Metabolic Characteristics of Gut Microbiota and Insomnia: Evidence from a Mendelian Randomization Analysis
Abstract
1. Introduction
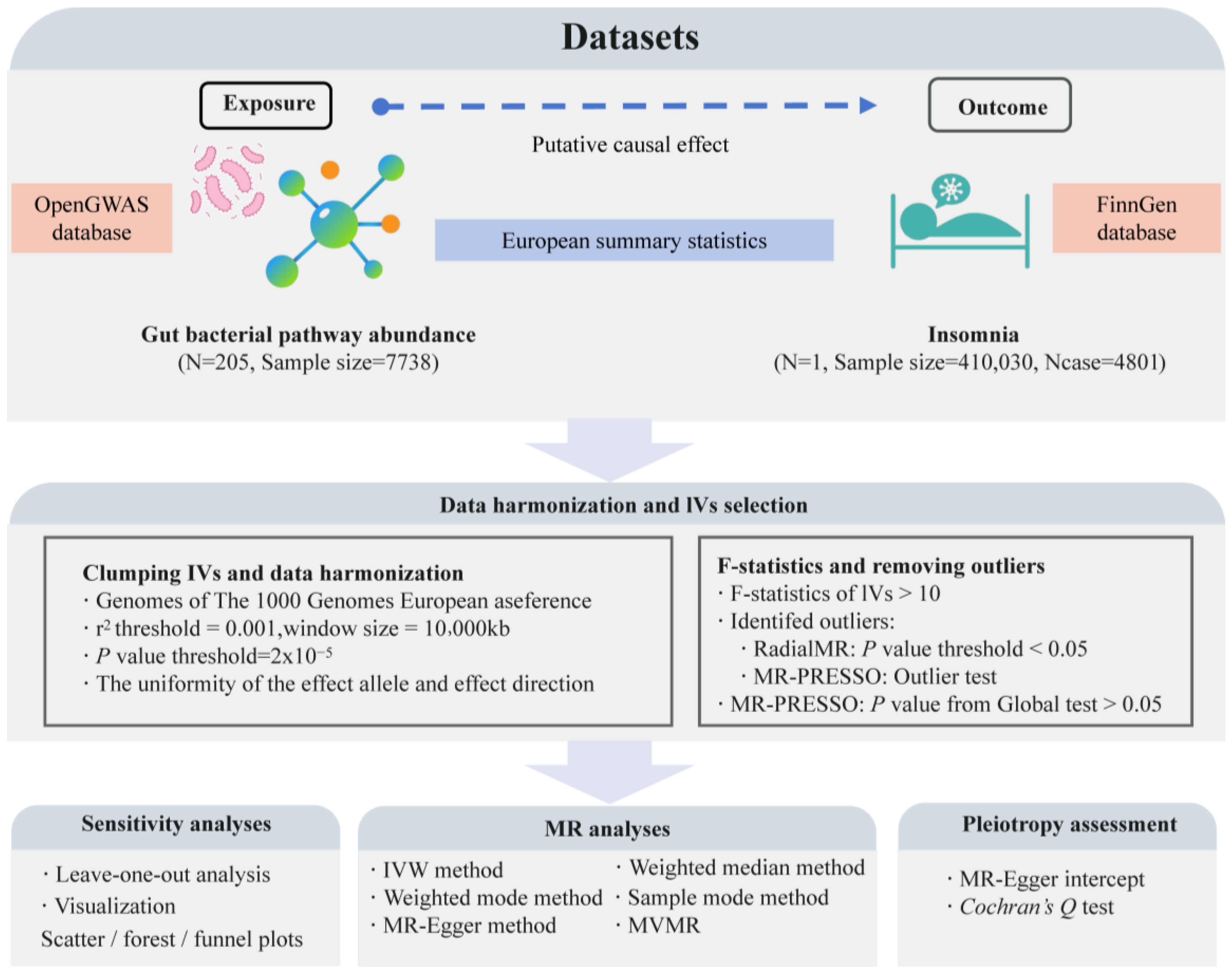
2. Materials and Methods
2.1. Data Sources
2.2. Instrumental Variable (IV) Selection
2.2.1. Selection of Exposure-Related IVs
2.2.2. Removing Confounding IVs
2.3. MR Analysis
2.4. Multivariable MR (MVMR) Analysis
2.5. Sensitivity Analysis
3. Results
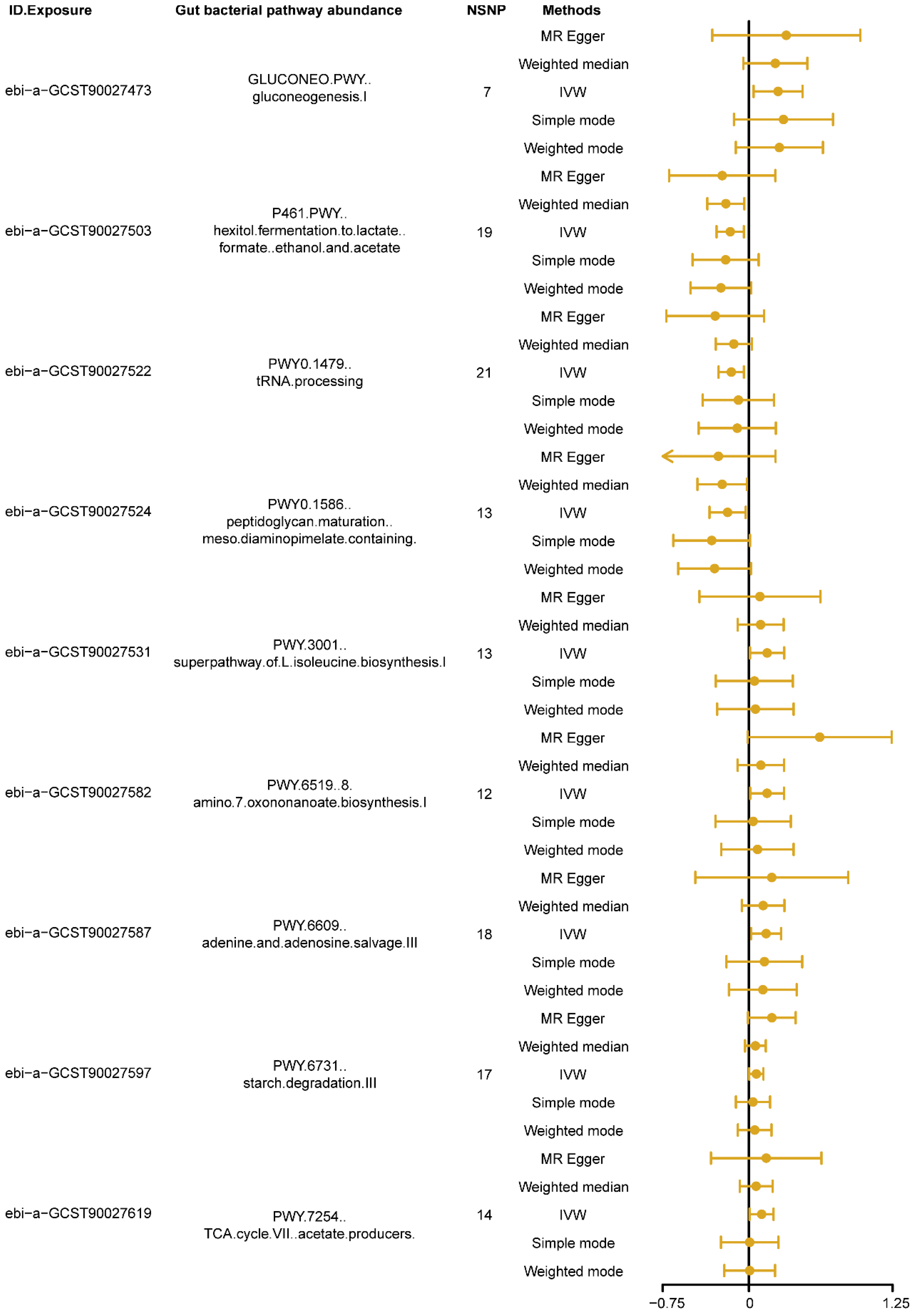
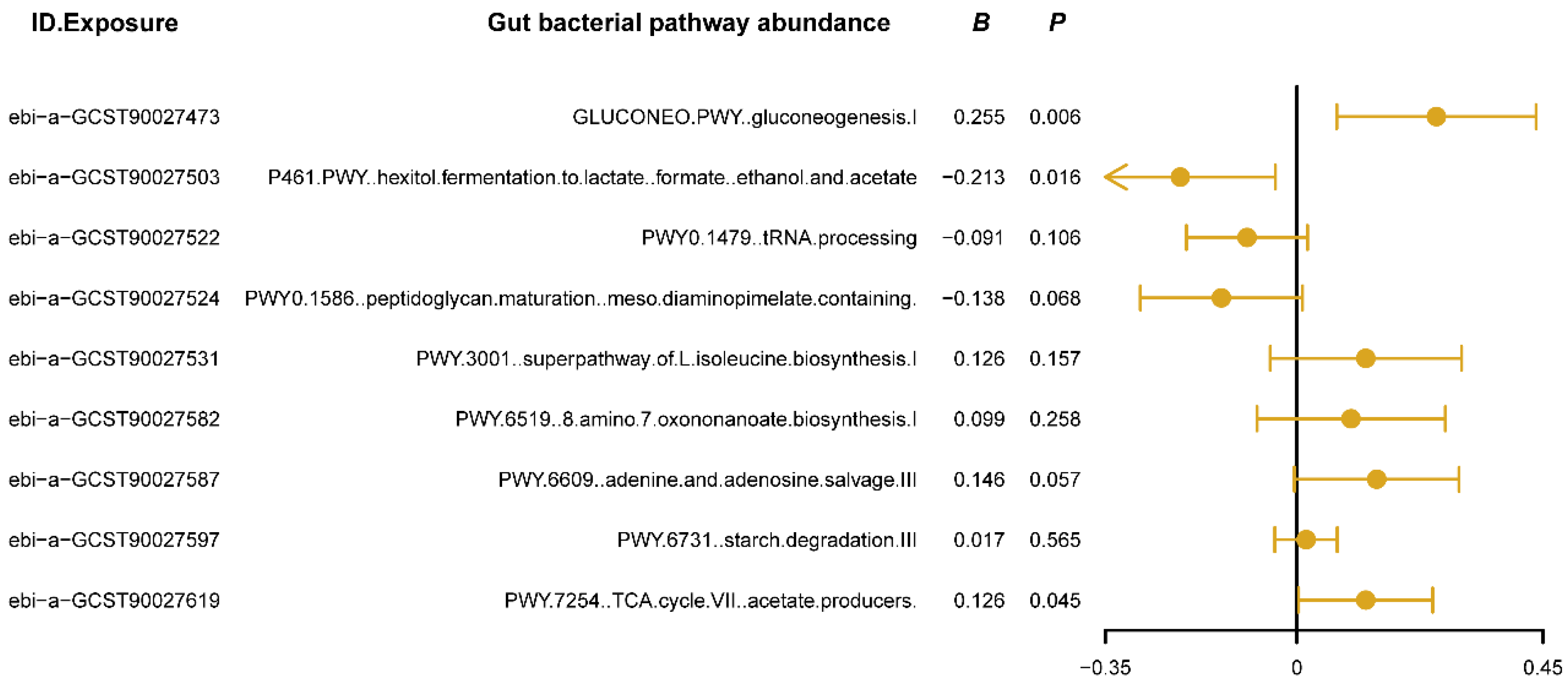
3.1. Causal Effects of Different Types of Gut Bacterial Pathway Abundance on Insomnia
3.2. MVMR Analysis
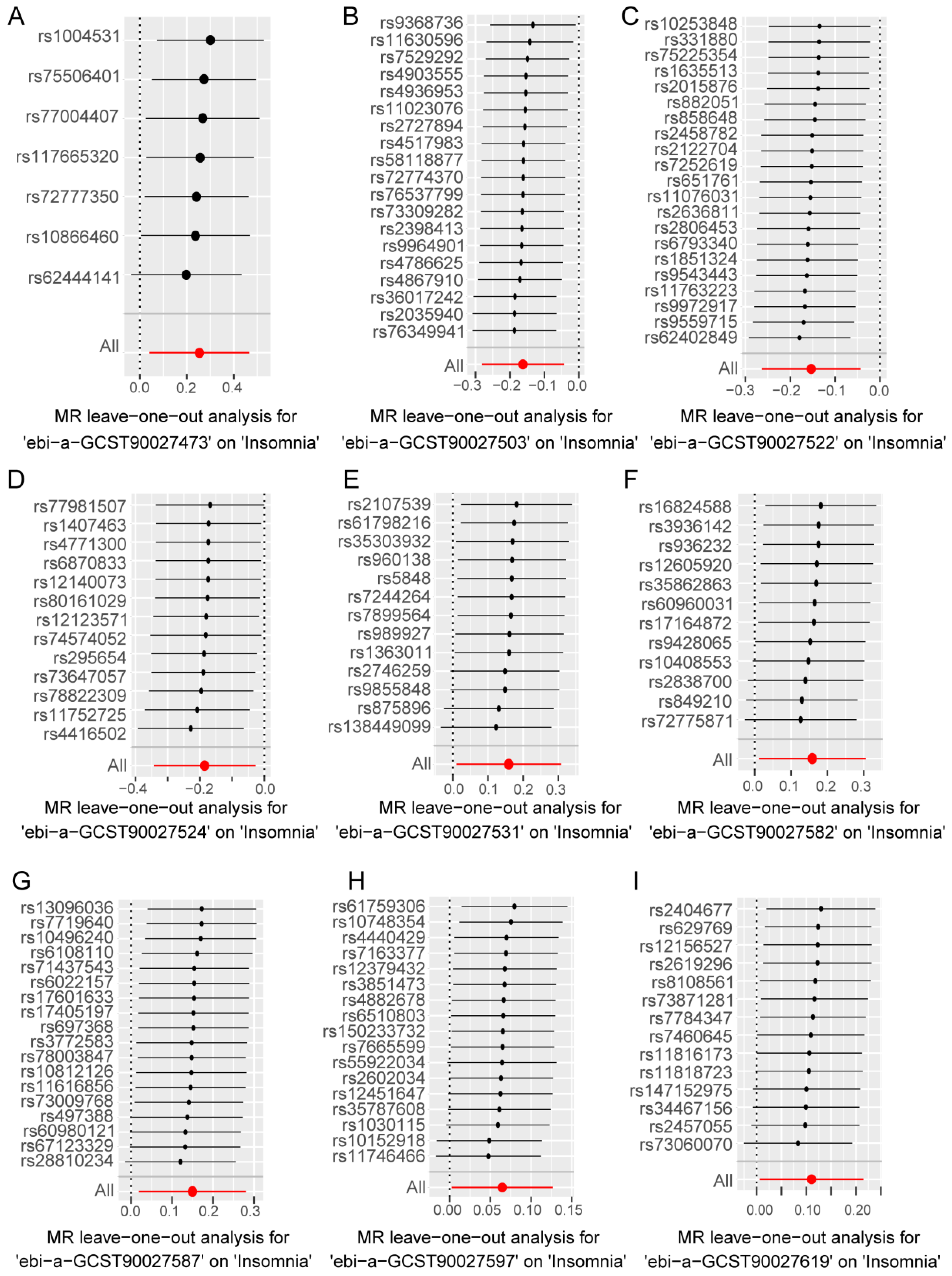
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iqbal, M.; Alshememry, A.; Imam, F.; Kalam, M.A.; Akhtar, A.; Ali, E.A. UPLC-MS/MS Based Identification and Quantification of a Novel Dual Orexin Receptor Antagonist in Plasma Samples by Validated SWGTOX Guidelines. Toxics 2023, 11, 109. [Google Scholar] [CrossRef]
- Jansen, P.R.; Watanabe, K.; Stringer, S.; Skene, N.; Bryois, J.; Hammerschlag, A.R.; de Leeuw, C.A.; Benjamins, J.S.; Muñoz-Manchado, A.B.; Nagel, M.; et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat. Genet. 2019, 51, 394–403. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Ma, T.; Wang, X.; Cheng, X.; Bai, Y. Association between longitudinal change of sleep patterns and the risk of cardiovascular diseases. Sleep 2024, 47, zsae084. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, S.; Redline, S. Insomnia and Risk of Cardiovascular Disease. Chest 2017, 152, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Markus, H.S. Genetic Liability to Insomnia and Cardiovascular Disease Risk. Circulation 2019, 140, 796–798. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhou, Z.; Li, X.; Yan, Z.; Ding, K.; Xiao, H.; Wu, Y.; Wu, T.; Chen, D. Integrative Identification of Genetic Loci Jointly Influencing Diabetes-Related Traits and Sleep Traits of Insomnia, Sleep Duration, and Chronotypes. Biomedicines 2022, 10, 368. [Google Scholar] [CrossRef]
- Tan, X.; van Egmond, L.; Chapman, C.D.; Cedernaes, J.; Benedict, C. Aiding sleep in type 2 diabetes: Therapeutic considerations. The lancet. Diabetes Endocrinol. 2018, 6, 60–68. [Google Scholar] [CrossRef]
- Blom, K.; Forsell, E.; Hellberg, M.; Svanborg, C.; Jernelöv, S.; Kaldo, V. Psychological Treatment of Comorbid Insomnia and Depression: A Double-Blind Randomized Placebo-Controlled Trial. Psychother. Psychosom. 2024, 93, 100–113. [Google Scholar] [CrossRef]
- Kunicki, Z.J.; Frietchen, R.; McGeary, J.E.; Jiang, L.; Duprey, M.S.; Bayer, T.; Singh, M.; Primack, J.M.; Kelso, C.M.; Wu, W.C.; et al. Prevalence of Comorbid Depression and Insomnia Among Veterans Hospitalized for Heart Failure with Alzheimer Disease and Related Disorders. Am. J. Geriatr. Psychiatry 2023, 31, 428–437. [Google Scholar] [CrossRef]
- Liverant, G.I.; Arditte Hall, K.A.; Wieman, S.T.; Pineles, S.L.; Pizzagalli, D.A. Associations between insomnia and reward learning in clinical depression. Psychol. Med. 2021, 52, 3540–3549. [Google Scholar] [CrossRef]
- Nielson, S.A.; Kay, D.B.; Dzierzewski, J.M. Sleep and Depression in Older Adults: A Narrative Review. Curr. Psychiatry Rep. 2023, 25, 643–658. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Oh, J.W.; Park, K.M.; Lee, S.; Lee, E. Digital cognitive behavioral therapy for insomnia on depression and anxiety: A systematic review and meta-analysis. NPJ Digit. Med. 2023, 6, 52. [Google Scholar] [CrossRef]
- Soltani, S.; Noel, M.; Bernier, E.; Kopala-Sibley, D.C. Pain and insomnia as risk factors for first lifetime onsets of anxiety, depression, and suicidality in adolescence. Pain 2023, 164, 1810–1819. [Google Scholar] [CrossRef]
- Sparasci, D.; Napoli, I.; Rossi, L.; Pereira-Mestre, R.; Manconi, M.; Treglia, G.; Marandino, L.; Ottaviano, M.; Turco, F.; Mangan, D.; et al. Prostate Cancer and Sleep Disorders: A Systematic Review. Cancers 2022, 14, 1784. [Google Scholar] [CrossRef]
- Schotanus, A.Y.; Dozeman, E.; Ikelaar, S.L.C.; van Straten, A.; Beekman, A.T.F.; van Nassau, F.; Bosmans, J.E.; van Schaik, A. Internet-delivered cognitive behavioural therapy for insomnia disorder in depressed patients treated at an outpatient clinic for mood disorders: Protocol of a randomised controlled trial. BMC Psychiatry 2023, 23, 75. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Dubey, V.; Ghosh, A.R. Obesity: An overview of possible role(s) of gut hormones, lipid sensing and gut microbiota. Metab. Clin. Exp. 2016, 65, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Hofman, D.; Kudla, U.; Miqdady, M.; Nguyen, T.V.H.; Morán-Ramos, S.; Vandenplas, Y. Faecal Microbiota in Infants and Young Children with Functional Gastrointestinal Disorders: A Systematic Review. Nutrients 2022, 14, 974. [Google Scholar] [CrossRef]
- Makrgeorgou, A.; Leonardi-Bee, J.; Bath-Hextall, F.J.; Murrell, D.F.; Tang, M.L.; Roberts, A.; Boyle, R.J. Probiotics for treating eczema. Cochrane Database Syst. Rev. 2018, 11, Cd006135. [Google Scholar] [CrossRef]
- Fan, S.; Guo, W.; Xiao, D.; Guan, M.; Liao, T.; Peng, S.; Feng, A.; Wang, Z.; Yin, H.; Li, M.; et al. Microbiota-gut-brain axis drives overeating disorders. Cell Metab. 2023, 35, 2011–2027.e2017. [Google Scholar] [CrossRef]
- Gheorghe, C.E.; Cryan, J.F.; Clarke, G. Debugging the gut-brain axis in depression. Cell Host Microbe 2022, 30, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Hou, S.; Xiong, B.; Wen, Y.; Wang, J.; Zeng, J.; Ma, X.; Wang, F. Therapeutic Effects of Baicalin on Diseases Related to Gut-Brain Axis Dysfunctions. Molecules 2023, 28, 6501. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Shin, Y.; Han, S.; Kwon, J.; Choi, T.G.; Kang, I.; Kim, S.S. The Gut-Brain Axis in Schizophrenia: The Implications of the Gut Microbiome and SCFA Production. Nutrients 2023, 15, 4391. [Google Scholar] [CrossRef]
- Lana, D.; Giovannini, M.G. The Microbiota-Gut-Brain Axis in Behaviour and Brain Disorders. Int. J. Mol. Sci. 2023, 24, 8460. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peña, A.; Tansey, M.G. The Alzheimer’s risk gene APOE modulates the gut-brain axis. Nature 2023, 614, 629–630. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, T.; Chen, W.; Yan, W.; Yuan, K.; Shi, L.; Liu, X.; Zhou, X.; Shi, J.; et al. The microbiota-gut-brain axis in sleep disorders. Sleep Med. Rev. 2022, 65, 101691. [Google Scholar] [CrossRef]
- Omond, S.E.T.; Hale, M.W.; Lesku, J.A. Neurotransmitters of sleep and wakefulness in flatworms. Sleep 2022, 45, zsac053. [Google Scholar] [CrossRef]
- Ursin, R. Serotonin and sleep. Sleep Med. Rev. 2002, 6, 55–69. [Google Scholar] [CrossRef]
- Fenk, L.A.; Riquelme, J.L.; Laurent, G. Interhemispheric competition during sleep. Nature 2023, 616, 312–318. [Google Scholar] [CrossRef]
- Tossell, K.; Yu, X.; Giannos, P.; Anuncibay Soto, B.; Nollet, M.; Yustos, R.; Miracca, G.; Vicente, M.; Miao, A.; Hsieh, B.; et al. Somatostatin neurons in prefrontal cortex initiate sleep-preparatory behavior and sleep via the preoptic and lateral hypothalamus. Nat. Neurosci. 2023, 26, 1805–1819. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, B.; Sheng, D.; Yang, J.; Fu, S.; Wang, J.; Zhao, C.; Wang, Y.; Gai, X.; Wang, J.; et al. Multiomics Analysis Reveals Aberrant Metabolism and Immunity Linked Gut Microbiota with Insomnia. Microbiol. Spectr. 2022, 10, e0099822. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, B.; Zhou, Y.; Wang, D.; Liu, X.; Li, L.; Wang, T.; Zhang, Y.; Jiang, M.; Tang, H.; et al. Gut Microbiota Changes and Their Relationship with Inflammation in Patients with Acute and Chronic Insomnia. Nat. Sci. Sleep 2020, 12, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Wang, L.; Shi, Y.Y.; Zhang, W.; Tan, L.W.; Wan, J.B.; Huang, W.H. Biotransformation of the saponins in Panax notoginseng leaves mediated by gut microbiota from insomniac patients. J. Sep. Sci. 2023, 46, e2200803. [Google Scholar] [CrossRef]
- dos Santos, A.; Galiè, S. The Microbiota–Gut–Brain Axis in Metabolic Syndrome and Sleep Disorders: A Systematic Review. Nutrients 2024, 16, 390. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Yao, T.; Li, W.; Pan, N.; Xu, H.; Zhao, Q.; Su, Y.; Xiong, K.; Wang, J. Efficacy and safety of fecal microbiota transplantation for chronic insomnia in adults: A real world study. Front. Microbiol. 2023, 14, 1299816. [Google Scholar] [CrossRef]
- Qi, X.; Ye, J.; Wen, Y.; Liu, L.; Cheng, B.; Cheng, S.; Yao, Y.; Zhang, F. Evaluating the Effects of Diet-Gut Microbiota Interactions on Sleep Traits Using the UK Biobank Cohort. Nutrients 2022, 14, 1134. [Google Scholar] [CrossRef]
- Zhu, R.; Fang, Y.; Li, H.; Liu, Y.; Wei, J.; Zhang, S.; Wang, L.; Fan, R.; Wang, L.; Li, S.; et al. Psychobiotic Lactobacillus plantarum JYLP-326 relieves anxiety, depression, and insomnia symptoms in test anxious college via modulating the gut microbiota and its metabolism. Front. Immunol. 2023, 14, 1158137. [Google Scholar] [CrossRef]
- Kann, S.; Eberhardt, K.; Hinz, R.; Schwarz, N.G.; Dib, J.C.; Aristizabal, A.; Mendoza, G.A.C.; Hagen, R.M.; Frickmann, H.; Barrantes, I.; et al. The Gut Microbiome of an Indigenous Agropastoralist Population in a Remote Area of Colombia with High Rates of Gastrointestinal Infections and Dysbiosis. Microorganisms 2023, 11, 625. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
- Zhai, M.; Song, W.; Liu, Z.; Cai, W.; Lin, G.N. Causality Investigation between Gut Microbiome and Sleep-Related Traits: A Bidirectional Two-Sample Mendelian Randomization Study. Genes 2024, 15, 769. [Google Scholar] [CrossRef]
- Ahmed, H.; Leyrolle, Q.; Koistinen, V.; Kärkkäinen, O.; Layé, S.; Delzenne, N.; Hanhineva, K. Microbiota-derived metabolites as drivers of gut-brain communication. Gut Microbes 2022, 14, 2102878. [Google Scholar] [CrossRef] [PubMed]
- Moțățăianu, A.; Șerban, G.; Andone, S. The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Cross-Talk with a Focus on Amyotrophic Lateral Sclerosis: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 15094. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.; Timpson, N.; Davey Smith, G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef]
- Lopera-Maya, E.A.; Kurilshikov, A.; van der Graaf, A.; Hu, S.; Andreu-Sánchez, S.; Chen, L.; Vila, A.V.; Gacesa, R.; Sinha, T.; Collij, V.; et al. Effect of host genetics on the gut microbiome in 7738 participants of the Dutch Microbiome Project. Nat. Genet. 2022, 54, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipilä, T.P.; Kristiansson, K.; Donner, K.M.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 2023, 613, 508–518. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Kutalik, Z.; Holmes, M.V.; Minelli, C.; et al. Guidelines for performing Mendelian randomization investigations: Update for summer 2023. Wellcome Open Res. 2019, 4, 186. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef]
- VanderWeele, T.J. Mediation Analysis: A Practitioner’s Guide. Annu. Rev. Public Health 2016, 37, 17–32. [Google Scholar] [CrossRef]
- Zhou, X.; Lian, P.; Liu, H.; Wang, Y.; Zhou, M.; Feng, Z. Causal Associations between Gut Microbiota and Different Types of Dyslipidemia: A Two-Sample Mendelian Randomization Study. Nutrients 2023, 15, 4445. [Google Scholar] [CrossRef]
- Byrska-Bishop, M.; Evani, U.S.; Zhao, X.; Basile, A.O.; Abel, H.J.; Regier, A.A.; Corvelo, A.; Clarke, W.E.; Musunuri, R.; Nagulapalli, K.; et al. High-coverage whole-genome sequencing of the expanded 1000 Genomes Project cohort including 602 trios. Cell 2022, 185, 3426–3440.e3419. [Google Scholar] [CrossRef]
- Machiela, M.J.; Chanock, S.J. LDlink: A web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 2015, 31, 3555–3557. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-H.; Brown, D.W.; Machiela, M.J. LDtrait: An Online Tool for Identifying Published Phenotype Associations in Linkage Disequilibrium. Cancer Res. 2020, 80, 3443–3446. [Google Scholar] [CrossRef]
- Kuppa, A.; Tripathi, H.; Al-Darraji, A.; Tarhuni, W.M.; Abdel-Latif, A. C-Reactive Protein Levels and Risk of Cardiovascular Diseases: A Two-Sample Bidirectional Mendelian Randomization Study. Int. J. Mol. Sci. 2023, 24, 9129. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.L.; Ahsan, H.; Vanderweele, T.J. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 2011, 40, 740–752. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Spiller, W.; Del Greco, M.F.; Sheehan, N.; Thompson, J.; Minelli, C.; Davey Smith, G. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. Int. J. Epidemiol. 2018, 47, 1264–1278. [Google Scholar] [CrossRef]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Morrison, J.; Knoblauch, N.; Marcus, J.H.; Stephens, M.; He, X. Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nat. Genet. 2020, 52, 740–747. [Google Scholar] [CrossRef]
- Burgess, S.; Foley, C.N.; Allara, E.; Staley, J.R.; Howson, J.M.M. A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat. Commun. 2020, 11, 376. [Google Scholar] [CrossRef]
- Yan, X.; Yang, P.; Li, Y.; Liu, T.; Zha, Y.; Wang, T.; Zhang, J.; Feng, Z.; Li, M. New insights from bidirectional Mendelian randomization: Causal relationships between telomere length and mitochondrial DNA copy number in aging biomarkers. Aging 2024, 16, 7387–7404. [Google Scholar] [CrossRef]
- Sanderson, E.; Spiller, W.; Bowden, J. Testing and correcting for weak and pleiotropic instruments in two-sample multivariable Mendelian randomization. Stat. Med. 2021, 40, 5434–5452. [Google Scholar] [CrossRef]
- Burgess, S.; Bowden, J.; Fall, T.; Ingelsson, E.; Thompson, S.G. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology 2017, 28, 30–42. [Google Scholar] [CrossRef]
- Ovcjak, A.; Pontello, R.; Miller, S.P.; Sun, H.S.; Feng, Z.P. Hypothermia combined with neuroprotective adjuvants shortens the duration of hospitalization in infants with hypoxic ischemic encephalopathy: Meta-analysis. Front. Pharmacol. 2022, 13, 1037131. [Google Scholar] [CrossRef]
- Lu, Y.; Tang, H.; Huang, P.; Wang, J.; Deng, P.; Li, Y.; Zheng, J.; Weng, L. Assessment of causal effects of visceral adipose tissue on risk of cancers: A Mendelian randomization study. Int. J. Epidemiol. 2022, 51, 1204–1218. [Google Scholar] [CrossRef]
- Wang, K.; Yang, F.; Liu, X.; Lin, X.; Yin, H.; Tang, Q.; Jiang, L.; Yao, K. Appraising the Effects of Metabolic Traits on the Risk of Glaucoma: A Mendelian Randomization Study. Metabolites 2023, 13, 109. [Google Scholar] [CrossRef]
- Wu, P.F.; Lu, H.; Zhou, X.; Liang, X.; Li, R.; Zhang, W.; Li, D.; Xia, K. Assessment of causal effects of physical activity on neurodegenerative diseases: A Mendelian randomization study. J. Sport Health Sci. 2021, 10, 454–461. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Q.; Li, Y.; Yuan, Z.; Liu, Z.; Guo, J.; Li, X.; Zhang, W.; Tao, Y.; Mei, J. Fructus gardeniae ameliorates anxiety-like behaviors induced by sleep deprivation via regulating hippocampal metabolomics and gut microbiota. Front. Cell. Infect. Microbiol. 2023, 13, 1167312. [Google Scholar] [CrossRef]
- Pardi, D.; Black, J. Gamma-Hydroxybutyrate/sodium oxybate: Neurobiology, and impact on sleep and wakefulness. CNS Drugs 2006, 20, 993–1018. [Google Scholar] [CrossRef]
- Yan, R.; Murphy, M.; Genoni, A.; Marlow, E.; Dunican, I.C.; Lo, J.; Andrew, L.; Devine, A.; Christophersen, C.T. Does Fibre-fix provided to people with irritable bowel syndrome who are consuming a low FODMAP diet improve their gut health, gut microbiome, sleep and mental health? A double-blinded, randomised controlled trial. BMJ Open Gastroenterol. 2020, 7, e000448. [Google Scholar] [CrossRef]
- Lan, Y.; Lu, J.; Qiao, G.; Mao, X.; Zhao, J.; Wang, G.; Tian, P.; Chen, W. Bifidobacterium breve CCFM1025 Improves Sleep Quality via Regulating the Activity of the HPA Axis: A Randomized Clinical Trial. Nutrients 2023, 15, 4700. [Google Scholar] [CrossRef]
- Ribera, C.; Sánchez-Ortí, J.V.; Clarke, G.; Marx, W.; Mörkl, S.; Balanzá-Martínez, V. Probiotic, prebiotic, synbiotic and fermented food supplementation in psychiatric disorders: A systematic review of clinical trials. Neurosci. Biobehav. Rev. 2024, 158, 105561. [Google Scholar] [CrossRef] [PubMed]
- Humer, E.; Pieh, C.; Brandmayr, G. Metabolomics in Sleep, Insomnia and Sleep Apnea. Int. J. Mol. Sci. 2020, 21, 7244. [Google Scholar] [CrossRef]
- Rogers, R.C.; Burke, S.J.; Collier, J.J.; Ritter, S.; Hermann, G.E. Evidence that hindbrain astrocytes in the rat detect low glucose with a glucose transporter 2-phospholipase C-calcium release mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R38–R48. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Cherta-Murillo, A.; Darimont, C.; Mantantzis, K.; Martin, F.P.; Owen, L. The interrelationship between sleep, diet, and glucose metabolism. Sleep Med. Rev. 2023, 69, 101788. [Google Scholar] [CrossRef]
- Magistretti, P.J. Synaptic plasticity and the Warburg effect. Cell Metab. 2014, 19, 4–5. [Google Scholar] [CrossRef]
- Medel, V.; Crossley, N.; Gajardo, I.; Muller, E.; Barros, L.F.; Shine, J.M.; Sierralta, J. Whole-brain neuronal MCT2 lactate transporter expression links metabolism to human brain structure and function. Proc. Natl. Acad. Sci. USA 2022, 119, e2204619119. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; Wei, J.; Yoshizawa, T.; Del Fattore, A.; DePinho, R.A.; Teti, A.; Ducy, P.; Karsenty, G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 2010, 142, 296–308. [Google Scholar] [CrossRef]
- Homem, C.C.F.; Steinmann, V.; Burkard, T.R.; Jais, A.; Esterbauer, H.; Knoblich, J.A. Ecdysone and mediator change energy metabolism to terminate proliferation in Drosophila neural stem cells. Cell 2014, 158, 874–888. [Google Scholar] [CrossRef]
- Seifert, J.; Chen, Y.; Schöning, W.; Mai, K.; Tacke, F.; Spranger, J.; Köhrle, J.; Wirth, E.K. Hepatic Energy Metabolism under the Local Control of the Thyroid Hormone System. Int. J. Mol. Sci. 2023, 24, 4861. [Google Scholar] [CrossRef]
- Lévy, P.; Bonsignore, M.R.; Eckel, J. Sleep, sleep-disordered breathing and metabolic consequences. Eur. Respir. J. 2009, 34, 243–260. [Google Scholar] [CrossRef]
- Stamatakis, K.A.; Punjabi, N.M. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 2010, 137, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Piovezan, R.D.; Abucham, J.; Dos Santos, R.V.; Mello, M.T.; Tufik, S.; Poyares, D. The impact of sleep on age-related sarcopenia: Possible connections and clinical implications. Ageing Res. Rev. 2015, 23, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Mokhlesi, B.; Tjaden, A.H.; Temple, K.A.; Edelstein, S.L.; Sam, S.; Nadeau, K.J.; Hannon, T.S.; Manchanda, S.; Mather, K.J.; Kahn, S.E.; et al. Obstructive Sleep Apnea, Glucose Tolerance, and β-Cell Function in Adults with Prediabetes or Untreated Type 2 Diabetes in the Restoring Insulin Secretion (RISE) Study. Diabetes Care 2021, 44, 993–1001. [Google Scholar] [CrossRef]
- Pack, A.I. Gut microbiome: Role in insulin resistance in obstructive sleep apnea. eBioMedicine 2021, 65, 103278. [Google Scholar] [CrossRef] [PubMed]
- Dahan, T.; Nassar, S.; Yajuk, O.; Steinberg, E.; Benny, O.; Abudi, N.; Plaschkes, I.; Benyamini, H.; Gozal, D.; Abramovitch, R.; et al. Chronic Intermittent Hypoxia during Sleep Causes Browning of Interscapular Adipose Tissue Accompanied by Local Insulin Resistance in Mice. Int. J. Mol. Sci. 2022, 23, 15462. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, Y.; Yuan, J.; Mu, Y.; Chen, P.; Srimoragot, M.; Li, Y.; Park, C.G.; Reutrakul, S. Associations between sleep variability and cardiometabolic health: A systematic review. Sleep Med. Rev. 2022, 66, 101688. [Google Scholar] [CrossRef]
- Feder, A.; Coplan, J.D.; Goetz, R.R.; Mathew, S.J.; Pine, D.S.; Dahl, R.E.; Ryan, N.D.; Greenwald, S.; Weissman, M.M. Twenty-four-hour cortisol secretion patterns in prepubertal children with anxiety or depressive disorders. Biol. Psychiatry 2004, 56, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.M.; Kamat, R.; Tomfohr, L.M.; Ancoli-Israel, S.; Dimsdale, J.E. Obstructive sleep apnea and neurocognitive performance: The role of cortisol. Sleep Med. 2014, 15, 27–32. [Google Scholar] [CrossRef]
- Østergaard Madsen, H.; Hageman, I.; Kolko, M.; Lund-Andersen, H.; Martiny, K.; Ba-Ali, S. Seasonal variation in neurohormones, mood and sleep in patients with primary open angle glaucoma—Implications of the ipRGC-system. Chronobiol. Int. 2021, 38, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Tong, J.; Hao, H.; Yang, Z.; Chen, K.; Xu, H.; Wang, A. Bile acid coordinates microbiota homeostasis and systemic immunometabolism in cardiometabolic diseases. Acta Pharm. Sin. B 2022, 12, 2129–2149. [Google Scholar] [CrossRef]
- Hasan, S.; Ghani, N.; Zhao, X.; Good, J.; Huang, A.; Wrona, H.L.; Liu, J.; Liu, C.J. Dietary pyruvate targets cytosolic phospholipase A2 to mitigate inflammation and obesity in mice. Protein Cell, 2024; pwae014, online ahead of print. [Google Scholar] [CrossRef]
- Wang, X.; Ota, N.; Manzanillo, P.; Kates, L.; Zavala-Solorio, J.; Eidenschenk, C.; Zhang, J.; Lesch, J.; Lee, W.P.; Ross, J.; et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 2014, 514, 237–241. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, G.; Chen, L.; Kim, S.; Yu, J.; Hu, G.; Chen, J.; Huang, Y.; Zheng, G.; Huang, S. The Role of NLRP3 and IL-1β in Refractory Epilepsy Brain Injury. Front. Neurol. 2019, 10, 1418. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhang, L.J.; Huang, T.Q.; Kim, J.; Gu, M.Y.; Yang, H.O. Narciclasine inhibits LPS-induced neuroinflammation by modulating the Akt/IKK/NF-κB and JNK signaling pathways. Phytomed. Int. J. Phytother. Phytopharm. 2021, 85, 153540. [Google Scholar] [CrossRef]
- Li, X.; Qiao, M.; Zhou, Y.; Peng, Y.; Wen, G.; Xie, C.; Zhang, Y. Modulating the RPS27A/PSMD12/NF-κB pathway to control immune response in mouse brain ischemia-reperfusion injury. Mol. Med. 2024, 30, 106. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Xu, M.; Fei, Q.; Gu, Y.; Luo, Y.; Wu, H. Efficient biosynthesis of 3-hydroxypropionic acid from ethanol in metabolically engineered Escherichia coli. Bioresour. Technol. 2022, 363, 127907. [Google Scholar] [CrossRef]
- Schink, S.J.; Christodoulou, D.; Mukherjee, A.; Athaide, E.; Brunner, V.; Fuhrer, T.; Bradshaw, G.A.; Sauer, U.; Basan, M. Glycolysis/gluconeogenesis specialization in microbes is driven by biochemical constraints of flux sensing. Mol. Syst. Biol. 2022, 18, e10704. [Google Scholar] [CrossRef]
- Guo, W.L.; Cao, Y.J.; You, S.Z.; Wu, Q.; Zhang, F.; Han, J.Z.; Lv, X.C.; Rao, P.F.; Ai, L.Z.; Ni, L. Ganoderic acids-rich ethanol extract from Ganoderma lucidum protects against alcoholic liver injury and modulates intestinal microbiota in mice with excessive alcohol intake. Curr. Res. Food Sci. 2022, 5, 515–530. [Google Scholar] [CrossRef]
- Lyu, J.; Yang, Z.; Wang, E.; Liu, G.; Wang, Y.; Wang, W.; Li, S. Possibility of Using By-Products with High NDF Content to Alter the Fecal Short Chain Fatty Acid Profiles, Bacterial Community, and Digestibility of Lactating Dairy Cows. Microorganisms 2022, 10, 1731. [Google Scholar] [CrossRef] [PubMed]
- Garbacz, K. Anticancer activity of lactic acid bacteria. Semin. Cancer Biol. 2022, 86, 356–366. [Google Scholar] [CrossRef]
- Karboune, S.; Seo, S.; Li, M.; Waglay, A.; Lagacé, L. Biotransformation of sucrose rich Maple syrups into fructooligosaccharides, oligolevans and levans using levansucrase biocatalyst: Bioprocess optimization and prebiotic activity assessment. Food Chem. 2022, 382, 132355. [Google Scholar] [CrossRef] [PubMed]
- Liotti, F.; Marotta, M.; Sorriento, D.; Pagliuca, C.; Caturano, V.; Mantova, G.; Scaglione, E.; Salvatore, P.; Melillo, R.M.; Prevete, N. Probiotic Lactobacillus rhamnosus GG (LGG) restrains the angiogenic potential of colorectal carcinoma cells by activating a proresolving program via formyl peptide receptor 1. Mol. Oncol. 2022, 16, 2959–2980. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.; Zhan, X.; Song, Y.; Xu, M.; Wang, S.; Huang, X.; Xu, Z.Z. Meta-analysis reveals Helicobacter pylori mutual exclusivity and reproducible gastric microbiome alterations during gastric carcinoma progression. Gut Microbes 2023, 15, 2197835. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.W.; Wu, C.H.; Jao, Y.C.; Tsai, Y.S.; Chen, Y.L.; Chen, C.C.; Fang, T.J.; Chau, C.F. Fermented Supernatants of Lactobacillus plantarum GKM3 and Bifidobacterium lactis GKK2 Protect against Protein Glycation and Inhibit Glycated Protein Ligation. Nutrients 2023, 15, 277. [Google Scholar] [CrossRef]
- Prasad, K.; de Vries, E.F.J.; Sijbesma, J.W.A.; Garcia-Varela, L.; Vazquez-Matias, D.A.; Moraga-Amaro, R.; Willemsen, A.T.M.; Dierckx, R.; van Waarde, A. Impact of an Adenosine A(2A) Receptor Agonist and Antagonist on Binding of the Dopamine D(2) Receptor Ligand [(11)C]raclopride in the Rodent Striatum. Mol. Pharm. 2022, 19, 2992–3001. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Z.; Chen, G.; Geng, D.; Gao, D. Low Levels of Adenosine and GDNF Are Potential Risk Factors for Parkinson’s Disease with Sleep Disorders. Brain Sci. 2023, 13, 200. [Google Scholar] [CrossRef]
- Quiquempoix, M.; Sauvet, F.; Erblang, M.; Van Beers, P.; Guillard, M.; Drogou, C.; Trignol, A.; Vergez, A.; Léger, D.; Chennaoui, M.; et al. Effects of Caffeine Intake on Cognitive Performance Related to Total Sleep Deprivation and Time on Task: A Randomized Cross-Over Double-Blind Study. Nat. Sci. Sleep 2022, 14, 457–473. [Google Scholar] [CrossRef]
- Peng, W.; Wu, Z.; Song, K.; Zhang, S.; Li, Y.; Xu, M. Regulation of sleep homeostasis mediator adenosine by basal forebrain glutamatergic neurons. Science 2020, 369, eabb0556. [Google Scholar] [CrossRef]
- Doke, M.; McLaughlin, J.P.; Baniasadi, H.; Samikkannu, T. Sleep Disorder and Cocaine Abuse Impact Purine and Pyrimidine Nucleotide Metabolic Signatures. Metabolites 2022, 12, 869. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Liu, X.; Ma, G.; Wu, Z.; Wang, Z.; Fei, X.; Qin, M.; Wang, L.; Li, Y.; Zhang, S.; et al. Adenosine-independent regulation of the sleep-wake cycle by astrocyte activity. Cell Discov. 2023, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Nayeem, M.A.; Hanif, A.; Geldenhuys, W.J.; Agba, S. Crosstalk between adenosine receptors and CYP450-derived oxylipins in the modulation of cardiovascular, including coronary reactive hyperemic response. Pharmacol. Ther. 2022, 240, 108213. [Google Scholar] [CrossRef]
- Mandal, A.K.; Merriman, T.R.; Choi, H.K.; Mount, D.B. Caffeine inhibits both basal and insulin-activated urate transport. Arthritis Rheumatol. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Norman, B.; Nygren, A.T.; Nowak, J.; Sabina, R.L. The effect of AMPD1 genotype on blood flow response to sprint exercise. Eur. J. Appl. Physiol. 2008, 103, 173–180. [Google Scholar] [CrossRef]
- Augustin, R.C.; Leone, R.D.; Naing, A.; Fong, L.; Bao, R.; Luke, J.J. Next steps for clinical translation of adenosine pathway inhibition in cancer immunotherapy. J. Immunother. Cancer 2022, 10, e004089. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, B.E.; Tyni-Lennè, R.; Svedenhag, J.; Hallin, R.; Jensen-Urstad, K.; Jensen-Urstad, M.; Bergman, K.; Selvén, C. Physical training in Syndrome X: Physical training counteracts deconditioning and pain in Syndrome X. J. Am. Coll. Cardiol. 2000, 36, 1619–1625. [Google Scholar] [CrossRef]
- Lu, S.; Tian, H.; Li, L.; Li, B.; Yang, M.; Zhou, L.; Jiang, H.; Li, Q.; Wang, W.; Nice, E.C.; et al. Nanoengineering a Zeolitic Imidazolate Framework-8 Capable of Manipulating Energy Metabolism against Cancer Chemo-Phototherapy Resistance. Small 2022, 18, e2204926. [Google Scholar] [CrossRef]
- Wu, L.; Xie, W.; Li, Y.; Ni, Q.; Timashev, P.; Lyu, M.; Xia, L.; Zhang, Y.; Liu, L.; Yuan, Y.; et al. Biomimetic Nanocarriers Guide Extracellular ATP Homeostasis to Remodel Energy Metabolism for Activating Innate and Adaptive Immunity System. Adv. Sci. 2022, 9, e2105376. [Google Scholar] [CrossRef]
- Micheva, K.D.; Taylor, C.P.; Smith, S.J. Pregabalin reduces the release of synaptic vesicles from cultured hippocampal neurons. Mol. Pharmacol. 2006, 70, 467–476. [Google Scholar] [CrossRef]
- Lorenzo, M.P.; Navarrete, A.; Balderas, C.; Garcia, A. Optimization and validation of a CE-LIF method for amino acid determination in biological samples. J. Pharm. Biomed. Anal. 2013, 73, 116–124. [Google Scholar] [CrossRef]
- Chun, S.W.; Hinze, M.E.; Skiba, M.A.; Narayan, A.R.H. Chemistry of a Unique Polyketide-like Synthase. J. Am. Chem. Soc. 2018, 140, 2430–2433. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Jiang, N.; Wu, H.; Mei, Y.; Yang, J.; Tan, R. Cytotoxic and antibacterial polyketide-indole hybrids synthesized from indole-3-carbinol by Daldinia eschscholzii. Acta Pharm. Sin. B 2019, 9, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Baixauli, F.; Piletic, K.; Puleston, D.J.; Villa, M.; Field, C.S.; Flachsmann, L.J.; Quintana, A.; Rana, N.; Edwards-Hicks, J.; Matsushita, M.; et al. An LKB1-mitochondria axis controls T(H)17 effector function. Nature 2022, 610, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.H.; Engels, D.; Locatelli, G.; Emmanouilidis, I.; Fecher, C.; Theodorou, D.; Müller, S.A.; Licht-Mayer, S.; Kreutzfeldt, M.; Wagner, I.; et al. Targeting the TCA cycle can ameliorate widespread axonal energy deficiency in neuroinflammatory lesions. Nat. Metab. 2023, 5, 1364–1381. [Google Scholar] [CrossRef]
- Doan, M.T.; Teitell, M.A. Krebs and an alternative TCA cycle! Cell Res. 2022, 32, 509–510. [Google Scholar] [CrossRef]
- Mateska, I.; Alexaki, V.I. Light shed on a non-canonical TCA cycle: Cell state regulation beyond mitochondrial energy production. Signal Transduct. Target. Ther. 2022, 7, 201. [Google Scholar] [CrossRef]
- Wu, F.; Sun, X.; Zou, B.; Zhu, P.; Lin, N.; Lin, J.; Ji, K. Transcriptional Analysis of Masson Pine (Pinus massoniana) under High CO2 Stress. Genes 2019, 10, 804. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jin, J.; Hu, S.; Shen, L.; Zhang, P.; Li, Z.; Fang, Z.; Liu, H. Metabolomics and proteomics reveal the toxicological mechanisms of florfenicol stress on wheat (Triticum aestivum L.) seedlings. J. Hazard. Mater. 2023, 443, 130264. [Google Scholar] [CrossRef]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Le, Z.; Yanxiang Guo, J.; et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef]
- Jakkamsetti, V.; Marin-Valencia, I.; Ma, Q.; Good, L.B.; Terrill, T.; Rajasekaran, K.; Pichumani, K.; Khemtong, C.; Hooshyar, M.A.; Sundarrajan, C.; et al. Brain metabolism modulates neuronal excitability in a mouse model of pyruvate dehydrogenase deficiency. Sci. Transl. Med. 2019, 11, eaan0457. [Google Scholar] [CrossRef] [PubMed]
- Sponagel, J.; Jones, J.K.; Frankfater, C.; Zhang, S.; Tung, O.; Cho, K.; Tinkum, K.L.; Gass, H.; Nunez, E.; Spitz, D.R.; et al. Sex differences in brain tumor glutamine metabolism reveal sex-specific vulnerabilities to treatment. Med 2022, 3, 792–811.e712. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef]
- Mews, P.; Egervari, G.; Nativio, R.; Sidoli, S.; Donahue, G.; Lombroso, S.I.; Alexander, D.C.; Riesche, S.L.; Heller, E.A.; Nestler, E.J.; et al. Alcohol metabolism contributes to brain histone acetylation. Nature 2019, 574, 717–721. [Google Scholar] [CrossRef]
- Zhu, S.; Pan, W. Microbial metabolite steers intestinal stem cell fate under stress. Cell Stem Cell 2024, 31, 591–592. [Google Scholar] [CrossRef]
- Simon, J.; Nuñez-García, M.; Fernández-Tussy, P.; Barbier-Torres, L.; Fernández-Ramos, D.; Gómez-Santos, B.; Buqué, X.; Lopitz-Otsoa, F.; Goikoetxea-Usandizaga, N.; Serrano-Macia, M.; et al. Targeting Hepatic Glutaminase 1 Ameliorates Non-alcoholic Steatohepatitis by Restoring Very-Low-Density Lipoprotein Triglyceride Assembly. Cell Metab. 2020, 31, 605–622.e610. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Daneshmandi, S.; Choi, J.E.; Yan, Q.; MacDonald, C.R.; Pandey, M.; Goruganthu, M.; Roberts, N.; Singh, P.K.; Higashi, R.M.; Lane, A.N.; et al. Myeloid-derived suppressor cell mitochondrial fitness governs chemotherapeutic efficacy in hematologic malignancies. Nat. Commun. 2024, 15, 2803. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, F.; Feng, Z.; Xu, B. Metabolic Characteristics of Gut Microbiota and Insomnia: Evidence from a Mendelian Randomization Analysis. Nutrients 2024, 16, 2943. https://doi.org/10.3390/nu16172943
Xie F, Feng Z, Xu B. Metabolic Characteristics of Gut Microbiota and Insomnia: Evidence from a Mendelian Randomization Analysis. Nutrients. 2024; 16(17):2943. https://doi.org/10.3390/nu16172943
Chicago/Turabian StyleXie, Fuquan, Zhijun Feng, and Beibei Xu. 2024. "Metabolic Characteristics of Gut Microbiota and Insomnia: Evidence from a Mendelian Randomization Analysis" Nutrients 16, no. 17: 2943. https://doi.org/10.3390/nu16172943
APA StyleXie, F., Feng, Z., & Xu, B. (2024). Metabolic Characteristics of Gut Microbiota and Insomnia: Evidence from a Mendelian Randomization Analysis. Nutrients, 16(17), 2943. https://doi.org/10.3390/nu16172943





