Patients with Inflammatory Bowel Disease Show Fewer Sex-Related Differences in Their Dietary Behavior Than the General Population: A Qualitative Analysis
Abstract
1. Introduction
Objectives
2. Materials and Methods
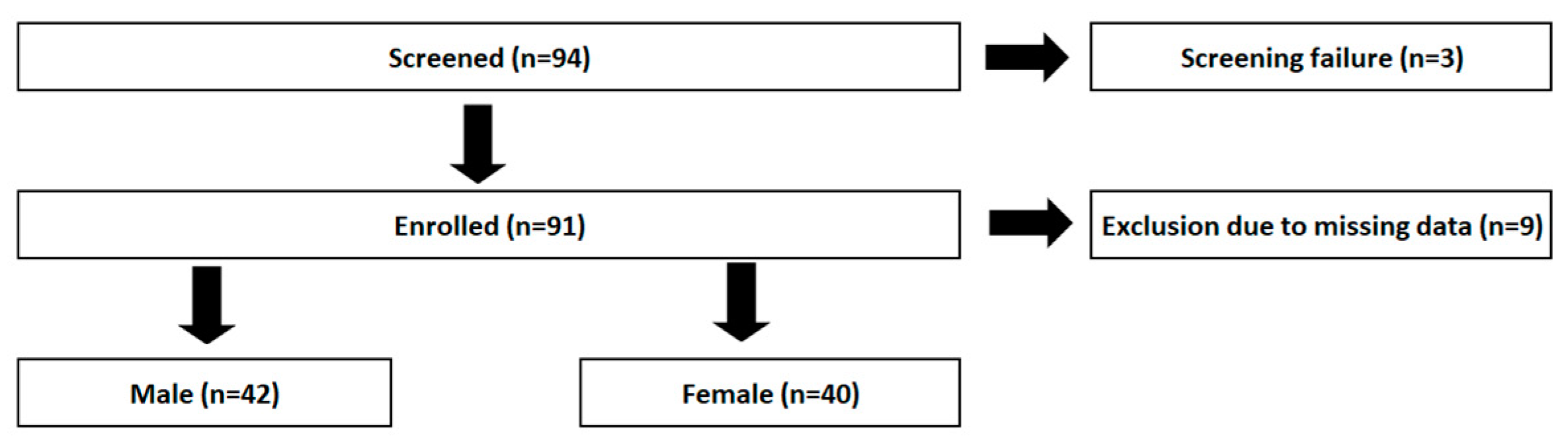
2.1. Participants and Setting
Control Group
2.2. Study Variables and Definitions
2.2.1. Dietary Behavior
2.2.2. Mediterranean Diet Score
2.2.3. Ultra-Processed Foods and Drinks
2.2.4. Food Groups of the German Nutrition Society
2.2.5. Food Frequency Questionnaire Variables and Macronutrients
2.3. Data Sources/Measurement
2.3.1. Questionnaires
2.3.2. Laboratory Values
2.4. Study Size
2.5. Statistical Analysis
2.5.1. Missing Data
2.5.2. Sampling Strategy
2.5.3. Bias
3. Results
3.1. Descriptive Data
3.2. Main Results
3.2.1. IBD Cohort Versus DEGS1 Cohort
Macronutrient Intake—Mean Daily Amount
Ultra-Processed Foods and Drinks—Mean Daily Amount
3.2.2. DGE Food Groups—Mean Daily Consumption
Fruits and Vegetables
Juices
Legumes and Pulses
Nuts and Seeds
Potato Products
Butter and Margarine
Dairy Products
Fish
Meat and Poultry
Cold Cuts
Eggs
Cereal Products and Rice
3.3. IBD: Sex-Related Trends and Differences in Correlation with Disease Parameters
4. Discussion
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Reznikov, E.A.; Suskind, D.L. Current Nutritional Therapies in Inflammatory Bowel Disease: Improving Clinical Remission Rates and Sustainability of Long-Term Dietary Therapies. Nutrients 2023, 15, 668. [Google Scholar] [CrossRef] [PubMed]
- Vissers, E.; Wellens, J.; Sabino, J. Ultra-processed foods as a possible culprit for the rising prevalence of inflammatory bowel diseases. Front. Med. 2022, 9, 1058373. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H.; Khandpur, N.; Rossato, S.L.; Lochhead, P.; Lopes, E.W.; Burke, K.E.; Richter, J.M.; Song, M.; Ardisson Korat, A.V.; Sun, Q.; et al. Ultra-processed Foods and Risk of Crohn’s Disease and Ulcerative Colitis: A Prospective Cohort Study. Clin. Gastroenterol. Hepatol. 2022, 20, e1323–e1337. [Google Scholar] [CrossRef]
- Narula, N.; Wong, E.C.L.; Dehghan, M.; Mente, A.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Rohatgi, P.; Lakshmi, P.V.M.; Varma, R.P.; et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: Prospective cohort study. BMJ 2021, 374, n1554. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth, L.; Machado, P.; Zinöcker, M.; Baker, P.; Lawrence, M. Ultra-Processed Foods and Health Outcomes: A Narrative Review. Nutrients 2020, 12, 1955. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.M.; Gamage, E.; Du, S.; Ashtree, D.N.; McGuinness, A.J.; Gauci, S.; Baker, P.; Lawrence, M.; Rebholz, C.M.; Srour, B.; et al. Ultra-processed food exposure and adverse health outcomes: Umbrella review of epidemiological meta-analyses. BMJ 2024, 384, e077310. [Google Scholar] [CrossRef]
- Roncoroni, L.; Gori, R.; Elli, L.; Tontini, G.E.; Doneda, L.; Norsa, L.; Cuomo, M.; Lombardo, V.; Scricciolo, A.; Caprioli, F.; et al. Nutrition in Patients with Inflammatory Bowel Diseases: A Narrative Review. Nutrients 2022, 14, 751. [Google Scholar] [CrossRef]
- Grzymisławska, M.; Puch, E.A.; Zawada, A.; Grzymisławski, M. Do nutritional behaviors depend on biological sex and cultural gender? Adv. Clin. Exp. Med. 2020, 29, 165–172. [Google Scholar] [CrossRef]
- Engler, D.; Schnabel, R.B.; Neumann, F.A.; Zyriax, B.C.; Makarova, N. Sex-Specific Dietary Patterns and Social Behaviour in Low-Risk Individuals. Nutrients 2023, 15, 1832. [Google Scholar] [CrossRef]
- Wiestler, M.; Kockelmann, F.; Kück, M.; Kerling, A.; Tegtbur, U.; Manns, M.P.; Attaran-Bandarabadi, M.; Bachmann, O. Quality of Life Is Associated with Wearable-Based Physical Activity in Patients with Inflammatory Bowel Disease: A Prospective, Observational Study. Clin. Transl. Gastroenterol. 2019, 10, e00094. [Google Scholar] [CrossRef] [PubMed]
- Kamtsiuris, P.; Lange, M.; Hoffmann, R.; Schaffrath Rosario, A.; Dahm, S.; Kuhnert, R.; Kurth, B.M. The first wave of the German Health Interview and Examination Survey for Adults (DEGS1): Sample design, response, weighting and representativeness. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013, 56, 620–630. [Google Scholar] [CrossRef] [PubMed]
- RKI German Health Interview and Examination Survey for Adults (DEGS11). In Scientific Use File First Version; Robert Koch Institute. Department of Epidemiology and Health Monitoring: Berlin, Germany, 2015. [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A. Nutrition and health. The issue is not food, nor nutrients, so much as processing. Public Health Nutr. 2009, 12, 729–731. [Google Scholar] [CrossRef]
- DGE. Referenzwerte für die Nährstoffzufuhr, 2nd ed.; 8th updated edition ed.; Deutsche Gesellschaft für Ernährung, Österreichische Gesellschaft für Ernährung: Bonn, Germany, 2024. [Google Scholar]
- Haftenberger, M.; Heuer, T.; Heidemann, C.; Kube, F.; Krems, C.; Mensink, G.B. Relative validation of a food frequency questionnaire for national health and nutrition monitoring. Nutr. J. 2010, 9, 36. [Google Scholar] [CrossRef]
- Mensink, G.B.M.; Truthmann, J.; Rabenberg, M.; Heidemann, C.; Haftenberger, M.; Schienkiewitz, A.; Richter, A. Obst- und Gemüsekonsum in Deutschland: Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1). Bundesgesundheitsblatt—Gesundheitsforschung—Gesundheitsschutz 2013, 56, 779–785. [Google Scholar] [CrossRef]
- Thieleking, R.; Schneidewind, L.; Kanyamibwa, A.; Hartmann, H.; Horstmann, A.; Witte, A.V.; Medawar, E. Nutrient scoring for the DEGS1-FFQ—From food intake to nutrient intake. BMC Nutr. 2023, 9, 12. [Google Scholar] [CrossRef]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s-disease activity. Lancet 1980, 1, 514. [Google Scholar] [CrossRef]
- Walmsley, R.S.; Ayres, R.C.S.; Pounder, R.E.; Allan, R.N. A simple clinical colitis activity index. Gut 1998, 43, 29–32. [Google Scholar] [CrossRef]
- Janke, K.-H.; Klump, B.; Steder-Neukamm, U.; Hoffmann, J.; Häuser, W. Validierung der Deutschen Version (Kompetenznetz “Chronisch entzündliche Darmerkrankungen”) des Inflammatory Bowel Disease Questionnaire IBDQ-D. Psychother. Psychosom. Med. Psychol. 2006, 56, 291–298. [Google Scholar] [CrossRef]
- Guyatt, G.; Mitchell, A.; Irvine, E.J.; Singer, J.; Williams, N.; Goodacre, R.; Tompkins, C. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989, 96, 804–810. [Google Scholar] [CrossRef]
- Black, A.E.; Goldberg, G.R.; Jebb, S.A.; Livingstone, M.B.; Cole, T.J.; Prentice, A.M. Critical evaluation of energy intake data using fundamental principles of energy physiology: 2. Evaluating the results of published surveys. Eur. J. Clin. Nutr. 1991, 45, 583–599. [Google Scholar] [PubMed]
- Black, A.E. Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, G.R.; Black, A.E.; Jebb, S.A.; Cole, T.J.; Murgatroyd, P.R.; Coward, W.A.; Prentice, A.M. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur. J. Clin. Nutr. 1991, 45, 569–581. [Google Scholar]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef]
- Moon, K.; Krems, C.; Heuer, T.; Roth, A.; Hoffmann, I. Predictors of BMI Vary along the BMI Range of German Adults—Results of the German National Nutrition Survey II. Obes. Facts 2017, 10, 38–49. [Google Scholar] [CrossRef]
- Godala, M.; Gaszyńska, E.; Durko, Ł.; Małecka-Wojciesko, E. Dietary Behaviors and Beliefs in Patients with Inflammatory Bowel Disease. J. Clin. Med. 2023, 12, 3455. [Google Scholar] [CrossRef]
- Zallot, C.; Quilliot, D.; Chevaux, J.-B.; Peyrin-Biroulet, C.; Guéant-Rodriguez, R.M.; Freling, E.; Collet-Fenetrier, B.; Williet, N.; Ziegler, O.; Bigard, M.-A.; et al. Dietary Beliefs and Behavior Among Inflammatory Bowel Disease Patients. Inflamm. Bowel Dis. 2013, 19, 66–72. [Google Scholar] [CrossRef]
- Bennett, E.; Peters, S.A.E.; Woodward, M. Sex differences in macronutrient intake and adherence to dietary recommendations: Findings from the UK Biobank. BMJ Open 2018, 8, e020017. [Google Scholar] [CrossRef]
- SACN. SACN Dietary Reference Values for Energy; TSO: London, UK, 2011. [Google Scholar]
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hébuterne, X.; Hvas, C.L.; Joly, F.; Klek, S.; Krznaric, Z.; Ockenga, J.; et al. ESPEN guideline on Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2023, 42, 352–379. [Google Scholar] [CrossRef] [PubMed]
- Knowles, S.R.; Keefer, L.; Wilding, H.; Hewitt, C.; Graff, L.A.; Mikocka-Walus, A. Quality of Life in Inflammatory Bowel Disease: A Systematic Review and Meta-analyses—Part II. Inflamm. Bowel Dis. 2018, 24, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Murtagh, A.; Cooney, L.; Higginbotham, C.; Heavey, P. Dietary practices, beliefs and behaviours of adults with inflammatory bowel disease: A cross-sectional study. Ir. J. Med. Sci. 2023, 192, 1115–1124. [Google Scholar] [CrossRef]
- Tomar, S.K.; Kedia, S.; Upadhyay, A.D.; Bopanna, S.; Yadav, D.P.; Goyal, S.; Jain, S.; Makharia, G.; Ahuja, V.; Singh, N. Impact of dietary beliefs and practices on patients with inflammatory bowel disease: An observational study from India. JGH Open 2017, 1, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, N.; Kumar, D. Food-specific IgG4-guided exclusion diets improve symptoms in Crohn’s disease: A pilot study. Color. Dis. 2011, 13, 1009–1013. [Google Scholar] [CrossRef]
- Sigall-Boneh, R.; Pfeffer-Gik, T.; Segal, I.; Zangen, T.; Boaz, M.; Levine, A. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 1353–1360. [Google Scholar] [CrossRef]
- Di Giorgio, F.M.; Modica, S.P.; Saladino, M.; Muscarella, S.; Ciminnisi, S.; Almasio, P.L.; Petta, S.; Cappello, M. Food Beliefs and the Risk of Orthorexia in Patients with Inflammatory Bowel Disease. Nutrients 2024, 16, 1193. [Google Scholar] [CrossRef]
- Donini, L.M.; Marsili, D.; Graziani, M.P.; Imbriale, M.; Cannella, C. Orthorexia nervosa: A preliminary study with a proposal for diagnosis and an attempt to measure the dimension of the phenomenon. Eat. Weight Disord. 2004, 9, 151–157. [Google Scholar] [CrossRef]
- Fidan, T.; Ertekin, V.; Işikay, S.; Kirpinar, I. Prevalence of orthorexia among medical students in Erzurum, Turkey. Compr. Psychiatry 2010, 51, 49–54. [Google Scholar] [CrossRef]
- Donini, L.M.; Marsili, D.; Graziani, M.P.; Imbriale, M.; Cannella, C. Orthorexia nervosa: Validation of a diagnosis questionnaire. Eat Weight Disord. 2005, 10, e28–e32. [Google Scholar] [CrossRef]

| Men (n = 42) | Women (n = 40) | psex | ||
|---|---|---|---|---|
| Entity [n (%)] | CD | 19 (45.2%) | 25 (62.5%) | 0.234 |
| UC | 23 (54.8%) | 15 (37.5%) | 0.233 | |
| Disease duration [median (IQR)] (years) | 9 [5, 17] | 8 [4, 16] | 0.781 | |
| HBI [mean (SD)] | 6.11 [5.92] | 7.84 [5.93] | 0.393 | |
| SCCAI [mean (SD)] | 5.7 [5.05] | 3.73 [4.68] | 0.404 | |
| Location of Crohn’s | L1: Ileal | 0 (0%) | 2 (8%) | 0.828 |
| L2: Colonic | 2 (10.5%) | 3 (12%) | 0.999 | |
| L3: Ileocolonic | 12 (63.2%) | 12 (48%) | 0.999 | |
| L4: Upper GI | 5 (26.3%) | 8 (32%) | 0.999 | |
| Crohn’s behavior | B1: Inflammatory | 5 (26.3%) | 11 (44%) | 0.681 |
| B2: Stricturing | 10 (52.6%) | 10 (40%) | 0.999 | |
| B3: Penetrating | 4 (21.1%) | 4 (16%) | 0.999 | |
| UC Montreal classification | E1: Proctitis | 2 (8.7%) | 1 (6.7%) | 0.999 |
| E2: Left-sided colitis | 12 (52.2%) | 4 (26.7%) | 0.358 | |
| E3: Pancolitis | 9 (39.1%) | 10 (66.7%) | 0.999 | |
| BMI [median (IQR)] (kg/m2) | 25.70 [23.08, 29.08] | 23.15 [20.90, 25.60] | 0.066 | |
| Age [median (IQR)] (years) | 36.50 [27.75, 49.25] | 33 [24.50, 47.25] | 0.492 | |
| Smoker [n (%)]: | yes | 38 (90.5%) | 5 (12.5%) | 0.999 |
| no | 4 (9.5%) | 35 (87.5%) | 0.999 | |
| Calprotectin [median (IQR)] (mg/L) | 1081 [233, 1880] | 1020 [269, 1556] | 0.588 | |
| C-reactive protein [median (IQR)] (mg/L) | 2.95 [1.08, 11.03] | 2.80 [0.90, 10.48] | 0.300 | |
| Hemoglobin [median (IQR)] (g/dL) | 14.10 [11.90, 14.95] | 12.65 [12.10, 13.60] | 0.164 | |
| Leucocytes [median (IQR)] (Tsd/µL) | 8.10 [6.15, 11.20] | 8.10 [6.50, 10.93] | 0.741 | |
| Vitamin D3 25-OH [median (IQR)] (µg/L) | 21.83 [13.80, 27.80] | 22.22 [13.50, 30.95] | 0.870 | |
| IBD Cohort | DEGS1-Cohort | IBD vs DEGS1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Macronutrients—Mean Daily Amount | Sex | n | Mean | 95% CI | pt-Test(twosided) | d | n | Mean | 95% CI | pt-Test(twosided) | d | pt-Test(twosided) | d |
| Energy (kJ) | Men | 42 | 9358 | 7658, 11,058 | 0.205 | 0.3 | 168 | 10,506 | 9763, 11,249 | <0.001 | 0.7 | 0.184 | −0.2 |
| Women | 40 | 8102 | 7082, 9122 | 160 | 7572 | 7125, 8019 | 0.307 | 0.2 | |||||
| Protein (g) | Men | 42 | 93 | 72.90, 113.0 | 0.181 | 0.3 | 168 | 95 | 87.83, 101.7 | <0.001 | 0.7 | 0.864 | 0.0 |
| Women | 40 | 78 | 67.89, 87.95 | 160 | 68 | 64.31, 72.31 | 0.044 | 0.4 | |||||
| Protein (%EN) | Men | 42 | 16 | 15.43, 17.48 | 0.689 | −0.1 | 168 | 16 | 15.15, 16.14 | 0.731 | 0.0 | 0.154 | 0.2 |
| Women | 40 | 17 | 15.46, 18.11 | 160 | 16 | 15.22, 16.33 | 0.121 | 0.3 | |||||
| Fat (g) | Men | 42 | 78 | 59.95, 95.32 | 0.169 | 0.3 | 168 | 81 | 74.98, 87.19 | <0.001 | 0.7 | 0.649 | −0.1 |
| Women | 40 | 64 | 54.30, 73.32 | 160 | 57 | 52.66, 60.77 | 0.135 | 0.3 | |||||
| Fat (%EN) | Men | 42 | 30 | 27.65, 31.72 | 0.764 | 0.1 | 168 | 29 | 27.71, 29.74 | 0.214 | 0.1 | 0.400 | 0.1 |
| Women | 40 | 29 | 27.07, 31.42 | 160 | 28 | 26.72, 28.87 | 0.234 | 0.2 | |||||
| Carbohydrates (g) | Men | 42 | 272 | 224.8, 318.4 | 0.466 | 0.2 | 168 | 323 | 296.1, 349.9 | <0.001 | 0.5 | 0.084 | −0.3 |
| Women | 40 | 250 | 212.8, 286.8 | 160 | 242 | 224.2, 259.1 | 0.682 | 0.1 | |||||
| Carbohydrates (%EN) | Men | 42 | 50 | 47.73, 53.25 | 0.497 | −0.2 | 168 | 52 | 50.17, 52.96 | 0.040 | −0.2 | 0.494 | −0.1 |
| Women | 40 | 52 | 48.80, 54.97 | 160 | 54 | 52.23, 55.04 | 0.281 | −0.2 | |||||
| Fiber (g) | Men | 42 | 21 | 15.70, 26.04 | 0.361 | 0.2 | 168 | 21 | 19.51, 23.08 | 0.093 | 0.2 | 0.847 | 0.0 |
| Women | 40 | 18 | 15.14, 21.15 | 160 | 19 | 17.61, 20.85 | 0.546 | −0.1 | |||||
| Fiber (%EN) | Men | 42 | 2 | 1.551, 2.098 | 0.708 | −0.1 | 168 | 2 | 1.590, 1.817 | <0.001 | −0.5 | 0.367 | 0.2 |
| Women | 40 | 2 | 1.635, 2.155 | 160 | 2 | 1.943, 2.190 | 0.224 | −0.2 | |||||
| Sugar (g) | Men | 42 | 127 | 98.83, 155.6 | 0.883 | 0.0 | 168 | 159 | 140.1, 178.5 | 0.006 | 0.3 | 0.123 | −0.3 |
| Women | 40 | 130 | 97.47, 163.3 | 160 | 126 | 112.8, 139.6 | 0.790 | 0.0 | |||||
| Cellulose (mg) | Men | 42 | 3631 | 2671, 4591 | 0.609 | 0.1 | 168 | 3056 | 2802, 3310 | 0.124 | −0.2 | 0.249 | 0.3 |
| Women | 40 | 3322 | 2587, 4057 | 160 | 3367 | 3057, 3676 | 0.901 | 0.0 | |||||
| Lignin (mg) | Men | 42 | 875 | 604.4, 1145 | 0.354 | 0.2 | 168 | 724 | 647.6, 801.0 | 0.268 | −0.1 | 0.286 | 0.3 |
| Women | 40 | 733 | 589.1, 877.4 | 160 | 794 | 696.2, 891.2 | 0.566 | −0.1 | |||||
| Soluble fats (mg) | Men | 42 | 6401 | 4799, 8003 | 0.308 | 0.2 | 168 | 6803 | 6247, 7359 | 0.014 | 0.3 | 0.559 | −0.1 |
| Women | 40 | 5479 | 4632, 6326 | 160 | 5868 | 5372, 6364 | 0.476 | −0.1 | |||||
| Insoluble fats (mg) | Men | 42 | 14,189 | 10,654, 17,724 | 0.386 | 0.2 | 168 | 14,426 | 13,225, 15,627 | 0.138 | 0.2 | 0.899 | 0.0 |
| Women | 40 | 12,390 | 10,188, 14,592 | 160 | 13,185 | 12,064, 14,306 | 0.529 | −0.1 | |||||
| Tyrosine (mg) | Men | 42 | 3408 | 2665, 4152 | 0.206 | 0.3 | 168 | 3482 | 3208, 3757 | <0.001 | 0.6 | 0.825 | 0.0 |
| Women | 40 | 2871 | 2458, 3283 | 160 | 2535 | 2370, 2699 | 0.087 | 0.3 | |||||
| Tryptophan (mg) | Men | 42 | 1098 | 861.5, 1335 | 0.193 | 0.3 | 168 | 1115 | 1032, 1198 | <0.001 | 0.7 | 0.894 | 0.0 |
| Women | 40 | 924 | 800.7, 1048 | 160 | 795 | 748.5, 842.5 | 0.024 | 0.4 | |||||
| Saturated fats (mg) | Men | 42 | 35,011 | 27,309, 42,713 | 0.231 | 0.3 | 168 | 37,108 | 34,092, 40,123 | <0.001 | 0.6 | 0.561 | −0.1 |
| Women | 40 | 29,386 | 24,128, 34,643 | 160 | 26,320 | 24,183, 28,457 | 0.226 | 0.2 | |||||
| Short-chain fatty acids (mg) | Men | 42 | 1586 | 1248, 1924 | 0.356 | 0.2 | 168 | 1607 | 1428, 1786 | 0.002 | 0.3 | 0.915 | 0.0 |
| Women | 40 | 1373 | 1060, 1687 | 160 | 1244 | 1105, 1383 | 0.422 | 0.1 | |||||
| Medium-chain fatty acids (mg) | Men | 42 | 1488 | 1170, 1805 | 0.419 | 0.2 | 168 | 1544 | 1378, 1709 | <0.001 | 0.5 | 0.762 | −0.1 |
| Women | 40 | 1307 | 989.7, 1624 | 160 | 1101 | 999.6, 1203 | 0.219 | 0.3 | |||||
| Long-chain fatty acids (mg) | Men | 42 | 67,875 | 52,087, 83,664 | 0.148 | 0.3 | 168 | 71,743 | 66,309, 77,177 | <0.001 | 0.7 | 0.567 | −0.1 |
| Women | 40 | 54,917 | 46,519, 63,316 | 160 | 49,630 | 46,035, 53,224 | 0.208 | 0.2 | |||||
| Omega-3 fatty acids (mg) | Men | 42 | 1955 | 1287, 2622 | 0.513 | 0.1 | 168 | 1792 | 1658, 1927 | <0.001 | 0.5 | 0.633 | 0.1 |
| Women | 40 | 1701 | 1320, 2082 | 160 | 1380 | 1236, 1524 | 0.065 | 0.3 | |||||
| Omega-6 fatty acids (mg) | Men | 42 | 10,755 | 8355, 13,155 | 0.134 | 0.3 | 168 | 11,047 | 10,265, 11,829 | <0.001 | 0.8 | 0.816 | −0.1 |
| Women | 40 | 8742 | 7551, 9933 | 160 | 7550 | 7043, 8056 | 0.045 | 0.4 | |||||
| IBD Cohort | DEGS1-Cohort | IBD vs DEGS1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ultra-Processed Foods and Drinks—Mean Daily Amount | Sex | n | Mean | 95% CI | pt-Test(twosided) | d | n | Mean | 95% CI | pt-Test(twosided) | d | pt-Test(twosided) | d |
| UPFD (g) | Men | 41 | 871 | 510.7, 1232 | 0.397 | 0.2 | 150 | 968 | 792.7, 1143 | <0.001 | 0.5 | 0.619 | −0.1 |
| Women | 40 | 664 | 328.9, 999.0 | 143 | 522 | 389.9, 654.0 | 0.356 | 0.2 | |||||
| UPFD (kJ) | Men | 41 | 3179 | 2421, 3938 | 0.170 | 0.3 | 148 | 3884 | 3452, 4317 | <0.001 | 0.6 | 0.127 | −0.3 |
| Women | 40 | 2514 | 1914, 3115 | 143 | 2447 | 2166, 2728 | 0.830 | 0.0 | |||||
| UPD (g) | Men | 42 | 685 | 338.1, 1032 | 0.483 | 0.2 | 156 | 838 | 654.7, 1020 | <0.001 | 0.5 | 0.445 | −0.1 |
| Women | 40 | 518 | 191.4, 845.4 | 152 | 366 | 244.2, 487.3 | 0.381 | 0.2 | |||||
| UPD (kJ) | Men | 42 | 987 | 535.0, 1440 | 0.233 | 0.3 | 156 | 1266 | 1008, 1523 | <0.001 | 0.6 | 0.316 | −0.3 |
| Women | 40 | 647 | 301.5, 991.7 | 152 | 504 | 377.8, 629.4 | 0.436 | 0.2 | |||||
| UPF (g) | Men | 41 | 172 | 131.8, 212.9 | 0.262 | 0.3 | 158 | 207 | 187.9, 226.7 | <0.001 | 0.6 | 0.111 | −0.3 |
| Women | 40 | 146 | 120.3, 170.8 | 150 | 148 | 134.1, 162.0 | 0.867 | 0.0 | |||||
| UPF (kJ) | Men | 41 | 2172 | 1614, 2729 | 0.37 | 0.2 | 156 | 2683 | 2416, 2950 | <0.001 | 0.5 | 0.089 | −0.3 |
| Women | 40 | 1867 | 1479, 2256 | 150 | 1924 | 1709, 2139 | 0.404 | 0.0 | |||||
| IBD Cohort | DEGS1-Cohort | IBD vs DEGS1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DGE Food Groups (g/d) | Sex | n | Mean | 95% CI | pt-Test(twosided) | d | n | Mean | 95% CI | pt-Test(twosided) | d | pt-Test(twosided) | d | |
| Fruits and vegetables | Fresh Vegetables | Men | 42 | 65.29 | 27.41, 103.2 | 0.224 | 0.3 | 168 | 56.47 | 44.85, 68.09 | 0.004 | −0.3 | 0.656 | 0.1 |
| Women | 40 | 40.92 | 28.01, 53.82 | 159 | 81.95 | 68.90, 95.01 | <0.001 | −0.5 | ||||||
| Processed Vegetables | Men | 42 | 68.26 | 43.70, 92.81 | 0.91 | 0.0 | 167 | 40.58 | 32.93, 48.23 | 0.053 | −0.2 | 0.035 | 0.5 | |
| Women | 40 | 66.45 | 45.64, 87.25 | 159 | 53.15 | 42.81, 63.48 | 0.127 | 0.2 | ||||||
| Fresh Fruits | Men | 42 | 168.08 | 104.2, 231.9 | 0.683 | −0.1 | 167 | 150.25 | 121.9, 178.6 | <0.001 | −0.4 | 0.586 | 0.1 | |
| Women | 40 | 193.09 | 86.13, 300.0 | 160 | 237.61 | 197.0, 278.2 | 0.363 | −0.2 | ||||||
| Processed Fruits | Men | 42 | 5.36 | 1.296, 9.418 | 0.278 | −0.2 | 166 | 6.39 | 3.757, 9.022 | 0.536 | 0.1 | 0.716 | −0.1 | |
| Women | 40 | 9.16 | 3.346, 14.97 | 160 | 5.43 | 3.921, 6.935 | 0.216 | 0.3 | ||||||
| Juices | Fruit Juice | Men | 42 | 120.96 | 69.07, 172.9 | 0.234 | −0.3 | 167 | 208.25 | 150.8, 265.7 | 0.476 | −0.1 | 0.144 | −0.3 |
| Women | 40 | 273.53 | 23.54, 523.5 | 159 | 245.74 | 158.2, 333.3 | 0.797 | 0.0 | ||||||
| Vegetable Juice | Men | 42 | 9.86 | −0.4242, 20.15 | 0.633 | 0.1 | 167 | 2.22 | 1.000, 3.448 | 0.145 | −0.2 | 0.144 | 0.5 | |
| Women | 39 | 6.68 | −1.748, 15.12 | 159 | 4.2 | 1.832, 6.569 | 0.435 | 0.1 | ||||||
| Legumes and pulses | Men | 42 | 19.45 | 6.867, 32.04 | 0.272 | 0.2 | 168 | 15.99 | 11.59, 20.39 | 0.024 | 0.2 | 0.525 | 0.1 | |
| Women | 39 | 11.62 | 5.613, 17.64 | 159 | 10.28 | 7.993, 12.58 | 0.314 | 0.1 | ||||||
| Nuts and seeds | Men | 42 | 2.9 | 1.473, 4.336 | 0.254 | 0.3 | 167 | 1.99 | 1.206, 2.767 | 0.842 | 0.0 | 0.290 | 0.2 | |
| Women | 40 | 1.79 | 0.4449, 3.126 | 159 | 2.09 | 1.377, 2.811 | 0.699 | −0.1 | ||||||
| Potatoes | Boiled Potatoes | Men | 42 | 62.31 | 39.31, 85.32 | 0.596 | −0.1 | 167 | 68.63 | 57.33, 79.93 | 0.167 | 0.2 | 0.621 | −0.1 |
| Women | 40 | 70.74 | 48.51, 92.98 | 160 | 58.86 | 50.69, 67.03 | 0.316 | 0.2 | ||||||
| Fried Potatoes | Men | 42 | 7.35 | 4.979, 9.721 | 0.475 | 0.2 | 168 | 10.08 | 8.114, 12.06 | 0.013 | 0.3 | 0.079 | −0.2 | |
| Women | 40 | 6.08 | 3.372, 8.782 | 159 | 6.86 | 5.267, 8.463 | 0.653 | −0.1 | ||||||
| French fries | Men | 42 | 13.27 | 5.886, 20.64 | 0.292 | 0.2 | 166 | 13.44 | 11.19, 15.69 | <0.001 | 0.4 | 0.953 | 0.0 | |
| Women | 40 | 8.81 | 4.852, 12.76 | 160 | 7.79 | 6.192, 9.385 | 0.591 | 0.1 | ||||||
| Butter and margarine | Men | 42 | 10.05 | 6.19, 13.91 | 0.492 | 0.2 | 168 | 9.36 | 7.77, 10.96 | 0.003 | 0.3 | 0.715 | 0.1 | |
| Women | 40 | 8.28 | 4.84, 11.72 | 160 | 6.36 | 5.18, 7.53 | 0.190 | 0.2 | ||||||
| Dairy products | Milk | Men | 41 | 247.17 | 157.5, 336.8 | 0.723 | 0.1 | 165 | 311.1 | 243.7, 378.5 | 0.015 | 0.3 | 0.376 | −0.2 |
| Women | 40 | 225.18 | 138.3, 312.0 | 158 | 213.33 | 172.7, 253.9 | 0.798 | 0.0 | ||||||
| Cream Cheese | Men | 42 | 7.23 | 3.345, 11.12 | 0.950 | 0.0 | 167 | 5.61 | 4.202, 7.017 | 0.635 | −0.1 | 0.433 | 0.2 | |
| Women | 40 | 7.07 | 3.485, 10.65 | 160 | 6.15 | 4.377, 7.928 | 0.648 | 0.1 | ||||||
| Cheese | Men | 42 | 40.65 | 24.16, 57.14 | 0.311 | 0.2 | 165 | 33.67 | 27.53, 39.80 | 0.882 | 0.0 | 0.346 | 0.2 | |
| Women | 40 | 30.5 | 19.30, 41.70 | 160 | 32.92 | 24.97, 40.86 | 0.777 | −0.1 | ||||||
| Yoghurt | Men | 42 | 78.53 | 39.63, 117.4 | 0.702 | −0.1 | 164 | 91.52 | 68.47, 114.6 | 0.230 | 0.1 | 0.605 | −0.1 | |
| Women | 40 | 90.49 | 40.40, 140.6 | 159 | 75.57 | 63.16, 87.99 | 0.562 | 0.1 | ||||||
| Fish | Cold fish | Men | 42 | 30.56 | 15.96, 45.16 | 0.692 | 0.1 | 168 | 18.51 | 15.32, 21.70 | 0.171 | 0.2 | 0.111 | 0.4 |
| Women | 40 | 26.63 | 12.96, 40.29 | 160 | 15.48 | 12.51, 18.44 | 0.114 | 0.4 | ||||||
| Warm fish | Men | 42 | 12.2 | 6.782, 17.52 | 0.675 | 0.1 | 167 | 9.4 | 7.605, 11.15 | 0.32 | 0.1 | 0.327 | 0.2 | |
| Women | 40 | 10.7 | 6.594, 14.86 | 160 | 8.8 | 7.316, 10.27 | 0.287 | 0.2 | ||||||
| Meat and poultry | Poultry | Men | 42 | 45.5 | 27.14, 63.87 | 0.996 | 0.0 | 168 | 32.6 | 26.02, 39.11 | 0.048 | 0.2 | 0.187 | 0.3 |
| Women | 40 | 45.6 | 27.20, 63.94 | 160 | 24.5 | 19.80, 29.16 | 0.030 | 0.6 | ||||||
| Meat | Men | 42 | 51.8 | 31.16, 72.51 | 0.104 | 0.4 | 167 | 53.8 | 43.86, 63.72 | <0.001 | 0.6 | 0.863 | 0.0 | |
| Women | 40 | 32.2 | 20.35, 44.04 | 160 | 26.5 | 22.83, 30.26 | 0.363 | 0.2 | ||||||
| Cold cuts | Men | 42 | 19.3 | 10.91, 27.73 | 0.335 | 0.2 | 168 | 34.0 | 28.50, 39.54 | <0.001 | 0.7 | 0.015 | −0.4 | |
| Women | 40 | 14.5 | 8.994, 19.93 | 160 | 14.4 | 11.55, 17.22 | 0.982 | 0.0 | ||||||
| Eggs | Men | 42 | 30.56 | 15.96, 45.16 | 0.692 | 0.1 | 168 | 18.51 | 15.32, 21.70 | 0.171 | 0.2 | 0.111 | 0.4 | |
| Women | 40 | 26.63 | 12.96, 40.29 | 160 | 15.48 | 12.51, 18.44 | 0.114 | 0.4 | ||||||
| Cereal products | Breakfast cereals | Men | 42 | 3.3 | 0.7905, 5.766 | 0.959 | 0.0 | 168 | 2.9 | 1.994, 3.881 | 0.063 | 0.2 | 0.765 | 0.1 |
| Women | 40 | 3.4 | −0.8803, 7.684 | 159 | 1.8 | 0.9100, 2.585 | 0.448 | 0.2 | ||||||
| Muesli | Men | 42 | 13.0 | −1.690, 27.58 | 0.372 | 0.2 | 166 | 5.7 | 3.731, 7.626 | 0.248 | 0.1 | 0.326 | 0.3 | |
| Women | 40 | 6.1 | 2.605, 9.605 | 158 | 4.3 | 2.798, 5.693 | 0.274 | 0.2 | ||||||
| Wholegrain bread and rolls | Men | 42 | 45.1 | 12.03, 78.09 | 0.357 | 0.2 | 157 | 55.7 | 43.15, 68.23 | 0.611 | −0.1 | 0.481 | −0.1 | |
| Women | 40 | 28.4 | 15.03, 41.80 | 160 | 60.5 | 46.90, 74.00 | 0.001 | −0.4 | ||||||
| Mixed bread | Men | 42 | 44.2 | 25.83, 62.52 | 0.897 | 0.0 | 166 | 78.2 | 60.72, 95.66 | <0.001 | 0.5 | 0.008 | −0.3 | |
| Women | 40 | 42.6 | 25.57, 59.56 | 159 | 37.0 | 29.30, 44.65 | 0.526 | 0.1 | ||||||
| White bread and rolls | Men | 42 | 48.2 | 32.88, 63.55 | 0.955 | 0.0 | 166 | 45.6 | 37.52, 55.77 | 0.006 | 0.3 | 0.875 | 0.0 | |
| Women | 39 | 49.1 | 20.11, 78.10 | 160 | 30.9 | 24.19, 37.57 | 0.222 | 0.3 | ||||||
| Pasta | Men | 42 | 38.7 | 20.70, 56.74 | 0.589 | 0.1 | 167 | 43.6 | 36.26, 50.84 | 0.013 | 0.3 | 0.575 | −0.1 | |
| Women | 40 | 33.2 | 23.79, 42.56 | 160 | 32.4 | 27.48, 37.32 | 0.888 | 0.0 | ||||||
| Rice | Men | 42 | 31.6 | 14.16, 49.04 | 0.357 | 0.2 | 168 | 18.9 | 14.77, 22.94 | 0.759 | 0.0 | 0.158 | 0.4 | |
| Women | 40 | 22.5 | 13.42, 31.51 | 160 | 19.7 | 16.54, 22.78 | 0.468 | 0.1 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pueschel, L.; Kockelmann, F.; Kueck, M.; Tegtbur, U.; Attaran-Bandarabadi, M.; Bachmann, O.; Wedemeyer, H.; Lenzen, H.; Wiestler, M. Patients with Inflammatory Bowel Disease Show Fewer Sex-Related Differences in Their Dietary Behavior Than the General Population: A Qualitative Analysis. Nutrients 2024, 16, 2954. https://doi.org/10.3390/nu16172954
Pueschel L, Kockelmann F, Kueck M, Tegtbur U, Attaran-Bandarabadi M, Bachmann O, Wedemeyer H, Lenzen H, Wiestler M. Patients with Inflammatory Bowel Disease Show Fewer Sex-Related Differences in Their Dietary Behavior Than the General Population: A Qualitative Analysis. Nutrients. 2024; 16(17):2954. https://doi.org/10.3390/nu16172954
Chicago/Turabian StylePueschel, Lea, Fabian Kockelmann, Momme Kueck, Uwe Tegtbur, Masoumeh Attaran-Bandarabadi, Oliver Bachmann, Heiner Wedemeyer, Henrike Lenzen, and Miriam Wiestler. 2024. "Patients with Inflammatory Bowel Disease Show Fewer Sex-Related Differences in Their Dietary Behavior Than the General Population: A Qualitative Analysis" Nutrients 16, no. 17: 2954. https://doi.org/10.3390/nu16172954
APA StylePueschel, L., Kockelmann, F., Kueck, M., Tegtbur, U., Attaran-Bandarabadi, M., Bachmann, O., Wedemeyer, H., Lenzen, H., & Wiestler, M. (2024). Patients with Inflammatory Bowel Disease Show Fewer Sex-Related Differences in Their Dietary Behavior Than the General Population: A Qualitative Analysis. Nutrients, 16(17), 2954. https://doi.org/10.3390/nu16172954







