Lactiplantibacillus plantarum P470 Isolated from Fermented Chinese Chives Has the Potential to Improve In Vitro the Intestinal Microbiota and Biological Activity in Feces of Coronary Heart Disease (CHD) Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Vitro Colonic Fermentation Models
2.3. Determination of Antioxidant Activity before and after Fermentation
2.4. In Vitro Assessment of Anti-Inflammatory Effects
2.4.1. Measurement of Cell Counting Kit-8 (CCK8) in RAW264.7 Cells
2.4.2. In Vitro Evaluation of Anti-Inflammatory Effects
2.5. DNA Extraction and Gut Microbiota Analysis
2.6. Gas Chromatography for SCFA Analysis
2.7. UPLC-MS/MS for Untargeted Metabolomics
2.8. Statistic Analysis
3. Results
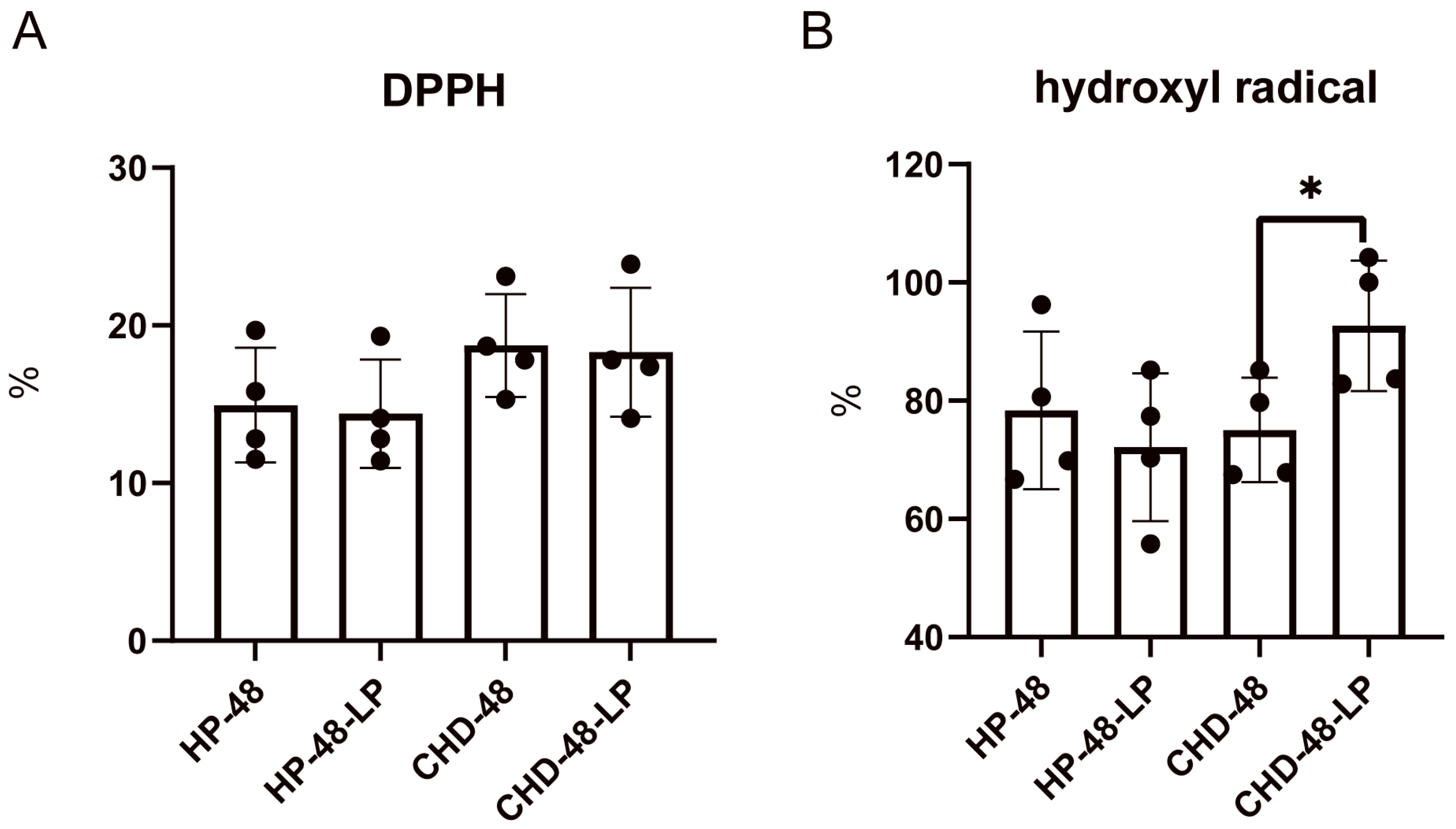
3.1. L. plantarum P470 Exerts an Antioxidant Activity in Feces from CHD Patients
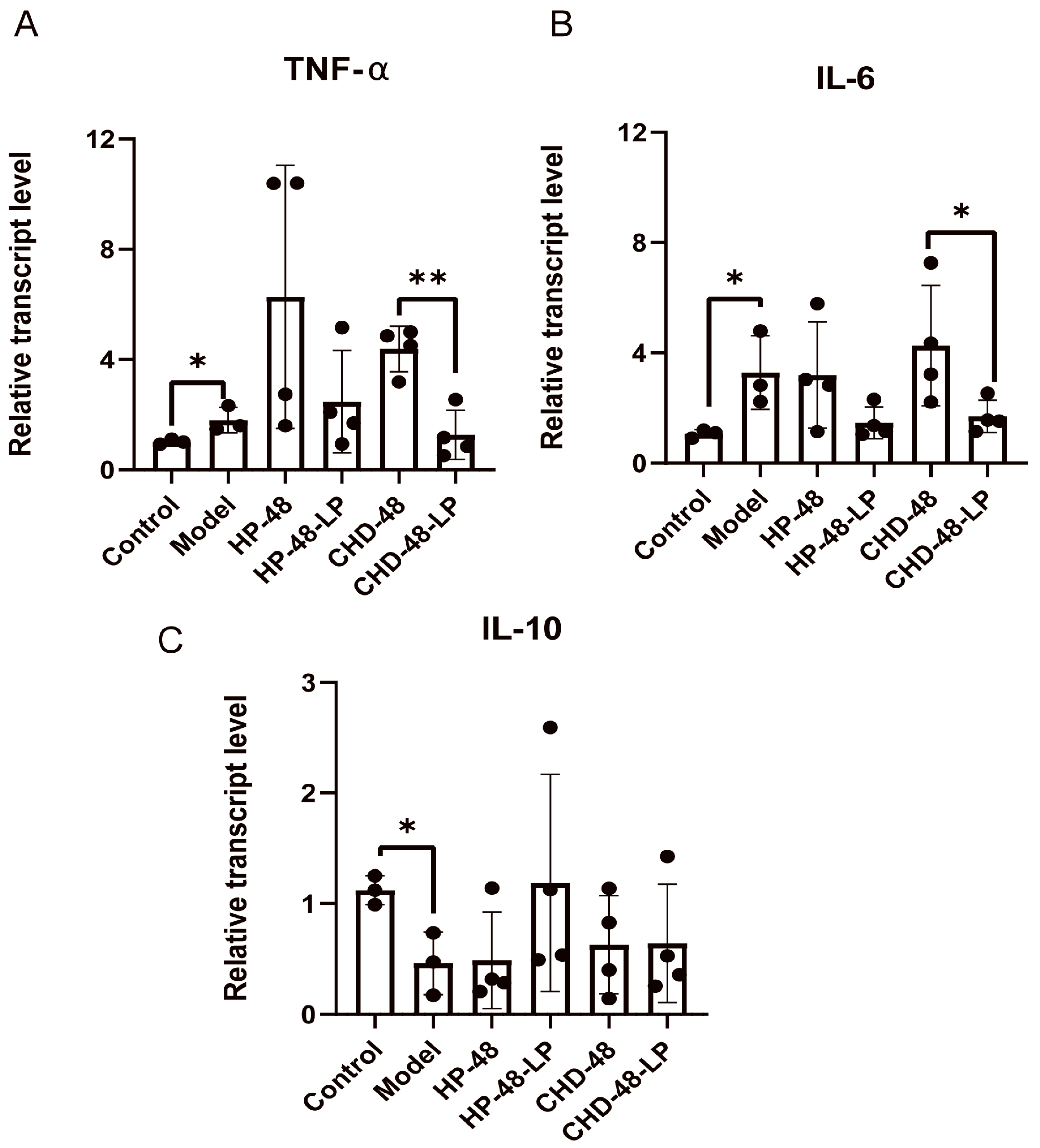
3.2. L. plantarum P470 Seems to Exert Some Anti-Inflammatory Activity in Feces of CHD Patients
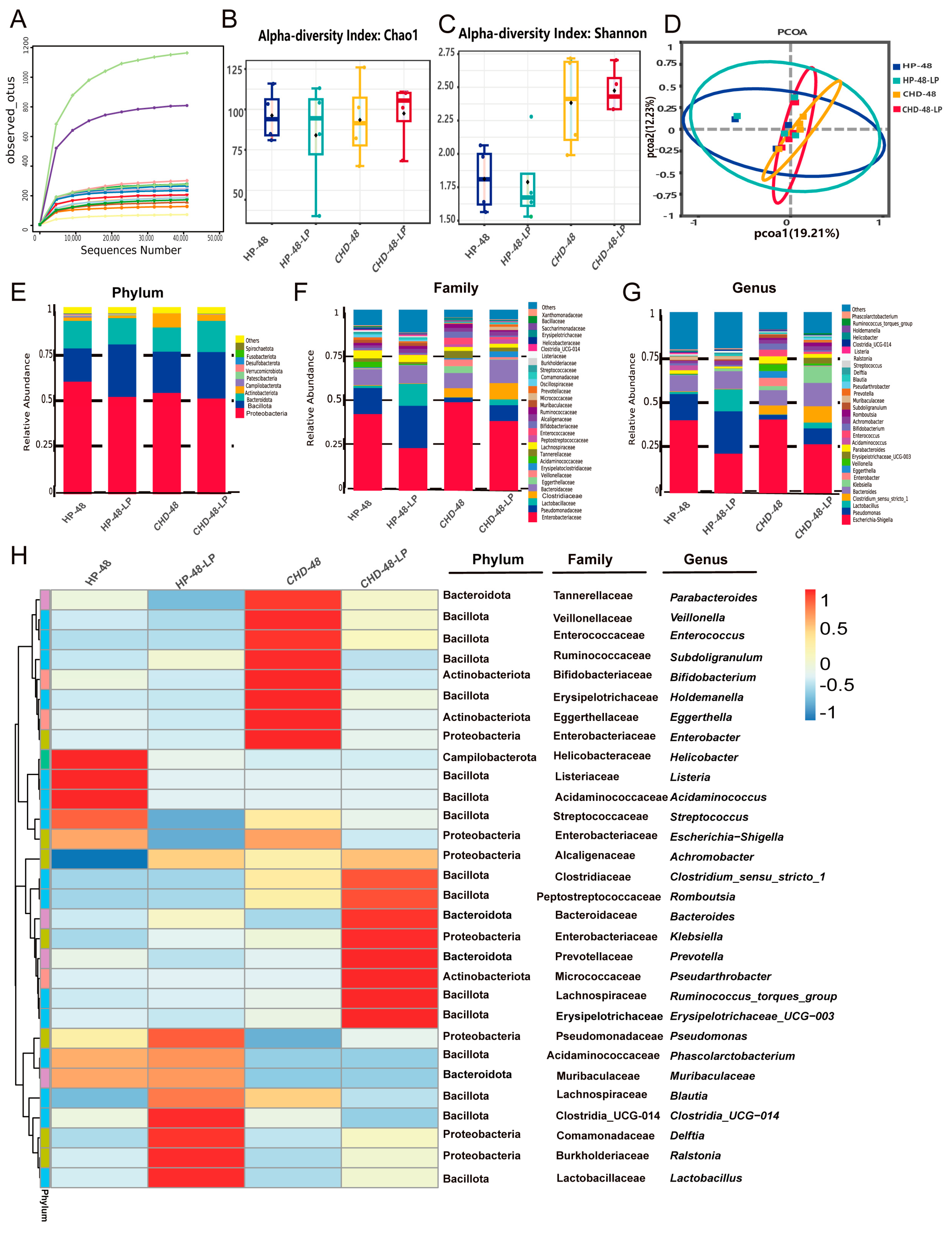
3.3. L. plantarum P470 Exerts Restricted Efficacy in Feces from CHD Patients In Vitro
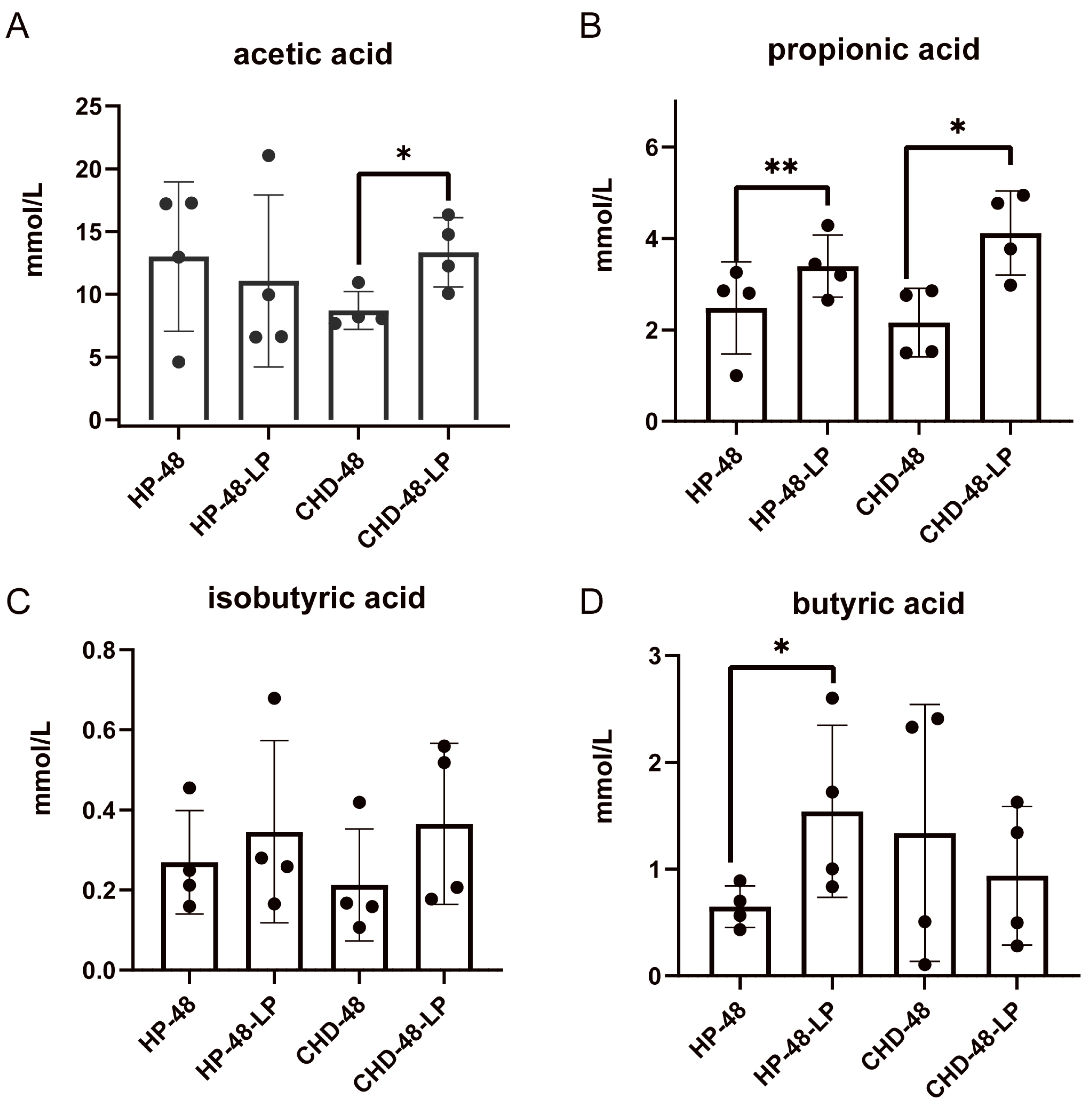
3.4. L. plantarum P470 Enhances Fecal SCFAs Content of CHD Patients In Vitro
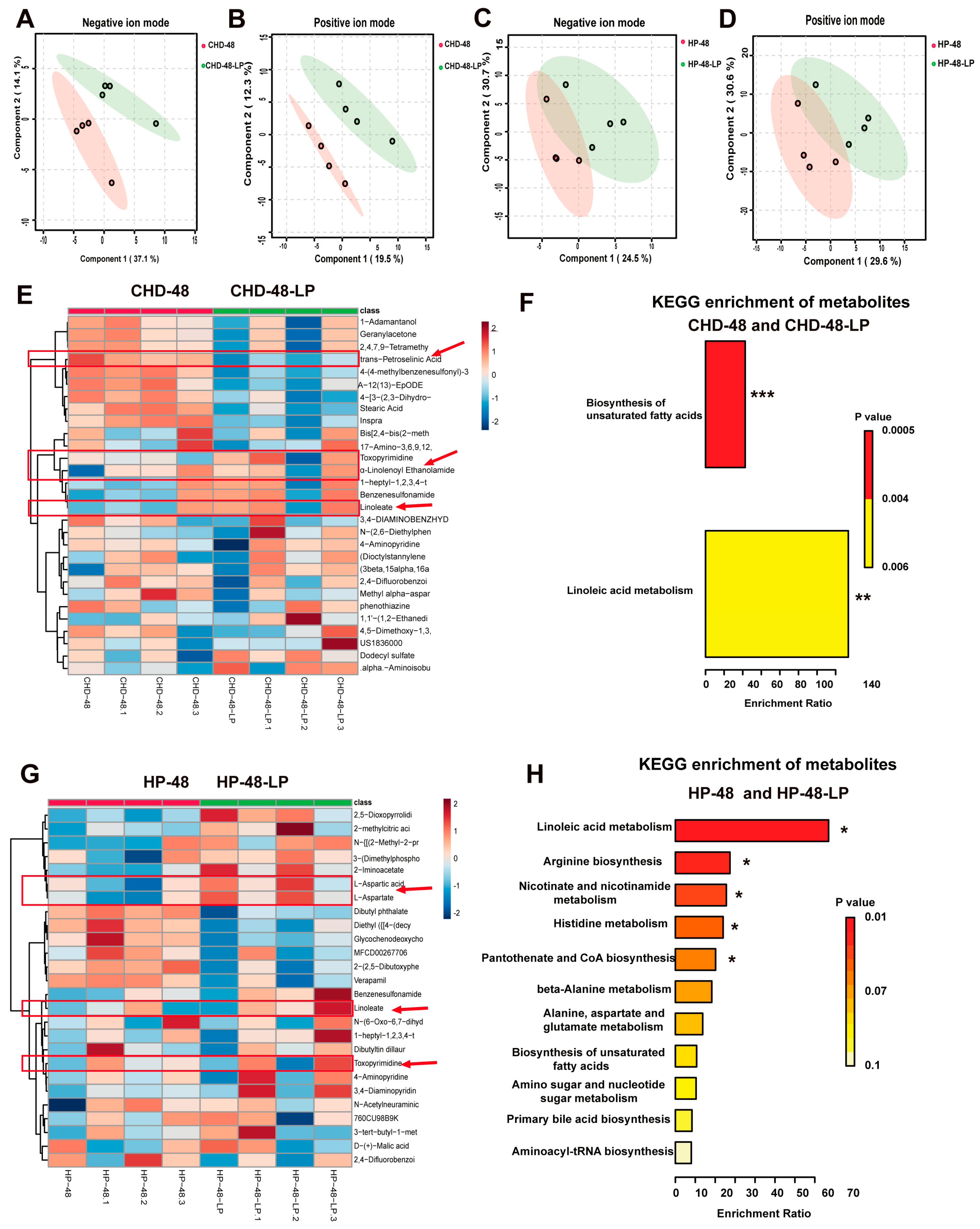
3.5. L. plantarum P470 Regulated Metabolism in Feces of CHD Patients In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mendis, S.; Davis, S.; Norrving, B. Organizational update: The world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke 2015, 46, e121–e122. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream to Identify Novel Targets for Atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Li, Q.; Wang, Y.W.; He, Y.; Li, X.J.; Sun, C.Y.; Onder, E.; Can, F.S.; Pang, Z.Q.; et al. LPS adsorption and inflammation alleviation by polymyxin B-modified liposomes for atherosclerosis treatment. Acta Pharm. Sin. B 2023, 13, 3817–3833. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.O.; Budoff, M. Effect of statins on atherosclerotic plaque. Trends Cardiovasc. Med. 2019, 29, 451–455. [Google Scholar] [CrossRef]
- Saiyitijiang, A.; Aizezi, M.; Zhao, Y.; Gao, Y. Efficacy and safety of new oral anticoagulants combined with antiplatelet drugs in the treatment of coronary heart disease: Systematic evaluation and meta-analysis. Ann. Noninvasive Electrocardiol. 2022, 27, e12977. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Tsui, P.F.; Chuang, Y.P.; Chiang, D.M.; Chen, L.W.; Liu, S.T.; Lin, F.Y.; Huang, S.M.; Lin, S.H.; Wu, W.L.; et al. Carvedilol Ameliorates Experimental Atherosclerosis by Regulating Cholesterol Efflux and Exosome Functions. Int. J. Mol. Sci. 2019, 20, 5202. [Google Scholar] [CrossRef]
- Padam, P.; Barton, L.; Wilson, S.; David, A.; Walji, S.; de Lorenzo, F.; Ray, K.K.; Jones, B.; Cegla, J. Lipid lowering with inclisiran: A real-world single-centre experience. Open Heart 2022, 9, e002184. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Fak, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Backhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef]
- Jin, L.; Shi, X.; Yang, J.; Zhao, Y.; Xue, L.; Xu, L.; Cai, J. Gut microbes in cardiovascular diseases and their potential therapeutic applications. Protein Cell 2021, 12, 346–359. [Google Scholar] [CrossRef]
- Ditano-Vazquez, P.; Torres-Pena, J.D.; Galeano-Valle, F.; Perez-Caballero, A.I.; Demelo-Rodriguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients 2019, 11, 2833. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Yang, S.C.; Chao, J.C.; Chen, J.R. Beneficial effects of catechin-rich green tea and inulin on the body composition of overweight adults. Br. J. Nutr. 2012, 107, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Mohamadshahi, M.; Veissi, M.; Haidari, F.; Shahbazian, H.; Kaydani, G.A.; Mohammadi, F. Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts 2014, 4, 83–88. [Google Scholar] [PubMed]
- Mokoena, M.P.; Mutanda, T.; Olaniran, A.O. Perspectives on the probiotic potential of lactic acid bacteria from African traditional fermented foods and beverages. Food Nutr. Res. 2016, 60, 29630. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ji, Y.; Park, H.; Lee, J.; Park, S.; Yeo, S.; Shin, H.; Holzapfel, W.H. Selection of functional lactic acid bacteria as starter cultures for the fermentation of Korean leek (Allium tuberosum Rottler ex Sprengel.). Int. J. Food Microbiol. 2014, 191, 164–171. [Google Scholar] [CrossRef]
- Kumar, R.; Grover, S.; Batish, V.K. Hypocholesterolaemic effect of dietary inclusion of two putative probiotic bile salt hydrolase-producing Lactobacillus plantarum strains in Sprague-Dawley rats. Br. J. Nutr. 2011, 105, 561–573. [Google Scholar] [CrossRef]
- Jeun, J.; Kim, S.; Cho, S.Y.; Jun, H.J.; Park, H.J.; Seo, J.G.; Chung, M.J.; Lee, S.J. Hypocholesterolemic effects of Lactobacillus plantarum KCTC3928 by increased bile acid excretion in C57BL/6 mice. Nutrition 2010, 26, 321–330. [Google Scholar]
- Song, D.F.; Zhu, M.Y.; Gu, Q. Purification and characterization of Plantaricin ZJ5, a new bacteriocin produced by Lactobacillus plantarum ZJ5. PLoS ONE 2014, 9, e105549. [Google Scholar] [CrossRef]
- Mo, S.J.; Lee, K.; Hong, H.J.; Hong, D.K.; Jung, S.H.; Park, S.D.; Shim, J.J.; Lee, J.L. Effects of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 on Overweight and the Gut Microbiota in Humans: Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Nutrients 2022, 14, 2484. [Google Scholar] [CrossRef]
- Hao, H.; Zhang, X.; Tong, L.; Liu, Q.; Liang, X.; Bu, Y.; Gong, P.; Liu, T.; Zhang, L.; Xia, Y.; et al. Effect of Extracellular Vesicles Derived From Lactobacillus plantarum Q7 on Gut Microbiota and Ulcerative Colitis in Mice. Front. Immunol. 2021, 12, 777147. [Google Scholar] [CrossRef]
- Zhu, R.; Fang, Y.; Li, H.; Liu, Y.; Wei, J.; Zhang, S.; Wang, L.; Fan, R.; Wang, L.; Li, S.; et al. Psychobiotic Lactobacillus plantarum JYLP-326 relieves anxiety, depression, and insomnia symptoms in test anxious college via modulating the gut microbiota and its metabolism. Front. Immunol. 2023, 14, 1158137. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Tao, X.; Xiong, H.; Yu, J.; Wei, H. Lactobacillus plantarum ZDY04 exhibits a strain-specific property of lowering TMAO via the modulation of gut microbiota in mice. Food Funct. 2018, 9, 4299–4309. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, M.; Zeng, Z.; Xie, M.; Xu, W.; Peng, Y.; Zhou, W.; Sun, Y.; Zeng, X.; Liu, Z. Fuzhuan brick tea polysaccharides serve as a promising candidate for remodeling the gut microbiota from colitis subjects in vitro: Fermentation characteristic and anti-inflammatory activity. Food Chem. 2022, 391, 133203. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Someya, T.; Sano, K.; Sagane, Y.; Watanabe, T.; Wijesekara, R.G.S. Antioxidant activities of traditional plants in Sri Lanka by DPPH free radical-scavenging assay. Data Brief 2018, 17, 870–875. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Xie, X.; Chen, M.; Xue, L.; Wang, J.; Ye, Q.; Wu, S.; Yang, R.; Zhao, H.; et al. Exploration of the Molecular Mechanisms Underlying the Anti-Photoaging Effect of Limosilactobacillus fermentum XJC60. Front. Cell Infect. Microbiol. 2022, 12, 838060. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xie, X.; Li, Y.; Wu, L.; Fan, C.; Liang, T.; Xi, Y.; Yang, S.; Li, H.; Zhang, J.; et al. Evaluation of the Cholesterol-Lowering Mechanism of Enterococcus faecium Strain 132 and Lactobacillus paracasei Strain 201 in Hypercholesterolemia Rats. Nutrients 2021, 13, 1982. [Google Scholar] [CrossRef]
- Li, J.; Cao, Y.; Lu, R.; Li, H.; Pang, Y.; Fu, H.; Fang, G.; Chen, Q.; Liu, B.; Wu, J.; et al. Integrated Fecal Microbiome and Serum Metabolomics Analysis Reveals Abnormal Changes in Rats with Immunoglobulin A Nephropathy and the Intervention Effect of Zhen Wu Tang. Front. Pharmacol. 2020, 11, 606689. [Google Scholar]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Low Wang, C.C.; Hess, C.N.; Hiatt, W.R.; Goldfine, A.B. Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus-Mechanisms, Management, and Clinical Considerations. Circulation 2016, 133, 2459–2502. [Google Scholar] [CrossRef]
- Brown, J.M.; Hazen, S.L. The gut microbial endocrine organ: Bacterially derived signals driving cardiometabolic diseases. Annu. Rev. Med. 2015, 66, 343–359. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Shang, N.; Li, P. In vitro and in vivo antioxidant activity of exopolysaccharide fractions from Bifidobacterium animalis RH. Anaerobe 2011, 17, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.F.; Moslehi, J.J.; Babaev, V.R. Akt Signaling in Macrophage Polarization, Survival, and Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 2703. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, W.; Liu, X.; Cheng, L. Metagenomic analysis of the gut microbiome in atherosclerosis patients identify cross-cohort microbial signatures and potential therapeutic target. FASEB J. 2020, 34, 14166–14181. [Google Scholar] [CrossRef]
- Emoto, T.; Yamashita, T.; Sasaki, N.; Hirota, Y.; Hayashi, T.; So, A.; Kasahara, K.; Yodoi, K.; Matsumoto, T.; Mizoguchi, T.; et al. Analysis of Gut Microbiota in Coronary Artery Disease Patients: A Possible Link between Gut Microbiota and Coronary Artery Disease. J. Atheroscler. Thromb. 2016, 23, 908–921. [Google Scholar] [CrossRef]
- Manco, M.; Putignani, L.; Bottazzo, G.F. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr. Rev. 2010, 31, 817–844. [Google Scholar] [CrossRef]
- Alard, J.; Lehrter, V.; Rhimi, M.; Mangin, I.; Peucelle, V.; Abraham, A.L.; Mariadassou, M.; Maguin, E.; Waligora-Dupriet, A.J.; Pot, B.; et al. Beneficial metabolic effects of selected probiotics on diet-induced obesity and insulin resistance in mice are associated with improvement of dysbiotic gut microbiota. Environ. Microbiol. 2016, 18, 1484–1497. [Google Scholar] [CrossRef]
- Boo, T.W.; Cryan, B.; O’Donnell, A.; Fahy, G. Prosthetic valve endocarditis caused by Veillonella parvula. J. Infect. 2005, 50, 81–83. [Google Scholar] [CrossRef]
- Alexander, M.; Ang, Q.Y.; Nayak, R.R.; Bustion, A.E.; Sandy, M.; Zhang, B.; Upadhyay, V.; Pollard, K.S.; Lynch, S.V.; Turnbaugh, P.J. Human gut bacterial metabolism drives Th17 activation and colitis. Cell Host Microbe 2022, 30, 17–30.e19. [Google Scholar] [CrossRef]
- Eicher, T.P.; Mohajeri, M.H. Overlapping Mechanisms of Action of Brain-Active Bacteria and Bacterial Metabolites in the Pathogenesis of Common Brain Diseases. Nutrients 2022, 14, 2661. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Ivey, K.L.; Wang, D.D.; Wilkinson, J.E.; Franke, A.; Lee, K.H.; Chan, A.; Huttenhower, C.; Hu, F.B.; et al. Interplay between diet and gut microbiome, and circulating concentrations of trimethylamine N-oxide: Findings from a longitudinal cohort of US men. Gut 2022, 71, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Xia, H.; Zhong, S.L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, M.; Ji, J.; Hu, X.; Chen, F. Gut microbiota determines the prevention effects of Luffa cylindrica (L.) Roem supplementation against obesity and associated metabolic disorders induced by high-fat diet. FASEB J. 2019, 33, 10339–10352. [Google Scholar] [CrossRef]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 2015, 6, e02481. [Google Scholar] [CrossRef]
- Shi, H.; Li, Y.; Dong, C.; Si, G.; Xu, Y.; Peng, M.; Li, Y. Helicobacter pylori infection and the progression of atherosclerosis: A systematic review and meta-analysis. Helicobacter 2022, 27, e12865. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.A.; Jackson, J.; Stanton, M.; Rojas-Triana, A.; Bober, L.; Laverty, M.; Yang, X.; Zhu, F.; Liu, J.; Wang, S.; et al. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E2 and cytokines. World J. Gastroenterol. 2009, 15, 5549–5557. [Google Scholar] [CrossRef]
- Aguilar, E.C.; dos Santos, L.C.; Leonel, A.J.; de Oliveira, J.S.; Santos, E.A.; Navia-Pelaez, J.M.; da Silva, J.F.; Mendes, B.P.; Capettini, L.S.A.; Teixeira, L.G.; et al. Oral butyrate reduces oxidative stress in atherosclerotic lesion sites by a mechanism involving NADPH oxidase down-regulation in endothelial cells. J. Nutr. Biochem. 2016, 34, 99–105. [Google Scholar] [CrossRef]
- Hu, J.N.; Taki, F.; Sugiyama, S.; Asai, J.; Izawa, Y.; Satake, T.; Ozawa, T. Neutrophil-derived epoxide, 9,10-epoxy-12-octadecenoate, induces pulmonary edema. Lung 1988, 166, 327–337. [Google Scholar] [PubMed]
- Sugiyama, S.; Hayakawa, M.; Nagai, S.; Ajioka, M.; Ozawa, T. Leukotoxin, 9, 10-epoxy-12-octadecenoate, causes cardiac failure in dogs. Life Sci. 1987, 40, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Toomey, S.; Harhen, B.; Roche, H.M.; Fitzgerald, D.; Belton, O. Profound resolution of early atherosclerosis with conjugated linoleic acid. Atherosclerosis 2006, 187, 40–49. [Google Scholar] [CrossRef]
- Toomey, S.; Roche, H.; Fitzgerald, D.; Belton, O. Regression of pre-established atherosclerosis in the apoE−/− mouse by conjugated linoleic acid. Biochem. Soc. Trans. 2003, 31, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Stachowska, E.; Siennicka, A.; Baskiewcz-Halasa, M.; Bober, J.; Machalinski, B.; Chlubek, D. Conjugated linoleic acid isomers may diminish human macrophages adhesion to endothelial surface. Int. J. Food Sci. Nutr. 2012, 63, 30–35. [Google Scholar] [CrossRef]
- Vahmani, P.; Meadus, W.J.; Duff, P.; Rolland, D.C.; Dugan, M.E.R. Comparing the lipogenic and cholesterolgenic effects of individual trans-18:1 isomers in liver cells. Eur. J. Lipid Sci. Technol. 2016, 119, 1600162. [Google Scholar] [CrossRef]

| Gene | Oligonucleotide Sequence (5′–3′) |
|---|---|
| TNF-α | GGACTAGCCAGGAGGGAGAA |
| CGCGGATCATGCTTTCTGTG | |
| IL-6 | TGGAGTACCATAGCTACCTGGA |
| TCTCTCTGAAGGACTCTGGCT | |
| IL-10 | GGGCGAGTGTAACAAGACCT |
| ATTTGCTGGGTTCCCACACT | |
| β-actin | AGGGAAATCGTGCGTGACAT |
| GGAAAAGAGCCTCAGGGCAT |
| CHD-48 vs. CHD-48-LP | |||||||||
| Compound | Formula | Molecular Weight | RT (min) | HMDB | PubChem | KEGG | p-Value | Fold Change | log2(FC) |
| Stearic acid | C18H36O2 | 284.27186 | 16.997 | HMDB0000827 | 5281 | C01530 | 0.044816 | 2.277 | 1.1871 |
| Toxopyrimidine | C6H9N3O | 139.07474 | 19.621 | METPA0166 | - | C01279 | 0.014444 | 0.15151 | −2.7225 |
| Geranylacetone | C13H22O | 194.16701 | 17.101 | HMDB0031846 | 1713001 | C13297 | 0.012086 | 0.49681 | −1.0092 |
| Linoleate | C18H32O2 | 280.24017 | 16.121 | HMDB0000673 | 5280450 | C01595 | 0.0081258 | 0.14277 | −2.8082 |
| Inspra | C24H30O6 | 414.20414 | 10.885 | HMDB0014838 | 443872 | C12512 | 0.014593 | 4.0497 | 2.0178 |
| A-12(13)-EpODE | C18H30O3 | 294.21941 | 16.398 | HMDB0010200 | 16061061 | 0.026316 | 5.2634 | 2.396 | |
| α-linolenoyl Ethanolamide | C20H35NO2 | 321.2668 | 16.105 | NA | NA | NA | 0.034482 | 0.24847 | −2.0089 |
| HP-48 vs. HP-48-LP | |||||||||
| Compound | Formula | Molecular Weight | RT (min) | HMDB | PubChem | KEGG | p-value | Fold Change | log2(FC) |
| L-aspartic acid | C4H7N O4 | 133.03669 | 0.772 | HMDB0000191 | 5960 | C00049 | 0.03759 | 0.077347 | −3.6925 |
| Dibutyl phthalate | C16H22O4 | 278.15181 | 17.236 | HMDB0033244 | 3026 | C14214 | 0.029904 | 2.6541 | 1.4082 |
| D-(+)-malic acid | C4H6O5 | 134.02082 | 0.783 | HMDB0031518 | 92824 | C00497 | 0.036464 | 0.21635 | −2.2086 |
| 2-methylcitric acid | C7H10O7 | 206.04345 | 0.847 | HMDB0000379 | 5290 | C02225 | 0.046553 | 0.059527 | −4.0703 |
| Linoleic acid | C18H32O2 | 280.24047 | 18.049 | HMDB0000673 | 5280450 | C01595 | 0.012485 | 0.17687 | −2.4992 |
| L-aspartic acid | C4H7NO4 | 133.03669 | 0.772 | HMDB0000191 | 5960 | C00049 | 0.038565 | 0.034422 | −4.8605 |
| Chenodeoxycholic acid | C24H40O4 | 438.29865 | 7.765 | HMDB0000637 | 22833540 | C05466 | 0.031712 | 26.812 | 4.7448 |
| N-acetylneuraminic acid | C11H19NO9 | 309.10584 | 1.004 | HMDB0000230 | 445063 | C19910 | 0.049937 | 0.24727 | −2.0159 |
| Verapamil | C27H38N2O4 | 454.28316 | 6.051 | HMDB0001850 | 2520 | C07188 | 0.015041 | 3.1745 | 1.6665 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Wu, Y.; Yang, J.; Li, Y.; Zhao, X.; Liang, T.; Li, L.; Jiang, T.; Zhang, T.; Zhang, J.; et al. Lactiplantibacillus plantarum P470 Isolated from Fermented Chinese Chives Has the Potential to Improve In Vitro the Intestinal Microbiota and Biological Activity in Feces of Coronary Heart Disease (CHD) Patients. Nutrients 2024, 16, 2945. https://doi.org/10.3390/nu16172945
Yang L, Wu Y, Yang J, Li Y, Zhao X, Liang T, Li L, Jiang T, Zhang T, Zhang J, et al. Lactiplantibacillus plantarum P470 Isolated from Fermented Chinese Chives Has the Potential to Improve In Vitro the Intestinal Microbiota and Biological Activity in Feces of Coronary Heart Disease (CHD) Patients. Nutrients. 2024; 16(17):2945. https://doi.org/10.3390/nu16172945
Chicago/Turabian StyleYang, Lingshuang, Yuwei Wu, Juan Yang, Ying Li, Xinyu Zhao, Tingting Liang, Longyan Li, Tong Jiang, Tiantian Zhang, Jumei Zhang, and et al. 2024. "Lactiplantibacillus plantarum P470 Isolated from Fermented Chinese Chives Has the Potential to Improve In Vitro the Intestinal Microbiota and Biological Activity in Feces of Coronary Heart Disease (CHD) Patients" Nutrients 16, no. 17: 2945. https://doi.org/10.3390/nu16172945
APA StyleYang, L., Wu, Y., Yang, J., Li, Y., Zhao, X., Liang, T., Li, L., Jiang, T., Zhang, T., Zhang, J., Zhong, H., Xie, X., & Wu, Q. (2024). Lactiplantibacillus plantarum P470 Isolated from Fermented Chinese Chives Has the Potential to Improve In Vitro the Intestinal Microbiota and Biological Activity in Feces of Coronary Heart Disease (CHD) Patients. Nutrients, 16(17), 2945. https://doi.org/10.3390/nu16172945







