The Combination of Exercise and Konjac Glucomannan More Effectively Prevents Antibiotics-Induced Dysbiosis in Mice Compared with Singular Intervention
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
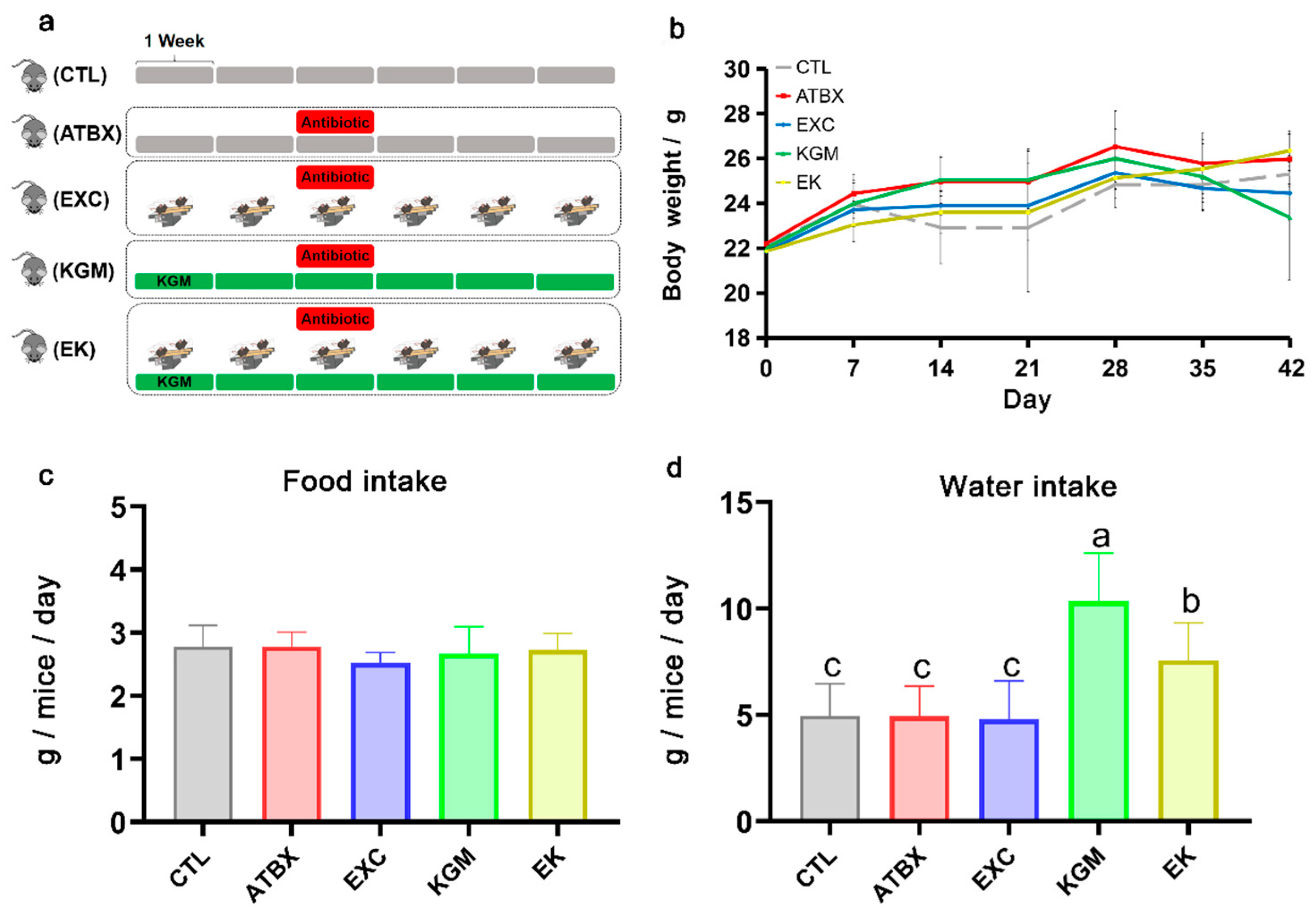
2.2. Animal Experiment Design
2.3. Endurance Test
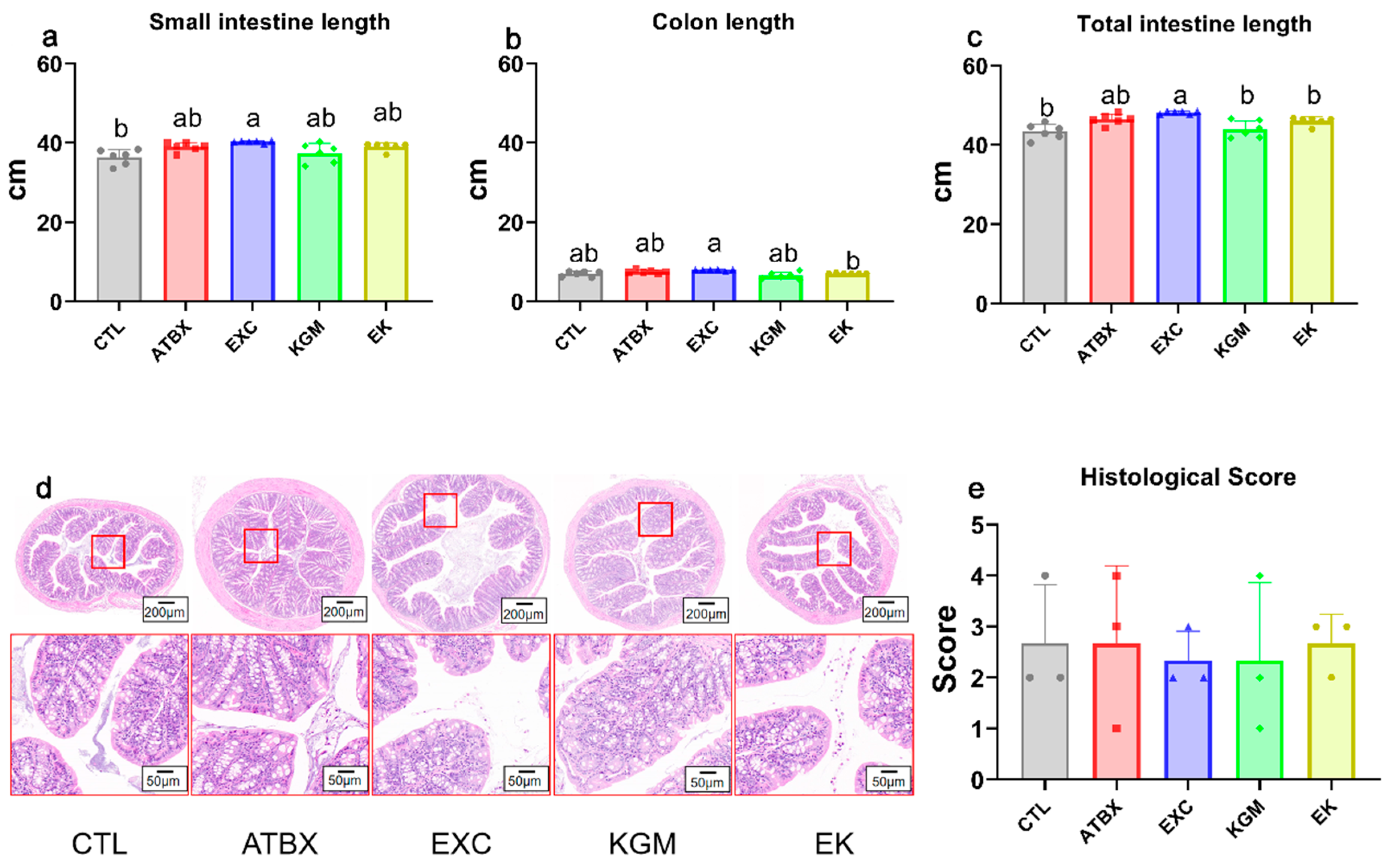
2.4. Histological Analysis
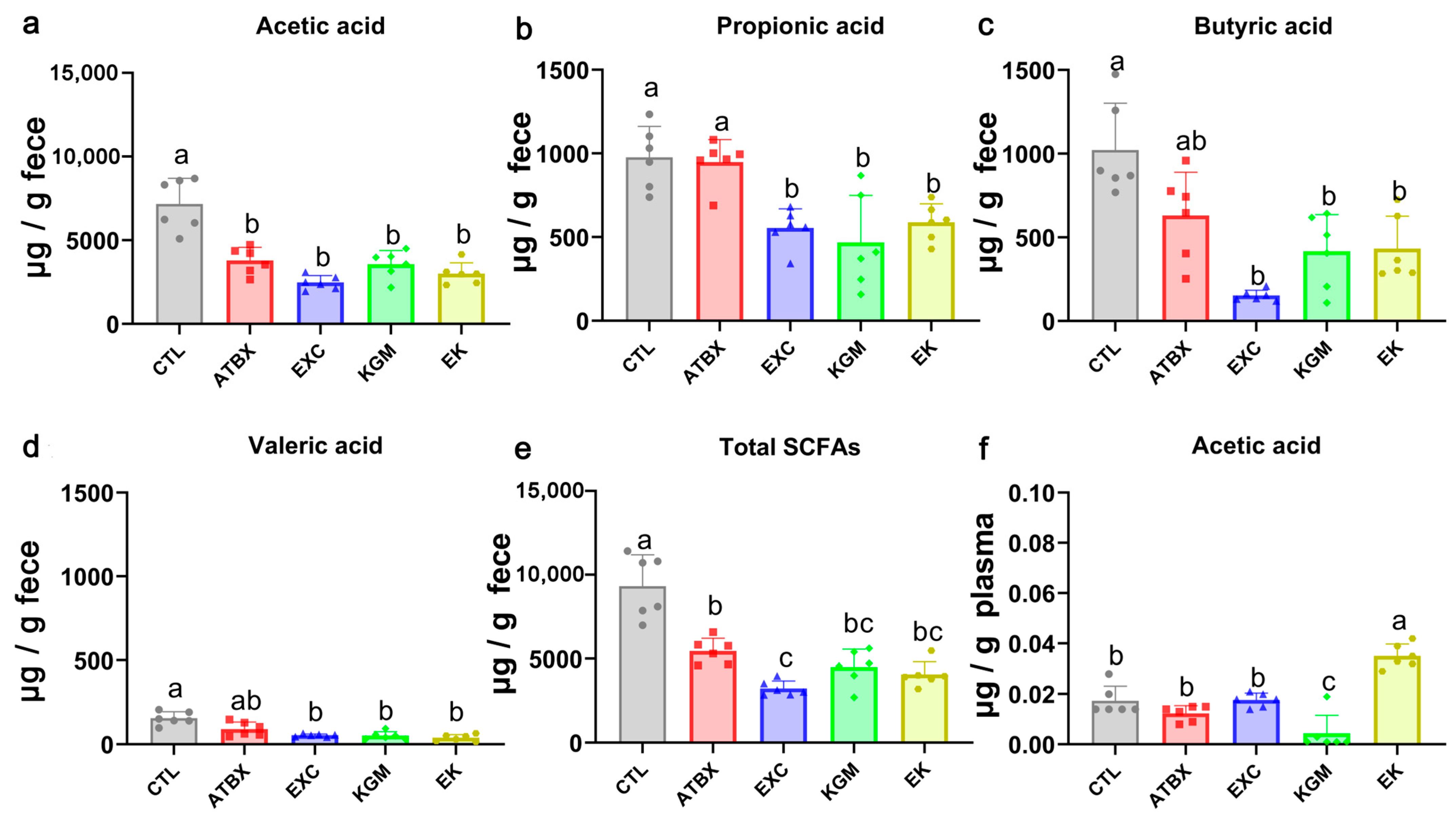
2.5. Quantification of Short-Chain Fatty Acids in Plasma and Fecal Samples
2.6. DNA Extraction and 16S rRNA Sequencing
2.7. Metagenomic Sequencing and Data Analysis of Fecal Samples
2.8. Statistical Analysis
3. Results
3.1. Body Weight and Water and Food Intake
3.2. Intestinal Morphography
3.3. Short-Chain Fatty Acids
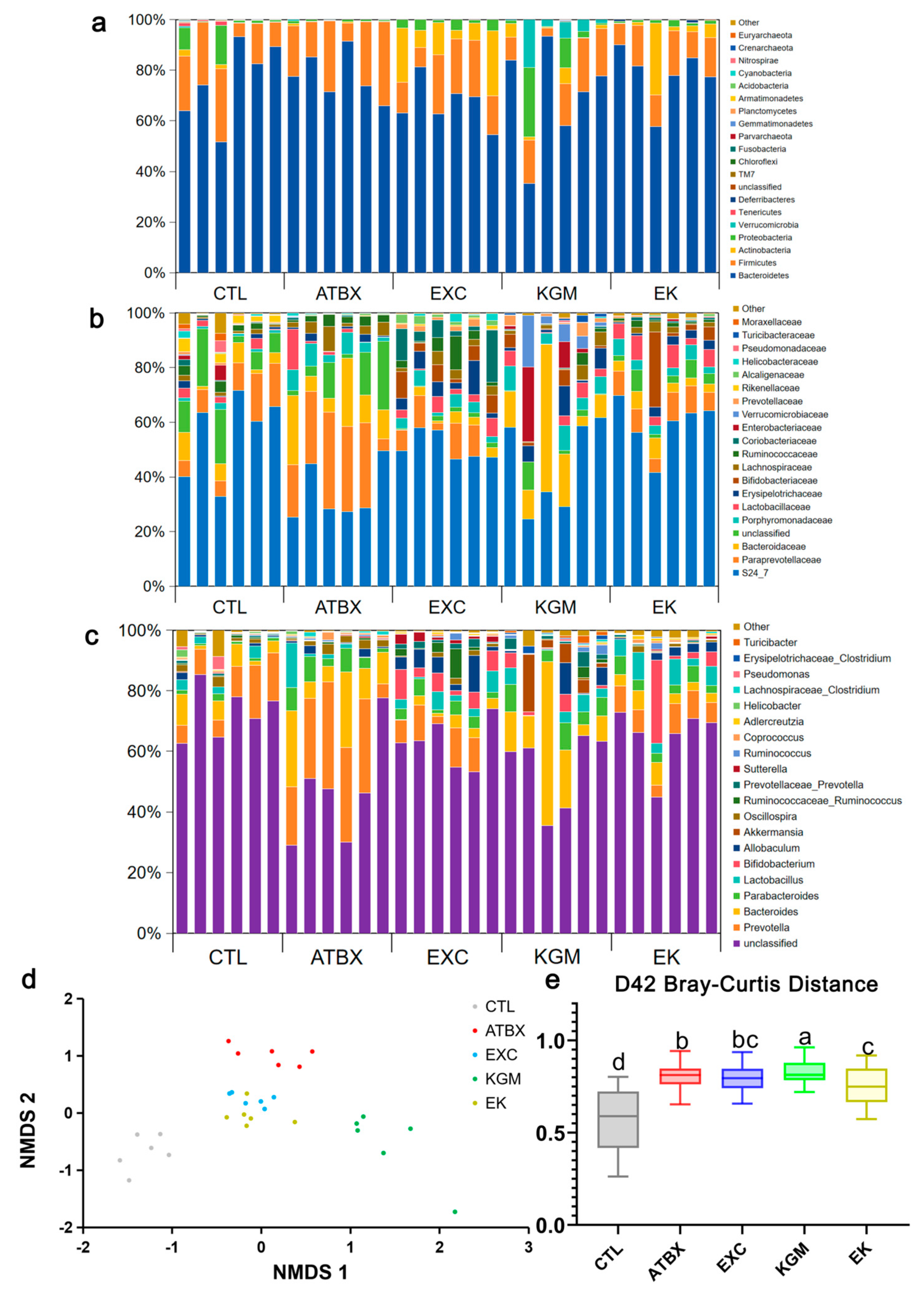
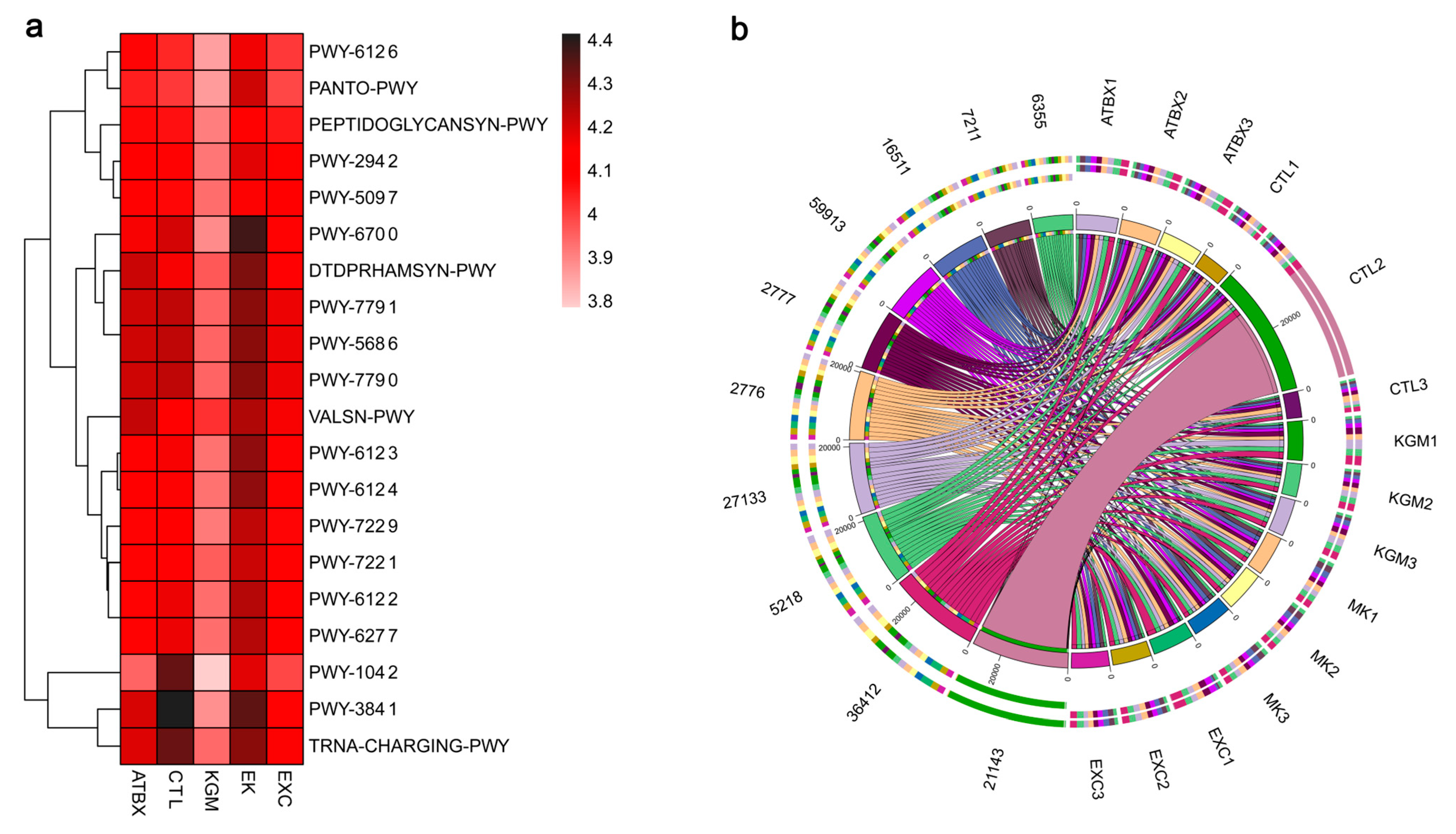
3.4. Gut Microbiome (16S rRNA Sequencing\Bioinformatic Analysis)
3.5. Gut Microbiota
3.6. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morello, W.; Baskin, E.; Jankauskiene, A.; Yalcinkaya, F.; Zurowska, A.; Puccio, G.; Serafinelli, J.; La Manna, A.; Krzemień, G.; Pennesi, M.; et al. Antibiotic prophylaxis in infants with grade III, IV, or V vesicoureteral reflux. N. Engl. J. Med. 2023, 389, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wei, L.; Li, H.; Zhang, M.; Cao, B.; Bian, J.; Zhan, S. Appropriateness of antibiotic prescriptions in ambulatory care in China: A nationwide descriptive database study. Lancet Infect. Dis. 2021, 21, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Zven, S.; Susi, A.; Nylund, C. Multiple Classes of Antibiotic Use in Infancy and Allergic Disease in Childhood-Reply. JAMA Pediatr. 2020, 174, 1003–1004. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Tiffany, C.R.; Mahan, S.P.; Kellom, M.; Rogers, A.W.L.; Nguyen, H.; Stevens, E.T.; Masson, H.L.P.; Yamazaki, K.; Marco, M.L.; et al. High fat intake sustains sorbitol intolerance after antibiotic-mediated Clostridia depletion from the gut microbiota. Cell 2024, 187, 1191–1205.e15. [Google Scholar] [CrossRef]
- Fishbein, S.R.S.; Mahmud, B.; Dantas, G. Antibiotic perturbations to the gut microbiome. Nat. Rev. Microbiol. 2023, 21, 772–788. [Google Scholar] [CrossRef]
- Hempel, S.; Newberry, S.J.; Maher, A.R.; Wang, Z.; Miles, J.N.; Shanman, R.; Johnsen, B.; Shekelle, P.G. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: A systematic review and meta-analysis. JAMA 2012, 307, 1959–1969. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Antibiotic-therapy-induced gut dysbiosis affecting gut microbiota-brain axis and cognition: Restoration by intake of probiotics and synbiotics. Int. J. Mol. Sci. 2023, 24, 3074. [Google Scholar] [CrossRef]
- Mao, Y.H.; Xu, Y.; Song, F.; Wang, Z.M.; Li, Y.H.; Zhao, M.; He, F.; Tian, Z.; Yang, Y. Protective effects of konjac glucomannan on gut microbiome with antibiotic perturbation in mice. Carbohydr. Polym. 2022, 290, 119476. [Google Scholar] [CrossRef]
- Conroy, G. Why is exercise good for you? Scientists are finding answers in our cells. Nature 2024, 629, 26–28. [Google Scholar] [CrossRef]
- Kamal, F.D.; Dagar, M.; Reza, T.; Karim Mandokhail, A.; Bakht, D.; Shahzad, M.W.; Silloca-Cabana, E.O.; Mohsin, S.N.; Chilla, S.P.; Bokhari, S.F.H. Beyond Diet and Exercise: The impact of gut microbiota on control of obesity. Cureus 2023, 15, e49339. [Google Scholar] [CrossRef] [PubMed]
- Wegierska, A.E.; Charitos, I.A.; Topi, S.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The connection between physical exercise and gut microbiota: Implications for competitive sports athletes. Sports Med. 2022, 52, 2355–2369. [Google Scholar] [CrossRef]
- Eglseer, D.; Traxler, M.; Embacher, S.; Reiter, L.; Schoufour, J.D.; Weijs, P.J.M.; Voortman, T.; Boirie, Y.; Cruz-Jentoft, A.; Bauer, S.; et al. Nutrition and Exercise Interventions to Improve Body Composition for Persons with Overweight or Obesity Near Retirement Age: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 516–538. [Google Scholar] [CrossRef] [PubMed]
- de Geus, M.; Dam, M.; Visser, W.J.; Ipema, K.J.R.; de Mik-van Egmond, A.M.E.; Tieland, M.; Weijs, P.J.M.; Kruizenga, H.M. The impact of combined nutrition and exercise interventions in patients with chronic kidney disease. Nutrients 2024, 16, 406. [Google Scholar] [CrossRef] [PubMed]
- Brauwers, B.; Machado, F.V.C.; Beijers, R.J.H.C.G.; Spruit, M.A.; Franssen, F.M.E. Combined exercise training and nutritional interventions or pharmacological treatments to improve exercise capacity and body composition in chronic obstructive pulmonary disease: A narrative review. Nutrients 2023, 15, 5136. [Google Scholar] [CrossRef]
- Guo, S.; Huang, Y.; Zhang, Y.; Huang, H.; Hong, S.; Liu, T. Impacts of exercise interventions on different diseases and organ functions in mice. J. Sport. Health Sci. 2020, 9, 53–73. [Google Scholar] [CrossRef]
- Xu, L.; Li, M.; Wei, A.; Yang, M.; Li, C.; Liu, R.; Zheng, Y.; Chen, Y.; Wang, Z.; Wang, K.; et al. Treadmill exercise promotes E3 ubiquitin ligase to remove amyloid β and P-tau and improve cognitive ability in APP/PS1 transgenic mice. J. Neuroinflamm. 2022, 19, 243. [Google Scholar] [CrossRef]
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome potentiates endurance exercise through intestinal acetate production. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E956–E966. [Google Scholar] [CrossRef]
- Bi, P.; Yue, F.; Sato, Y.; Wirbisky, S.; Liu, W.; Shan, T.; Wen, Y.; Zhou, D.; Freeman, J.; Kuang, S. Stage-specific effects of Notch activation during skeletal myogenesis. Elife 2016, 5, e17355. [Google Scholar] [CrossRef]
- Mao, Y.H.; Wang, M.; Yuan, Y.; Yan, J.K.; Peng, Y.; Xu, G.; Weng, X. Konjac glucomannan counteracted the side effects of excessive exercise on gut microbiome, endurance, and strength in an overtraining mice model. Nutrients 2023, 15, 4206. [Google Scholar] [CrossRef]
- Chen, Z.H.; Yuan, X.H.; Tu, T.T.; Wang, L.; Mao, Y.H.; Luo, Y.; Qiu, S.Y.; Song, A.X. Characterization and prebiotic potential of polysaccharides from Rosa roxburghii Tratt pomace by ultrasound-assisted extraction. Int. J. Biol. Macromol. 2024, 268 Pt 2, 131910. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhou, X.; Wang, C.; Zhang, J.; Chang, D.; Liu, W.; Zhu, M.; Zhuang, S.; Shi, H.; Wang, X.; et al. Effect of tanshinone IIA on gut microbiome in diabetes-induced cognitive impairment. Front. Pharmacol. 2022, 13, 890444. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, X.; Lin, Q.; Cai, J.; Tang, L.; Liang, Y. Active peptide KF-8 from rice bran attenuates oxidative stress in a mouse model of aging induced by d-Galactose. J. Agric. Food Chem. 2020, 68, 12271–12283. [Google Scholar] [CrossRef]
- Simpson, A.M.R.; De Souza, M.J.; Damani, J.; Rogers, C.J.; Williams, N.I.; Weaver, C.M.; Ferruzzi, M.G.; Nakatsu, C.H. Gut microbes differ in postmenopausal women responding to prunes to maintain hip bone mineral density. Front. Nutr. 2024, 11, 1389638. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.H.; Xu, Y.X.; Li, Y.H.; Cao, J.; Song, F.L.; Zhao, D.; Zhao, Y.; Wang, Z.M.; Yang, Y. Effects of konjac glucomannan with different molecular weights on gut microflora with antibiotic perturbance in in vitro fecal fermentation. Carbohydr. Polym. 2021, 273, 118546. [Google Scholar] [CrossRef] [PubMed]
- Tordoff, M.G.; Bachmanov, A.A.; Reed, D.R. Forty mouse strain survey of water and sodium intake. Physiol. Behav. 2007, 91, 620–631. [Google Scholar] [CrossRef]
- Ng, K.M.; Aranda-Díaz, A.; Tropini, C.; Frankel, M.R.; Van Treuren, W.; O’Loughlin, C.T.; Merrill, B.D.; Yu, F.B.; Pruss, K.M.; Oliveira, R.A.; et al. Recovery of the gut microbiota after antibiotics depends on host diet, community context, and environmental reservoirs. Cell Host Microbe 2019, 26, 650–665.e4. [Google Scholar] [CrossRef]
- Duan, H.; Yu, L.; Tian, F.; Zhai, Q.; Fan, L.; Chen, W. Antibiotic-induced gut dysbiosis and barrier disruption and the potential protective strategies. Crit. Rev. Food Sci. Nutr. 2022, 62, 1427–1452. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4554–4561. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Ben-Zeev Brik, R.; Federici, S.; et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 2018, 174, 1406–1423.e16. [Google Scholar] [CrossRef]
- McKee, L.S.; La Rosa, S.L.; Westereng, B.; Eijsink, V.G.; Pope, P.B.; Larsbrink, J. Polysaccharide degradation by the Bacteroidetes: Mechanisms and nomenclature. Environ. Microbiol. Rep. 2021, 13, 559–581. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Tillotson, G.; MacKenzie, T.N.; Warren, C.A.; Wexler, H.M.; Goldstein, E.J.C. Bacteroides and related species: The keystone taxa of the human gut microbiota. Anaerobe 2024, 85, 102819. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Ablitip, A.; Wang, R.; Luciana, T.; Wei, M.; Ma, X. Effects of exercise on gut microbiota of adults: A systematic review and meta-analysis. Nutrients 2024, 16, 1070. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- ter Steege, R.W.; Kolkman, J.J. Review article: The pathophysiology and management of gastrointestinal symptoms during physical exercise, and the role of splanchnic blood flow. Aliment. Pharmacol. Ther. 2012, 35, 516–528. [Google Scholar] [CrossRef]
- You, M. Role of physical activity in the prevention and treatment of influenza: A review. Sports Med. Open 2023, 9, 115. [Google Scholar] [CrossRef]
- Pereira, T.C.R.; Fidale, T.M.; Guimarães, L.C.; Deconte, S.R.; Herrera, G.C.; Mundim, A.V.; de Sales Cabral, E.; Lopes, P.R.; de Souza, F.R.; de Ulhôa Rocha Júnior, L.D.; et al. Cardioprotective effects of the 4-week aerobic running exercises before treatment with doxorubicin in rats. Cardiovasc. Toxicol. 2023, 23, 265–277. [Google Scholar] [CrossRef]
- Santos-Alves, E.; Rizo-Roca, D.; Marques-Aleixo, I.; Coxito, P.; Martins, S.; Guimarães, J.T.; Oliveira, P.J.; Torrella, J.R.; Magalhães, J.; Ascensão, A. Physical exercise positively modulates DOX-induced hepatic oxidative stress, mitochondrial dysfunction and quality control signaling. Mitochondrion 2019, 47, 103–113. [Google Scholar] [CrossRef]
- Boeno, F.P.; Patel, J.; Montalvo, R.N.; Lapierre-Nguyen, S.S.; Schreiber, C.M.; Smuder, A.J. Effects of exercise preconditioning on doxorubicin-induced liver and kidney toxicity in male and female rats. Int. J. Mol. Sci. 2023, 24, 10222. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Yang, Y.; Wang, Z.; Wang, B.; Zhang, B.; Yu, J.; Lu, W.; Pan, M.; Zhao, J.; et al. The diversity of gut microbiota in type 2 diabetes with or without cognitive impairment. Aging Clin. Exp. Res. 2021, 33, 589–601. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, R.; McCormick, K.L.; Zhang, Y.; Lin, X.; Yang, X. The role of the gut microbiota on the metabolic status of obese children. Microb. Cell Fact. 2021, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Karamzin, A.M.; Ropot, A.V.; Sergeyev, O.V.; Khalturina, E.O. Akkermansia muciniphila and host interaction within the intestinal tract. Anaerobe 2021, 72, 102472. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Garcia-Weber, D.; Lashermes, A.; Larraufie, P.; Marinelli, L.; Teixeira, V.; Rolland, A.; Béguet-Crespel, F.; Brochard, V.; Quatremare, T.; et al. Akkermansia muciniphila upregulates genes involved in maintaining the intestinal barrier function via ADP-heptose-dependent activation of the ALPK1/TIFA pathway. Gut Microbes 2022, 14, 2110639. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Zhai, X.; Lin, D.; Zhao, Y.; Li, W.; Yang, X. Effects of dietary fiber supplementation on fatty acid metabolism and intestinal microbiota diversity in C57BL/6J mice fed with a high-fat diet. J. Agric. Food Chem. 2018, 66, 12706–12718. [Google Scholar] [CrossRef]
- Mao, Y.H.; Song, A.X.; Yao, Z.P.; Wu, J.Y. Protective effects of natural and partially degraded konjac glucomannan on Bifidobacteria against antibiotic damage. Carbohydr. Polym. 2018, 181, 368–375. [Google Scholar] [CrossRef]
- Bi, L.; Triadafilopoulos, G. Exercise and gastrointestinal function and disease: An evidence-based review of risks and benefits. Clin. Gastroenterol. Hepatol. 2003, 1, 345–355. [Google Scholar] [CrossRef]
- Kobayashi, S.; Morino, K.; Okamoto, T.; Tanaka, M.; Ida, S.; Ohashi, N.; Murata, K.; Yanagimachi, T.; Sakai, J.; Maegawa, H.; et al. Acetate derived from the intestinal tract has a critical role in maintaining skeletal muscle mass and strength in mice. Physiol. Rep. 2024, 12, e16047. [Google Scholar] [CrossRef]
- Sakakibara, I.; Fujino, T.; Ishii, M.; Tanaka, T.; Shimosawa, T.; Miura, S.; Zhang, W.; Tokutake, Y.; Yamamoto, J.; Awano, M.; et al. Fasting-induced hypothermia and reduced energy production in mice lacking acetyl-CoA synthetase 2. Cell Metab. 2009, 9, 191–202. [Google Scholar] [CrossRef]
- Cammann, D.; Lu, Y.; Cummings, M.J.; Zhang, M.L.; Cue, J.M.; Do, J.; Ebersole, J.; Chen, X.; Oh, E.C.; Cummings, J.L.; et al. Genetic correlations between Alzheimer’s disease and gut microbiome genera. Sci. Rep. 2023, 13, 5258. [Google Scholar] [CrossRef]
- Bordin, D.; Livzan, M. History of chronic gastritis: How our perceptions have changed. World J. Gastroenterol. 2024, 30, 1851–1858. [Google Scholar] [CrossRef]
- Kasahara, K.; Kerby, R.L.; Zhang, Q.; Pradhan, M.; Mehrabian, M.; Lusis, A.J.; Bergström, G.; Bäckhed, F.; Rey, F.E. Gut bacterial metabolism contributes to host global purine homeostasis. Cell Host Microbe 2023, 31, 1038–1053.e10. [Google Scholar] [CrossRef]
- Liu, X.; Ke, L.; Lei, K.; Yu, Q.; Zhang, W.; Li, C.; Tian, Z. Antibiotic-induced gut microbiota dysbiosis has a functional impact on purine metabolism. BMC Microbiol. 2023, 23, 187. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.W.; Hsieh, S.H.; Chen, J.F.; Liu, C.R.; Chen, C.W.; Huang, Y.F.; Ho, H.H. Lactobacillus reuteri TSR332 and Lactobacillus fermentum TSF331 stabilize serum uric acid levels and prevent hyperuricemia in rats. PeerJ 2021, 9, e11209. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zeng, Y.; Wang, R.; Pang, J.; Wang, X.; Pan, Z.; Jin, Y.; Chen, Y.; Yang, Y.; Ling, W. Resveratrol improves hyperuricemia and ameliorates renal injury by modulating the gut microbiota. Nutrients 2024, 16, 1086. [Google Scholar] [CrossRef] [PubMed]
- Moradell, A.; Casajús, J.A.; Moreno, L.A.; Vicente-Rodríguez, G.; Gómez-Cabello, A. Effects of diet-exercise interaction on human health across a lifespan. Nutrients 2023, 15, 2520. [Google Scholar] [CrossRef]
- Lopatkin, A.J.; Yang, J.H. Digital insights into nucleotide metabolism and antibiotic treatment failure. Front. Digit. Health 2021, 3, 583468. [Google Scholar] [CrossRef]




| Week | Velocity (m/min) | Duration (min) |
|---|---|---|
| 1 | 13 | 30 |
| 2, 3 | 13 | 40 |
| 4, 5, 6 | 14 | 40 |
| Groups. | Chao1 | Faith_pd | Observed_otus | Shannon | Simpson |
|---|---|---|---|---|---|
| CTL | 224 ± 43.67 a | 20.16 ± 4.98 a | 224 ± 43.67 a | 4.62 ± 1.19 | 0.86 ± 0.17 |
| ATBX | 156.68 ± 28.12 b | 12.81 ± 1.27 bc | 156.68 ± 28.12 b | 4.22 ± 0.57 | 0.88 ± 0.04 |
| EXC | 147.17 ± 35.05 b | 13.47 ± 1.76 bc | 147.17 ± 35.05 b | 5.14 ± 0.40 | 0.95 ± 0.01 |
| KGM | 96.83 ± 27.78 c | 10.55 ± 2.13 c | 96.83 ± 27.78 c | 4.19 ± 0.84 | 0.89 ± 0.09 |
| EK | 183 ± 32.54 a | 15.75 ± 2.14 b | 183 ± 32.54 a | 4.85 ± 0.39 | 0.93 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Chen, Y.; Song, A.-X.; Weng, X.; Meng, Y.; Lin, J.; Mao, Y.-H. The Combination of Exercise and Konjac Glucomannan More Effectively Prevents Antibiotics-Induced Dysbiosis in Mice Compared with Singular Intervention. Nutrients 2024, 16, 2942. https://doi.org/10.3390/nu16172942
Wang M, Chen Y, Song A-X, Weng X, Meng Y, Lin J, Mao Y-H. The Combination of Exercise and Konjac Glucomannan More Effectively Prevents Antibiotics-Induced Dysbiosis in Mice Compared with Singular Intervention. Nutrients. 2024; 16(17):2942. https://doi.org/10.3390/nu16172942
Chicago/Turabian StyleWang, Minghan, Yonglin Chen, Ang-Xin Song, Xiquan Weng, Yan Meng, Jieru Lin, and Yu-Heng Mao. 2024. "The Combination of Exercise and Konjac Glucomannan More Effectively Prevents Antibiotics-Induced Dysbiosis in Mice Compared with Singular Intervention" Nutrients 16, no. 17: 2942. https://doi.org/10.3390/nu16172942
APA StyleWang, M., Chen, Y., Song, A.-X., Weng, X., Meng, Y., Lin, J., & Mao, Y.-H. (2024). The Combination of Exercise and Konjac Glucomannan More Effectively Prevents Antibiotics-Induced Dysbiosis in Mice Compared with Singular Intervention. Nutrients, 16(17), 2942. https://doi.org/10.3390/nu16172942






