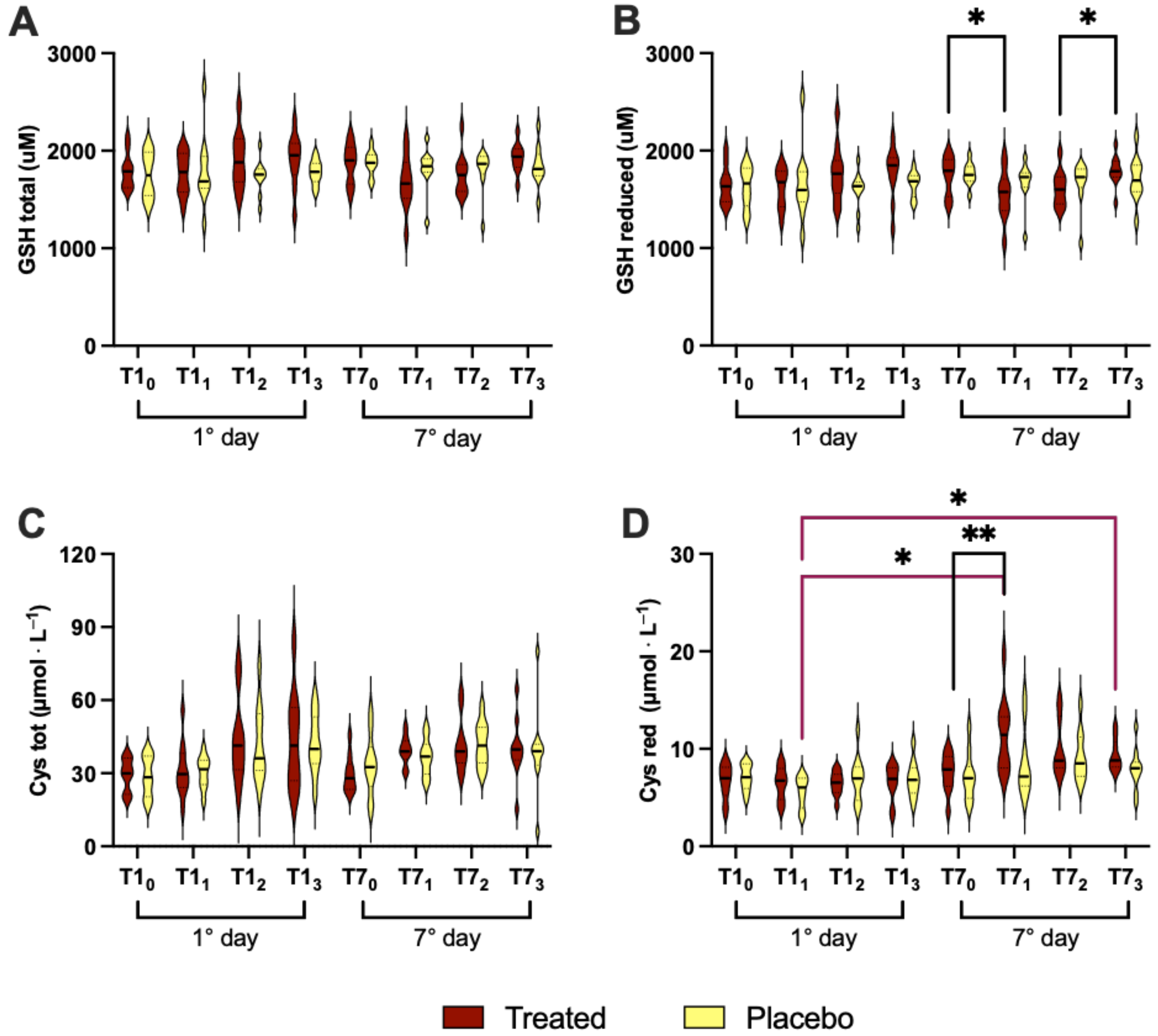
3.4. Redox Status
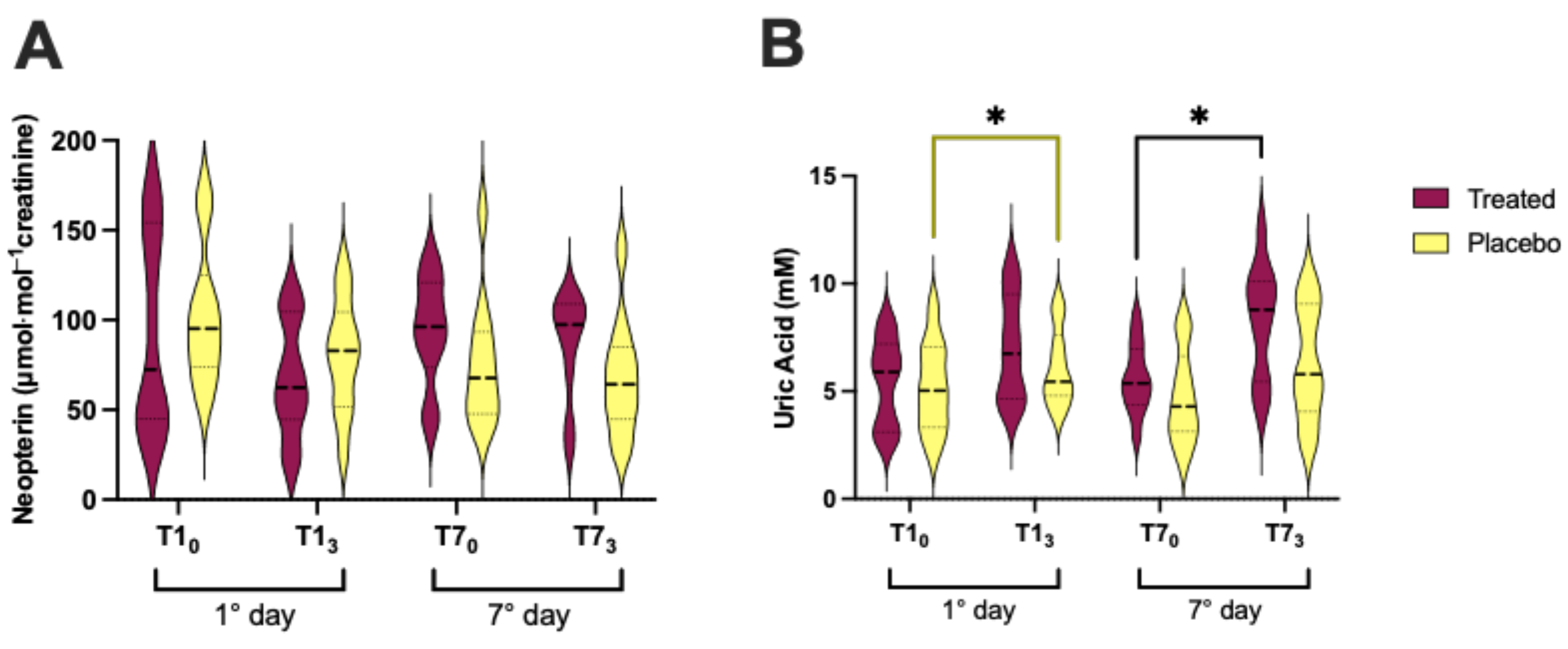
3.5. Neopterin and Uric Acid Concentration
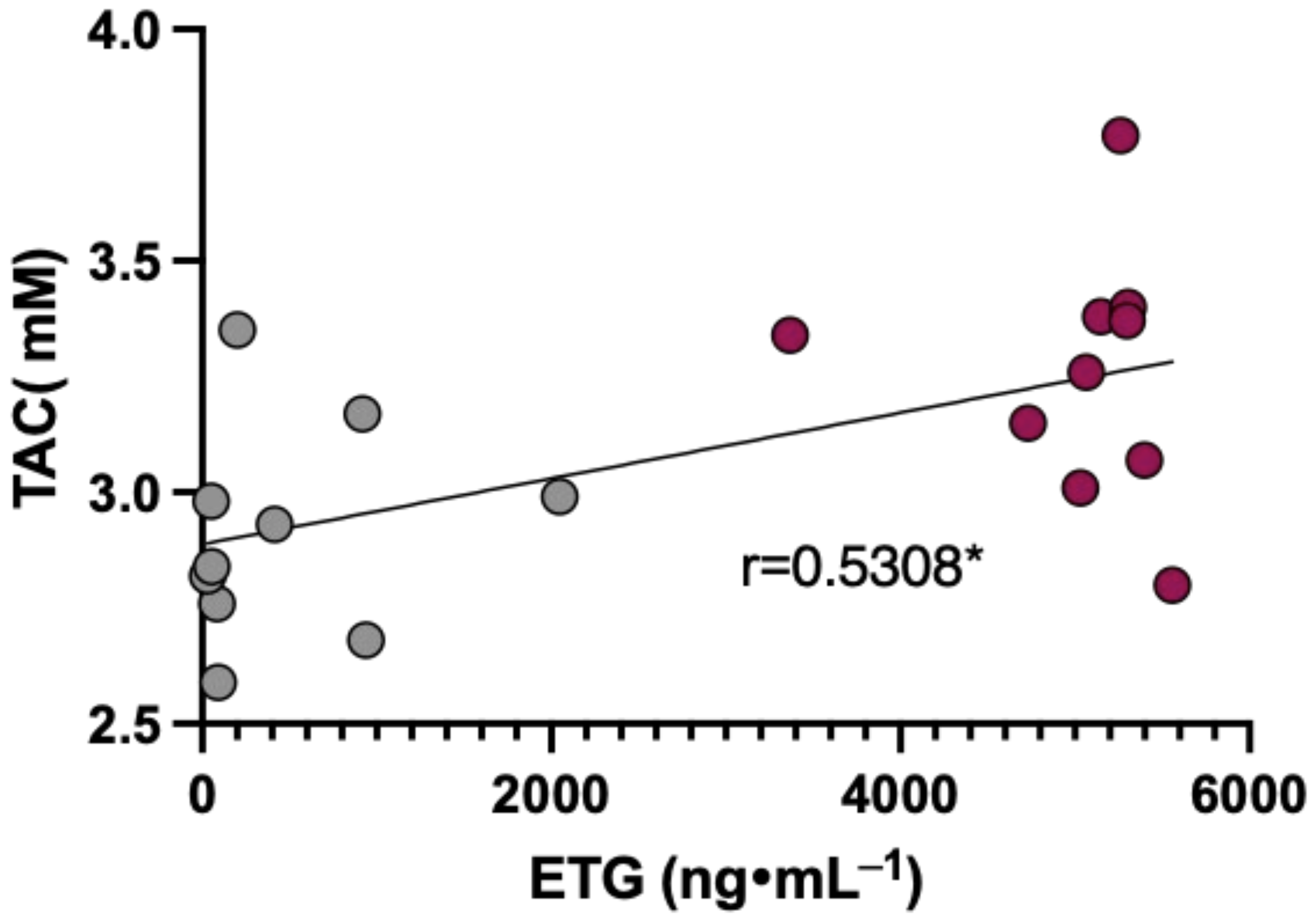
3.6. Data Correlation
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuster, D.; Samet, J.H. Alcohol Use in Patients with Chronic Liver Disease. N. Engl. J. Med. 2018, 379, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.T.; Salajegheh, A.; Brown, R.S., Jr. A Call to Standardize Definitions, Data Collection, and Outcome Assessment to Improve Care in Alcohol-Related Liver Disease. Hepatology 2019, 70, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Plapp, B.V. Rate-limiting steps in ethanol metabolism and approaches to changing these rates biochemically. Adv. Exp. Med. Biol. 1975, 56, 77–109. [Google Scholar] [PubMed]
- Loguercio, C.; Federico, A. Oxidative stress in viral and alcoholic hepatitis. Free Radic. Biol. Med. 2003, 34, 1–10. [Google Scholar] [CrossRef]
- Albano, E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol. Aspects Med. 2008, 29, 9–16. [Google Scholar] [CrossRef]
- Munzel, T.; Gori, T.; Bruno, R.M.; Taddei, S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur. Heart J. 2010, 31, 2741–2748. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Arosio, P.; Levi, S. Ferritin, iron homeostasis, and oxidative damage. Free Radic. Biol. Med. 2002, 33, 457–463. [Google Scholar] [CrossRef]
- Reed, D.J. Mitochondrial glutathione and chemically induced stress including ethanol. Drug Metab. Rev. 2004, 36, 569–582. [Google Scholar] [CrossRef]
- Tug, T.; Karatas, F.; Terzi, S.M. Antioxidant vitamins (A, C and E) and malondialdehyde levels in acute exacerbation and stable periods of patients with chronic obstructive pulmonary disease. Clin. Investig. Med. 2004, 27, 123–128. [Google Scholar]
- Kono, H.; Bradford, B.U.; Yin, M.; Sulik, K.K.; Koop, D.R.; Peters, J.M.; Gonzalez, F.J.; McDonald, T.; Dikalova, A.; Kadiiska, M.B.; et al. CYP2E1 is not involved in early alcohol-induced liver injury. Am. J. Physiol. 1999, 277, G1259–G1267. [Google Scholar] [CrossRef]
- Dawson, D.A. Methodological issues in measuring alcohol use. Alcohol Res. Health 2003, 27, 18–29. [Google Scholar] [PubMed]
- Jones, A.W.; Andersson, L. Variability of the blood/breath alcohol ratio in drinking drivers. J. Forensic Sci. 1996, 41, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Verster, J.C.; Penning, R. Treatment and prevention of alcohol hangover. Curr. Drug Abus. Rev. 2010, 3, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.T.; Wang, Y.Y.; Hu, X.Y.; Wang, S.B. The Protective Effects of Water Extracts of Compound Turmeric Recipe on Acute Alcoholism: An Experimental Research Using a Mouse Model. Evid.-Based Complement. Altern. Med. 2021, 2021, 6641919. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.S.; Holeckova, V.; Petraskova, L.; Biedermann, D.; Valentova, K.; Buchta, M.; Kren, V. The silymarin composition... and why does it matter??? Food Res. Int. 2017, 100, 339–353. [Google Scholar] [CrossRef]
- Song, Z.; Deaciuc, I.; Song, M.; Lee, D.Y.; Liu, Y.; Ji, X.; McClain, C. Silymarin protects against acute ethanol-induced hepatotoxicity in mice. Alcohol Clin. Exp. Res. 2006, 30, 407–413. [Google Scholar] [CrossRef]
- Guo, C.; Xue, G.; Pan, B.; Zhao, M.; Chen, S.; Gao, J.; Chen, T.; Qiu, L. Myricetin Ameliorates Ethanol-Induced Lipid Accumulation in Liver Cells by Reducing Fatty Acid Biosynthesis. Mol. Nutr. Food Res. 2019, 63, e1801393. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, Y.; Zhang, Y.; Tian, D.; Jiang, S.; Tang, Y. Hepatoprotective effect of pyrroloquinoline quinone against alcoholic liver injury through activating Nrf2-mediated antioxidant and inhibiting TLR4-mediated inflammation responses. Process Biochem. 2020, 92, 303–312. [Google Scholar] [CrossRef]
- Sarafian, D.; Maufrais, C.; Montani, J.P. Early and Late Cardiovascular and Metabolic Responses to Mixed Wine: Effect of Drink Temperature. Front. Physiol. 2018, 9, 1334. [Google Scholar] [CrossRef]
- Baraona, E.; Abittan, C.S.; Dohmen, K.; Moretti, M.; Pozzato, G.; Chayes, Z.W.; Schaefer, C.; Lieber, C.S. Gender differences in pharmacokinetics of alcohol. Alcohol. Clin. Exp. Res. 2001, 25, 502–507. [Google Scholar] [CrossRef] [PubMed]
- World Medical, A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- Kerr, W.C.; Greenfield, T.K.; Tujague, J.; Brown, S.E. A drink is a drink? Variation in the amount of alcohol contained in beer, wine and spirits drinks in a US methodological sample. Alcohol. Clin. Exp. Res. 2005, 29, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Savini, F.; Tartaglia, A.; Coccia, L.; Palestini, D.; D’Ovidio, C.; de Grazia, U.; Merone, G.M.; Bassotti, E.; Locatelli, M. Ethanol Determination in Post-Mortem Samples: Correlation between Blood and Vitreous Humor Concentration. Molecules 2020, 25, 2724. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. Assessment of a standardized ROS production profile in humans by electron paramagnetic resonance. Oxidative Med. Cell. Longev. 2012, 2012, 973927. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. A quantitative method to monitor reactive oxygen species production by electron paramagnetic resonance in physiological and pathological conditions. Oxidative Med. Cell. Longev. 2014, 2014, 306179. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Moretti, S.; Pratali, L.; Giardini, G.; Tacchini, P.; Dellanoce, C.; Tonacci, A.; Mastorci, F.; Borghini, A.; et al. Effects of Mountain Ultra-Marathon Running on ROS Production and Oxidative Damage by Micro-Invasive Analytic Techniques. PLoS ONE 2015, 10, e0141780. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; Garetto, G.; Paganini, M.; Camporesi, E.; Giacon, T.A.; Dellanoce, C.; Agrimi, J.; Bosco, G. Hyperbaric Oxygen Therapy Counters Oxidative Stress/Inflammation-Driven Symptoms in Long COVID-19 Patients: Preliminary Outcomes. Metabolites 2023, 13, 1032. [Google Scholar] [CrossRef]
- Strapazzon, G.; Malacrida, S.; Vezzoli, A.; Dal Cappello, T.; Falla, M.; Lochner, P.; Moretti, S.; Procter, E.; Brugger, H.; Mrakic-Sposta, S. Oxidative stress response to acute hypobaric hypoxia and its association with indirect measurement of increased intracranial pressure: A field study. Sci. Rep. 2016, 6, 32426. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Dellanoce, C.; Marzorati, M.; Montorsi, M.; Rasica, L.; Pratali, L.; D’Angelo, G.; Martinelli, M.; Bastiani, L.; et al. Effects of acute and sub-acute hypobaric hypoxia on oxidative stress: A field study in the Alps. Eur. J. Appl. Physiol. 2021, 121, 297–306. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Brizzolari, A.; Bosco, G.; Vezzoli, A.; Dellanoce, C.; Barassi, A.; Paganini, M.; Cialoni, D.; Mrakic-Sposta, S. Seasonal Oxy-Inflammation and Hydration Status in Non-Elite Freeskiing Racer: A Pilot Study by Non-Invasive Analytic Method. Int. J. Environ. Res. Public Health 2023, 20, 3157. [Google Scholar] [CrossRef] [PubMed]
- Dellanoce, C.; Cozzi, L.; Zuddas, S.; Pratali, L.; Accinni, R. Determination of different forms of aminothiols in red blood cells without washing erythrocytes. Biomed. Chromatogr. 2014, 28, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Vezzoli, A.; Dellanoce, C.; Mrakic-Sposta, S.; Montorsi, M.; Moretti, S.; Tonini, A.; Pratali, L.; Accinni, R. Oxidative Stress Assessment in Response to Ultraendurance Exercise: Thiols Redox Status and ROS Production according to Duration of a Competitive Race. Oxidative Med. Cell. Longev. 2016, 2016, 6439037. [Google Scholar] [CrossRef]
- Biswas, P.; Dellanoce, C.; Vezzoli, A.; Mrakic-Sposta, S.; Malnati, M.; Beretta, A.; Accinni, R. Antioxidant Activity with Increased Endogenous Levels of Vitamin C, E and A Following Dietary Supplementation with a Combination of Glutathione and Resveratrol Precursors. Nutrients 2020, 12, 3224. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; Rizzato, A.; Della Noce, C.; Malacrida, S.; Montorsi, M.; Paganini, M.; Cancellara, P.; Bosco, G. Oxidative stress assessment in breath-hold diving. Eur. J. Appl. Physiol. 2019, 119, 2449–2456. [Google Scholar] [CrossRef]
- Glantzounis, G.K.; Tsimoyiannis, E.C.; Kappas, A.M.; Galaris, D.A. Uric acid and oxidative stress. Curr. Pharm. Des. 2005, 11, 4145–4151. [Google Scholar] [CrossRef]
- Demartini, B.; Nistico, V.; Benayoun, C.; Cigognini, A.C.; Ferrucci, R.; Vezzoli, A.; Dellanoce, C.; Gambini, O.; Priori, A.; Mrakic-Sposta, S. Glutamatergic dysfunction, neuroplasticity, and redox status in the peripheral blood of patients with motor conversion disorders (functional movement disorders): A first step towards potential biomarkers discovery. Transl. Psychiatry 2023, 13, 212. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; Maderna, L.; Gregorini, F.; Montorsi, M.; Moretti, S.; Greco, F.; Cova, E.; Gussoni, M. R(+)-Thioctic Acid Effects on Oxidative Stress and Peripheral Neuropathy in Type II Diabetic Patients: Preliminary Results by Electron Paramagnetic Resonance and Electroneurography. Oxidative Med. Cell. Longev. 2018, 2018, 1767265. [Google Scholar] [CrossRef]
- Bosco, G.; Paganini, M.; Giacon, T.A.; Oppio, A.; Vezzoli, A.; Dellanoce, C.; Moro, T.; Paoli, A.; Zanotti, F.; Zavan, B.; et al. Oxidative Stress and Inflammation, MicroRNA, and Hemoglobin Variations after Administration of Oxygen at Different Pressures and Concentrations: A Randomized Trial. Int. J Environ. Res. Public Health 2021, 18, 9755. [Google Scholar] [CrossRef] [PubMed]
- Vezzoli, A.; Mrakic-Sposta, S.; Dellanoce, C.; Montorsi, M.; Vietti, D.; Ferrero, M.E. Chelation Therapy Associated with Antioxidant Supplementation Can Decrease Oxidative Stress and Inflammation in Multiple Sclerosis: Preliminary Results. Antioxidants 2023, 12, 1338. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S.; Leo, M.A.; Cao, Q.; Ren, C.; DeCarli, L.M. Silymarin retards the progression of alcohol-induced hepatic fibrosis in baboons. J. Clin. Gastroenterol. 2003, 37, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Pares, A.; Planas, R.; Torres, M.; Caballeria, J.; Viver, J.M.; Acero, D.; Panes, J.; Rigau, J.; Santos, J.; Rodes, J. Effects of silymarin in alcoholic patients with cirrhosis of the liver: Results of a controlled, double-blind, randomized and multicenter trial. J. Hepatol. 1998, 28, 615–621. [Google Scholar] [CrossRef]
- Lucena, M.I.; Andrade, R.J.; de la Cruz, J.P.; Rodriguez-Mendizabal, M.; Blanco, E.; Sanchez de la Cuesta, F. Effects of silymarin MZ-80 on oxidative stress in patients with alcoholic cirrhosis. Results of a randomized, double-blind, placebo-controlled clinical study. Int. J. Clin. Pharmacol. Ther. 2002, 40, 2–8. [Google Scholar] [CrossRef]
- Rosano, T.G.; Lin, J. Ethyl glucuronide excretion in humans following oral administration of and dermal exposure to ethanol. J. Anal. Toxicol. 2008, 32, 594–600. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, J.; Yao, J.; Zhang, B.; Duan, W.; Zhao, C.; Du, P.; Song, J.; Zheng, Y.; Wang, M. Shanxi Aged Vinegar Protects against Alcohol-Induced Liver Injury via Activating Nrf2-Mediated Antioxidant and Inhibiting TLR4-Induced Inflammatory Response. Nutrients 2018, 10, 805. [Google Scholar] [CrossRef]
- Haorah, J.; Ramirez, S.H.; Floreani, N.; Gorantla, S.; Morsey, B.; Persidsky, Y. Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic. Biol. Med. 2008, 45, 1542–1550. [Google Scholar] [CrossRef]
- Amerongen, A.V.; Veerman, E.C. Saliva-the defender of the oral cavity. Oral Dis. 2002, 8, 12–22. [Google Scholar] [CrossRef]
- Balaji, T.M.; Vasanthi, H.R.; Rao, S.R. Gingival, plasma and salivary levels of melatonin in periodontally healthy individuals and chronic periodontitis patients: A pilot study. J. Clin. Diagn. Res. 2015, 9, ZC23–ZC25. [Google Scholar] [CrossRef]
- Battino, M.; Ferreiro, M.S.; Gallardo, I.; Newman, H.N.; Bullon, P. The antioxidant capacity of saliva. J. Clin. Periodontol. 2002, 29, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontology 2020, 84, 45–68. [Google Scholar] [CrossRef]
- Detaille, D.; Sanchez, C.; Sanz, N.; Lopez-Novoa, J.M.; Leverve, X.; El-Mir, M.Y. Interrelation between the inhibition of glycolytic flux by silibinin and the lowering of mitochondrial ROS production in perifused rat hepatocytes. Life Sci. 2008, 82, 1070–1076. [Google Scholar] [CrossRef]
- Grattagliano, I.; Diogo, C.V.; Mastrodonato, M.; de Bari, O.; Persichella, M.; Wang, D.Q.; Liquori, A.; Ferri, D.; Carratu, M.R.; Oliveira, P.J.; et al. A silybin-phospholipids complex counteracts rat fatty liver degeneration and mitochondrial oxidative changes. World J. Gastroenterol. 2013, 19, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Mukherjee, S. Biochemical and immunological basis of silymarin effect, a milk thistle (Silybum marianum) against ethanol-induced oxidative damage. Toxicol. Mech. Methods 2012, 22, 409–413. [Google Scholar] [CrossRef]
- Cores, Á.; Carmona-Zafra, N.; Clerigué, J.; Villacampa, M.; Menéndez, J.C. Quinones as Neuroprotective Agents. Antioxidants 2023, 12, 1464. [Google Scholar] [CrossRef] [PubMed]
- Ciuclan, L.; Ehnert, S.; Ilkavets, I.; Weng, H.L.; Gaitantzi, H.; Tsukamoto, H.; Ueberham, E.; Meindl-Beinker, N.M.; Singer, M.V.; Breitkopf, K.; et al. TGF-beta enhances alcohol dependent hepatocyte damage via down-regulation of alcohol dehydrogenase I. J. Hepatol. 2010, 52, 407–416. [Google Scholar] [CrossRef]
- Zhu, H.J.; Brinda, B.J.; Chavin, K.D.; Bernstein, H.J.; Patrick, K.S.; Markowitz, J.S. An assessment of pharmacokinetics and antioxidant activity of free silymarin flavonolignans in healthy volunteers: A dose escalation study. Drug Metab. Dispos. 2013, 41, 1679–1685. [Google Scholar] [CrossRef]
- Adachi, M.; Ishii, H. Role of mitochondria in alcoholic liver injury. Free Radic. Biol. Med. 2002, 32, 487–491. [Google Scholar] [CrossRef]
- Bailey, S.M.; Cunningham, C.C. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radic. Biol. Med. 2002, 32, 11–16. [Google Scholar] [CrossRef]
- Vidyashankar, S.; Nandakumar, K.S.; Patki, P.S. Alcohol depletes coenzyme-Q(10) associated with increased TNF-alpha secretion to induce cytotoxicity in HepG2 cells. Toxicology 2012, 302, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Checa, J.C.; Kaplowitz, N. Hepatic mitochondrial glutathione: Transport and role in disease and toxicity. Toxicol. Appl. Pharmacol. 2005, 204, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Kurose, I.; Higuchi, H.; Kato, S.; Miura, S.; Ishii, H. Ethanol-induced oxidative stress in the liver. Alcohol. Clin Exp. Res. 1996, 20, 77A–85A. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, C.; Morales, A.; Colell, A.; Ballesta, A.; Rodes, J.; Kaplowitz, N.; Fernandez-Checa, J.C. Feeding S-adenosyl-L-methionine attenuates both ethanol-induced depletion of mitochondrial glutathione and mitochondrial dysfunction in periportal and perivenous rat hepatocytes. Hepatology 1995, 21, 207–214. [Google Scholar] [CrossRef]
- Iimuro, Y.; Bradford, B.U.; Yamashina, S.; Rusyn, I.; Nakagami, M.; Enomoto, N.; Kono, H.; Frey, W.; Forman, D.; Brenner, D.; et al. The glutathione precursor L-2-oxothiazolidine-4-carboxylic acid protects against liver injury due to chronic enteral ethanol exposure in the rat. Hepatology 2000, 31, 391–398. [Google Scholar] [CrossRef]
- Mari, M.; Morales, A.; Colell, A.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Mitochondrial glutathione, a key survival antioxidant. Antioxid. Redox Signal. 2009, 11, 2685–2700. [Google Scholar] [CrossRef]
- Das, S.K.; Vasudevan, D.M. Alcohol-induced oxidative stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef]
- Foronjy, R.; D’Armiento, J. The Effect of Cigarette Smoke-derived Oxidants on the Inflammatory Response of the Lung. Clin. Appl. Immunol. Rev. 2006, 6, 53–72. [Google Scholar] [CrossRef]
- Mudway, I.S.; Kelly, F.J.; Holgate, S.T. Oxidative stress in air pollution research. Free Radic. Biol. Med. 2020, 151, 2–6. [Google Scholar] [CrossRef]
| Parameter | T0 | T7 | ||
|---|---|---|---|---|
| Treated | Placebo | Treated | Placebo | |
| MCV (fL) range * (80–100) | 90.4 ± 2.7 | 90.2 ± 3.0 | 90.4 ± 2.1 | 90.0 ± 3.2 |
| AST (U/L) Range (17–59) | 32.8 ± 10.1 | 23.8 ± 9.8 | 32.0 ± 17.0 | 20.8 ± 5.1 |
| ALT (U/L) Range (7–55) | 21.8 ± 10.8 | 19.2 ± 10.0 | 28.2 ± 34.7 | 15.4 ± 8.6 |
| γ-GT (U/L) range (5–40) | 15.2 ± 4.3 | 10.6 ± 7.7 | 16.4 ± 3.8 | 13.8 ± 4.7 |
| TBL (mg/dL) Range (0.1–1.2) | 0.67 ± 0.09 | 0.56 ± 0.07 | 1.03 ± 0.35 | 0.47 ± 0.15 |
| DBIL (mg/dL) Range (0.1–0.3) | 0.25 ± 0.07 | 0.24 ± 0.10 | 0.26 ± 0.14 | 0.25 ± 0.11 |
| UBIL (mg/dL) Range (0.2–0.8) | 0.39 ± 0.40 | 0.36 ± 0.22 | 0.41 ± 0.25 | 0.37 ± 0.11 |
| Parameter | T10 | T13 | ||
|---|---|---|---|---|
| Treated | Placebo | Treated | Placebo | |
| Alcohol level (g/L) | - | - | 0.015 ± 0.007 | 0.020 ± 0.008 |
| ETG (ng/mL) | 483.1 ± 650.9 | 790.4 ± 1197 | 5017 ± 622.1 | 4466 ± 823.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosco, G.; Vezzoli, A.; Brizzolari, A.; Paganini, M.; Giacon, T.A.; Savini, F.; Gussoni, M.; Montorsi, M.; Dellanoce, C.; Mrakic-Sposta, S. Consumption of Sylimarin, Pyrroloquinoline Quinone Sodium Salt and Myricetin: Effects on Alcohol Levels and Markers of Oxidative Stress—A Pilot Study. Nutrients 2024, 16, 2965. https://doi.org/10.3390/nu16172965
Bosco G, Vezzoli A, Brizzolari A, Paganini M, Giacon TA, Savini F, Gussoni M, Montorsi M, Dellanoce C, Mrakic-Sposta S. Consumption of Sylimarin, Pyrroloquinoline Quinone Sodium Salt and Myricetin: Effects on Alcohol Levels and Markers of Oxidative Stress—A Pilot Study. Nutrients. 2024; 16(17):2965. https://doi.org/10.3390/nu16172965
Chicago/Turabian StyleBosco, Gerardo, Alessandra Vezzoli, Andrea Brizzolari, Matteo Paganini, Tommaso Antonio Giacon, Fabio Savini, Maristella Gussoni, Michela Montorsi, Cinzia Dellanoce, and Simona Mrakic-Sposta. 2024. "Consumption of Sylimarin, Pyrroloquinoline Quinone Sodium Salt and Myricetin: Effects on Alcohol Levels and Markers of Oxidative Stress—A Pilot Study" Nutrients 16, no. 17: 2965. https://doi.org/10.3390/nu16172965
APA StyleBosco, G., Vezzoli, A., Brizzolari, A., Paganini, M., Giacon, T. A., Savini, F., Gussoni, M., Montorsi, M., Dellanoce, C., & Mrakic-Sposta, S. (2024). Consumption of Sylimarin, Pyrroloquinoline Quinone Sodium Salt and Myricetin: Effects on Alcohol Levels and Markers of Oxidative Stress—A Pilot Study. Nutrients, 16(17), 2965. https://doi.org/10.3390/nu16172965











