Dietary Patterns, Gut Microbiota and Sports Performance in Athletes: A Narrative Review
Abstract
1. Introduction
2. The Overview of Gut Microbiota
2.1. Gut Microbiota
2.2. The Main Function of Gut Microbiota on Health
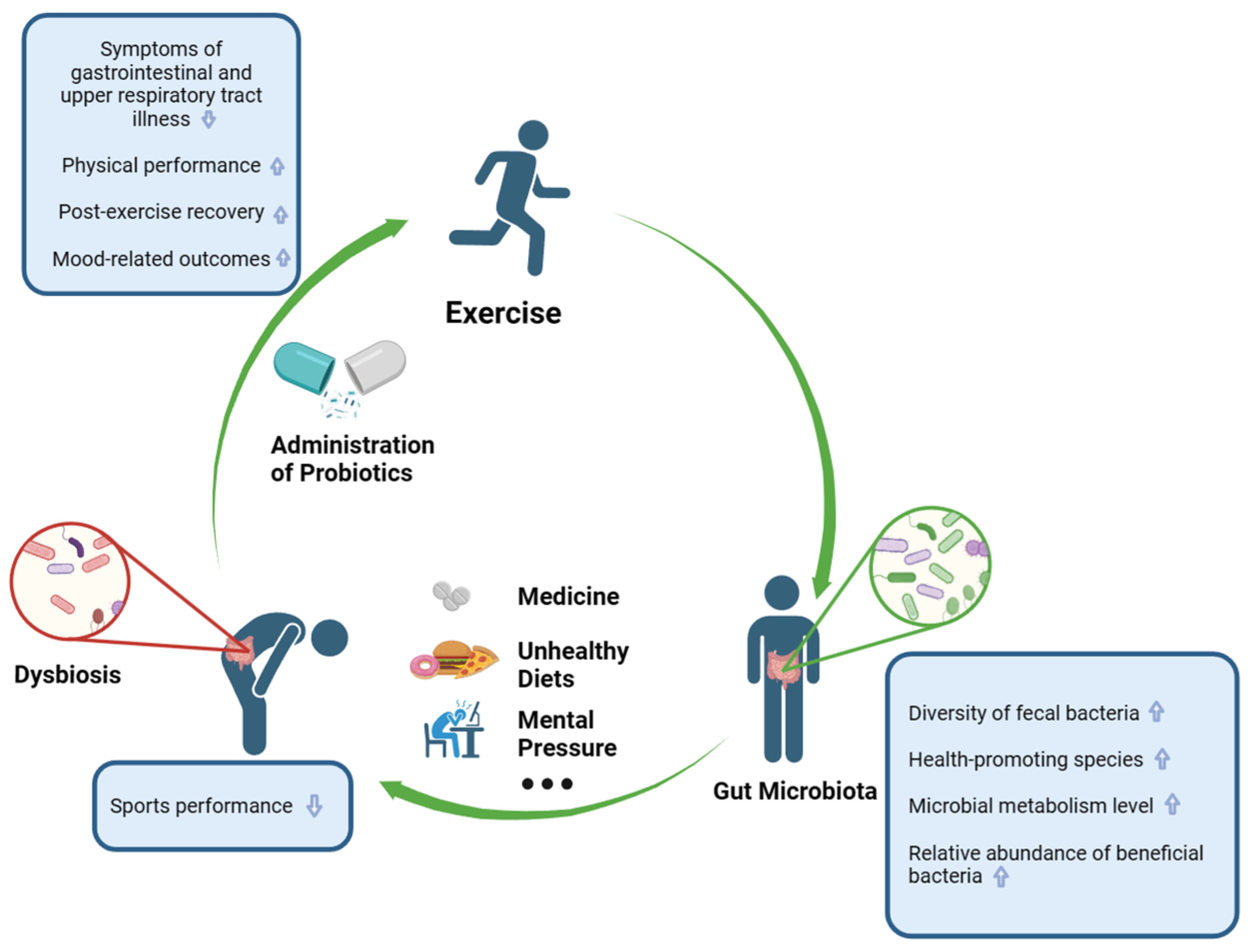
3. The Relation between Gut Microbiota and Exercise
3.1. Gut Microbiota in Athletes
3.2. Impact of Exercise Interventions on Gut Microbiota
3.3. The Influence of Gut Microbiota on Sports Performance
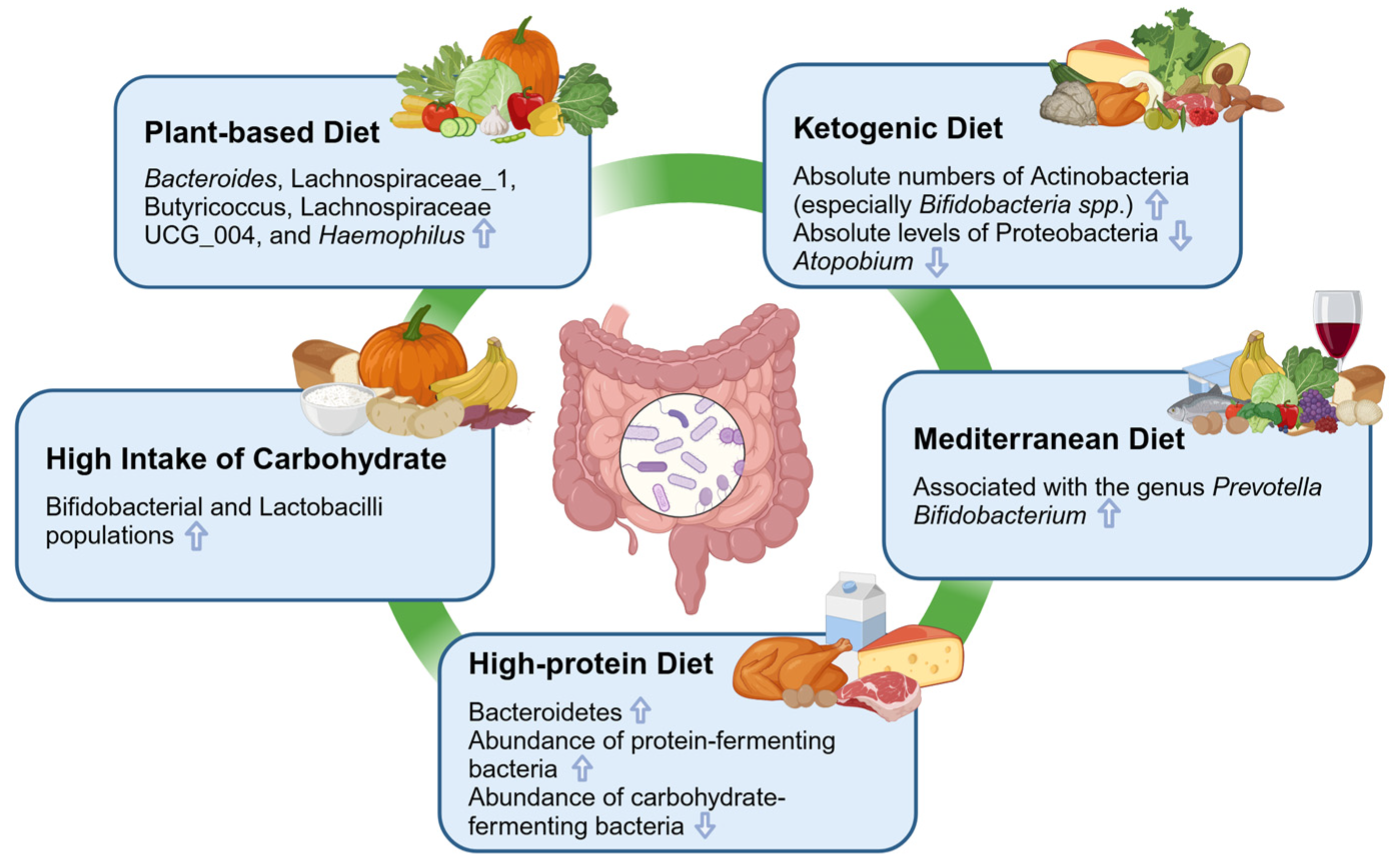
4. The Influence of Several Typical Dietary Patterns on the Gut Microbiota
4.1. Ketogenic Diet
4.2. Plant-Based Diet
4.3. High-Protein Diet
4.4. Mediterranean Diet
4.5. High Intake of Carbohydrate
5. Different Dietary Patterns and Sports Performance—Gut Microbiota as the Mediator
5.1. Gut Microbiota as the Mediator
5.2. Practical Application
6. Conclusions
- Key Points
- The interactions between exercise and the gut microbiota play a role in the sports performance of athletes.
- The ketogenic diet, plant-based diet, high-protein diet, and Mediterranean diet may improve sports performance from different aspects.
- The gut microbiota and its metabolites play an important role in the effects of dietary patterns on sports performance.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burke, L.M.; Read, R.S. Sports Nutrition. Approaching the Nineties. Sports Med. 1989, 8, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Kiens, B.; Ivy, J.L. Carbohydrates and Fat for Training and Recovery. J. Sports Sci. 2004, 22, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M.; Nguyen, C.; Shetty, M.; Oppezzo, M.; Barrack, M.; Fredericson, M. Popular Dietary Trends’ Impact on Athletic Performance: A Critical Analysis Review. Nutrients 2023, 15, 3511. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, A.; Matu, J.; Whyte, E.; Akin-Nibosun, P.; Clifford, T.; Stevenson, E.; Shannon, O.M. The Mediterranean Dietary Pattern for Optimising Health and Performance in Competitive Athletes: A Narrative Review. Br. J. Nutr. 2022, 128, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C. Vegetarian Dietary Practices and Endurance Performance. Am. J. Clin. Nutr. 1988, 48, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Marttinen, M.; Ala-Jaakkola, R.; Laitila, A.; Lehtinen, M.J. Gut Microbiota, Probiotics and Physical Performance in Athletes and Physically Active Individuals. Nutrients 2020, 12, 2936. [Google Scholar] [CrossRef] [PubMed]
- Bedu-Ferrari, C.; Biscarrat, P.; Langella, P.; Cherbuy, C. Prebiotics and the Human Gut Microbiota: From Breakdown Mechanisms to the Impact on Metabolic Health. Nutrients 2022, 14, 2096. [Google Scholar] [CrossRef] [PubMed]
- Whitman, J.A.; Doherty, L.A.; Pantoja-Feliciano de Goodfellow, I.G.; Racicot, K.; Anderson, D.J.; Kensil, K.; Karl, J.P.; Gibson, G.R.; Soares, J.W. In Vitro Fermentation Shows Polyphenol and Fiber Blends Have an Additive Beneficial Effect on Gut Microbiota States. Nutrients 2024, 16, 1159. [Google Scholar] [CrossRef] [PubMed]
- Mancin, L.; Amatori, S.; Caprio, M.; Sattin, E.; Bertoldi, L.; Cenci, L.; Sisti, D.; Bianco, A.; Paoli, A. Effect of 30 Days of Ketogenic Mediterranean Diet with Phytoextracts on Athletes’ Gut Microbiome Composition. Front. Nutr. 2022, 9, 979651. [Google Scholar] [CrossRef]
- Willems, M.E.T.; Blacker, S.D. Anthocyanin-Rich Supplementation: Emerging Evidence of Strong Potential for Sport and Exercise Nutrition. Front. Nutr. 2022, 9, 864323. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Matijašić, M.; Meštrović, T.; Čipčić Paljetak, H.; Perić, M.; Barešić, A.; Verbanac, D. Gut Microbiota beyond Bacteria—Mycobiome, Virome, Archaeome, and Eukaryotic Parasites in IBD. Int. J. Mol. Sci. 2020, 21, 2668. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef]
- Santacroce, L.; Man, A.; Charitos, I.A.; Haxhirexha, K.; Topi, S. Current Knowledge about the Connection between Health Status and Gut Microbiota from Birth to Elderly. A Narrative Review. Front. Biosci. Landmark Ed. 2021, 26, 135–148. [Google Scholar]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; Pace, N.R. Gastrointestinal Microbiology Enters the Metagenomics Era. Curr. Opin. Gastroenterol. 2008, 24, 4. [Google Scholar] [CrossRef]
- Sung, J.; Kim, N.; Kim, J.; Jo, H.J.; Park, J.H.; Nam, R.H.; Seok, Y.-J.; Kim, Y.-R.; Lee, D.H.; Jung, H.C. Comparison of Gastric Microbiota Between Gastric Juice and Mucosa by Next Generation Sequencing Method. J. Cancer Prev. 2016, 21, 60–65. [Google Scholar] [CrossRef]
- Nardone, G.; Compare, D. The Human Gastric Microbiota: Is It Time to Rethink the Pathogenesis of Stomach Diseases? United Eur. Gastroenterol. J. 2015, 3, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Wegierska, A.E.; Charitos, I.A.; Topi, S.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The Connection Between Physical Exercise and Gut Microbiota: Implications for Competitive Sports Athletes. Sports Med. 2022, 52, 2355–2369. [Google Scholar] [CrossRef]
- Fouhy, F.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C.; Cotter, P.D. Composition of the Early Intestinal Microbiota: Knowledge, Knowledge Gaps and the Use of High-Throughput Sequencing to Address These Gaps. Gut Microbes 2012, 3, 203–220. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, W.; Schink, M.; Zopf, Y. Microbiota in the Gastrointestinal Tract. Med. Sci. 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Bäckhed, F. The Gut Microbiota—Masters of Host Development and Physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Blaser, M.J. The Human Microbiome: At the Interface of Health and Disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- O’Hara, A.M.; Shanahan, F. The Gut Flora as a Forgotten Organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C. “Gut Health”: A New Objective in Medicine? BMC Med. 2011, 9, 24. [Google Scholar] [CrossRef]
- Santacroce, L.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Luperto, P.; De Nitto, E.; Topi, S. The Human Respiratory System and Its Microbiome at a Glimpse. Biology 2020, 9, 318. [Google Scholar] [CrossRef]
- Alam, A.; Neish, A. Role of Gut Microbiota in Intestinal Wound Healing and Barrier Function. Tissue Barriers 2018, 6, 1539595. [Google Scholar] [CrossRef] [PubMed]
- Wegienka, G.; Havstad, S.; Zoratti, E.M.; Woodcroft, K.J.; Bobbitt, K.R.; Ownby, D.R.; Johnson, C.C. Regulatory T Cells in Prenatal Blood Samples: Variability with Pet Exposure and Sensitization. J. Reprod. Immunol. 2009, 81, 74–81. [Google Scholar] [CrossRef]
- Santacroce, L.; Sardaro, N.; Topi, S.; Pettini, F.; Bottalico, L.; Cantore, S.; Cascella, G.; Del Prete, R.; Dipalma, G.; Inchingolo, F. The Pivotal Role of Oral Microbiota in Health and Disease. J. Biol. Regul. Homeost. Agents 2020, 34, 733–737. [Google Scholar] [PubMed]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 91, 1835. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Reddy, D.N. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Hawrelak, J.A.; Myers, S.P. The Causes of Intestinal Dysbiosis: A Review. Altern. Med. Rev. 2004, 9, 180–197. [Google Scholar]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [Google Scholar]
- Almeida, A.; Mitchell, A.L.; Boland, M.; Forster, S.C.; Gloor, G.B.; Tarkowska, A.; Lawley, T.D.; Finn, R.D. A New Genomic Blueprint of the Human Gut Microbiota. Nature 2019, 568, 499–504. [Google Scholar] [CrossRef]
- Tamboli, C.P.; Neut, C.; Desreumaux, P.; Colombel, J.F. Dysbiosis in Inflammatory Bowel Disease. Gut 2004, 53, 1–4. [Google Scholar] [CrossRef]
- Perler, B.K.; Friedman, E.S.; Wu, G.D. The Role of the Gut Microbiota in the Relationship Between Diet and Human Health. Annu. Rev. Physiol. 2023, 85, 449–468. [Google Scholar] [CrossRef]
- Purdel, C.; Margină, D.; Adam-Dima, I.; Ungurianu, A. The Beneficial Effects of Dietary Interventions on Gut Microbiota-An Up-to-Date Critical Review and Future Perspectives. Nutrients 2023, 15, 5005. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.L.; Holscher, H.D. Fueling Gut Microbes: A Review of the Interaction between Diet, Exercise, and the Gut Microbiota in Athletes. Adv. Nutr. 2021, 12, 2190–2215. [Google Scholar] [CrossRef] [PubMed]
- Ping, Z. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 231, 9588. [Google Scholar]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and Associated Dietary Extremes Impact on Gut Microbial Diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.M.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G.M. Community Characteristics of the Gut Microbiomes of Competitive Cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.-D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-Omics Analysis of Elite Athletes Identifies a Performance-Enhancing Microbe That Functions via Lactate Metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The Microbiome of Professional Athletes Differs from That of More Sedentary Subjects in Composition and Particularly at the Functional Metabolic Level. Gut 2018, 67, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, D.; Bressa, C.; Bailén, M.; Hamed-Bousdar, S.; Naclerio, F.; Carmona, M.; Pérez, M.; González-Soltero, R.; Montalvo-Lominchar, M.G.; Carabaña, C.; et al. Effect of a Protein Supplement on the Gut Microbiota of Endurance Athletes: A Randomized, Controlled, Double-Blind Pilot Study. Nutrients 2018, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Jang, L.-G.; Choi, G.; Kim, S.-W.; Kim, B.-Y.; Lee, S.; Park, H. The Combination of Sport and Sport-Specific Diet Is Associated with Characteristics of Gut Microbiota: An Observational Study. J. Int. Soc. Sports Nutr. 2019, 16, 21. [Google Scholar] [CrossRef]
- Munukka, E.; Ahtiainen, J.P.; Puigbó, P.; Jalkanen, S.; Pahkala, K.; Keskitalo, A.; Kujala, U.M.; Pietilä, S.; Hollmén, M.; Elo, L.; et al. Six-Week Endurance Exercise Alters Gut Metagenome That Is Not Reflected in Systemic Metabolism in Over-Weight Women. Front. Microbiol. 2018, 9, 2323. [Google Scholar] [CrossRef]
- Fernández, J.; Fernández-Sanjurjo, M.; Iglesias-Gutiérrez, E.; Martínez-Camblor, P.; Villar, C.J.; Tomás-Zapico, C.; Fernández-García, B.; Lombó, F. Resistance and Endurance Exercise Training Induce Differential Changes in Gut Microbiota Composition in Murine Models. Front. Physiol. 2021, 12, 748854. [Google Scholar] [CrossRef] [PubMed]
- Rohwer, N.; El Hage, R.; Smyl, C.; Ocvirk, S.; Goris, T.; Grune, T.; Swidsinski, A.; Weylandt, K.-H. Ketogenic Diet Has Moderate Effects on the Fecal Microbiota of Wild-Type Mice. Nutrients 2023, 15, 4629. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhong, F.; Zheng, X.; Lai, H.Y.; Wu, C.; Huang, C. Disparity of Gut Microbiota Composition Among Elite Athletes and Young Adults with Different Physical Activity Independent of Dietary Status: A Matching Study. Front. Nutr. 2022, 9, 843076. [Google Scholar] [CrossRef]
- Hintikka, J.E.; Munukka, E.; Valtonen, M.; Luoto, R.; Ihalainen, J.K.; Kallonen, T.; Waris, M.; Heinonen, O.J.; Ruuskanen, O.; Pekkala, S. Gut Microbiota and Serum Metabolome in Elite Cross-Country Skiers: A Controlled Study. Metabolites 2022, 12, 335. [Google Scholar] [CrossRef]
- Humińska-Lisowska, K.; Zielińska, K.; Mieszkowski, J.; Michałowska-Sawczyn, M.; Cięszczyk, P.; Łabaj, P.P.; Wasąg, B.; Frączek, B.; Grzywacz, A.; Kochanowicz, A.; et al. Microbiome Features Associated with Performance Measures in Athletic and Non-Athletic Individuals: A Case-Control Study. PLoS ONE 2024, 19, e0297858. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia Muciniphila and Improved Metabolic Health during a Dietary Intervention in Obesity: Relationship with Gut Microbiome Richness and Ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.S.; Morris, M.M.; Morrow, C.D.; Novak, J.R.; Roberts, M.D.; Frugé, A.D. Associations between Changes in Fat-Free Mass, Fecal Microbe Diversity, and Mood Disturbance in Young Adults after 10-Weeks of Resistance Training. Microorganisms 2022, 10, 2344. [Google Scholar] [CrossRef]
- Dupuit, M.; Rance, M.; Morel, C.; Bouillon, P.; Boscaro, A.; Martin, V.; Vazeille, E.; Barnich, N.; Chassaing, B.; Boisseau, N. Effect of Concurrent Training on Body Composition and Gut Microbiota in Postmenopausal Women with Overweight or Obesity. Med. Sci. Sports Exerc. 2022, 54, 517–529. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Chiu, C.C.; Li, Y.P.; Huang, W.C.; Huang, Y.T.; Huang, C.C.; Chuang, H.L. Effect of Intestinal Microbiota on Exercise Performance in Mice. J. Strength Cond. Res. 2015, 29, 552. [Google Scholar] [CrossRef]
- Coffey, V.G.; Hawley, J.A. Concurrent Exercise Training: Do Opposites Distract? J. Physiol. 2017, 595, 2883–2896. [Google Scholar] [CrossRef] [PubMed]
- West, N.P.; Horn, P.L.; Pyne, D.B.; Gebski, V.J.; Lahtinen, S.J.; Fricker, P.A.; Cripps, A.W. Probiotic Supplementation for Respiratory and Gastrointestinal Illness Symptoms in Healthy Physically Active Individuals. Clin. Nutr. 2014, 33, 581–587. [Google Scholar] [CrossRef]
- Huang, W.-C.; Lee, M.-C.; Lee, C.-C.; Ng, K.-S.; Hsu, Y.-J.; Tsai, T.-Y.; Young, S.-L.; Lin, J.-S.; Huang, C.-C. Effect of Lactobacillus Plantarum TWK10 on Exercise Physiological Adaptation, Performance, and Body Composition in Healthy Humans. Nutrients 2019, 11, 2836. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Wei, C.-C.; Huang, C.-C.; Chen, W.-L.; Huang, H.-Y. The Beneficial Effects of Lactobacillus Plantarum PS128 on High-Intensity, Exercise-Induced Oxidative Stress, Inflammation, and Performance in Triathletes. Nutrients 2019, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Sawada, D.; Kuwano, Y.; Tanaka, H.; Hara, S.; Uchiyama, Y.; Sugawara, T.; Fujiwara, S.; Rokutan, K.; Nishida, K. Daily Intake of Lactobacillus Gasseri CP2305 Relieves Fatigue and Stress-Related Symptoms in Male University Ekiden Runners: A Double-Blind, Randomized, and Placebo-Controlled Clinical Trial. J. Funct. Foods 2019, 57, 465–476. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Medawar, E.; Huhn, S.; Villringer, A.; Veronica Witte, A. The Effects of Plant-Based Diets on the Body and the Brain: A Systematic Review. Transl. Psychiatry 2019, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Przewłócka, K.; Folwarski, M.; Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Kaczor, J.J. Gut-Muscle AxisExists and May Affect Skeletal Muscle Adaptation to Training. Nutrients 2020, 12, 1451. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Narasimhan, S.; Marchesi, J.R.; Benson, A.; Wong, F.S.; Wen, L. Long Term Effect of Gut Microbiota Transfer on Diabetes Development. J. Autoimmun. 2014, 53, 85–94. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef]
- Cullen, A.E.; Centner, A.M.; Deitado, R.; Fernandez, J.; Salazar, G. The Impact of Dietary Supplementation of Whole Foods and Polyphenols on Atherosclerosis. Nutrients 2020, 12, 2069. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.M.; Sesso, H.D. Whole Food versus Supplement: Comparing the Clinical Evidence of Tomato Intake and Lycopene Supplementation on Cardiovascular Risk Factors. Adv. Nutr. 2014, 5, 457–485. [Google Scholar] [CrossRef] [PubMed]
- Seel, W.; Reiners, S.; Kipp, K.; Simon, M.-C.; Dawczynski, C. Role of Dietary Fiber and Energy Intake on Gut Microbiome in Vegans, Vegetarians, and Flexitarians in Comparison to Omnivores—Insights from the Nutritional Evaluation (NuEva) Study. Nutrients 2023, 15, 1914. [Google Scholar] [CrossRef]
- Szurkowska, J.; Wiącek, J.; Laparidis, K.; Karolkiewicz, J. A Comparative Study of Selected Gut Bacteria Abundance and Fecal pH in Bodybuilders Eating High-Protein Diet and More Sedentary Controls. Nutrients 2021, 13, 4093. [Google Scholar] [CrossRef] [PubMed]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef]
- Jin, Q.; Black, A.; Kales, S.N.; Vattem, D.; Ruiz-Canela, M.; Sotos-Prieto, M. Metabolomics and Microbiomes as Potential Tools to Evaluate the Effects of the Mediterranean Diet. Nutrients 2019, 11, 207. [Google Scholar] [CrossRef]
- Haro, C.; Garcia-Carpintero, S.; Alcala-Diaz, J.F.; Gomez-Delgado, F.; Delgado-Lista, J.; Perez-Martinez, P.; Rangel Zuñiga, O.A.; Quintana-Navarro, G.M.; Landa, B.B.; Clemente, J.C.; et al. The Gut Microbial Community in Metabolic Syndrome Patients Is Modified by Diet. J. Nutr. Biochem. 2016, 27, 27–31. [Google Scholar] [CrossRef]
- Odell, O.J.; Wallis, G.A. The Application of Lactose in Sports Nutrition. Int. Dairy J. 2021, 116, 104970. [Google Scholar] [CrossRef]
- Whitney, R.; Nair, R.R. Expanding Dietary Therapy Beyond the Classic Ketogenic Diet: On the Use of the Modified Atkins Diet and Low Glycemic Index Treatment in Pediatric Epilepsy. Indian Pediatr. 2021, 58, 811–812. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Guisado, J.; Muñoz-Serrano, A.; Alonso-Moraga, Á. Spanish Ketogenic Mediterranean Diet: A Healthy Cardiovascular Diet for Weight Loss. Nutr. J. 2008, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.; Likhodii, S.S.; Hatamian, M.; Burnham, W.M. Effect of the Ketogenic Diet on the Activity Level of Wistar Rats. Pediatr. Res. 2005, 57, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Tinsley, G.M.; Mattson, M.P.; De Vivo, I.; Dhawan, R.; Moro, T. Common and Divergent Molecular Mechanisms of Fasting and Ketogenic Diets. Trends Endocrinol. Metab. 2024, 35, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Antonio Paoli, A.; Mancin, L.; Caprio, M.; Monti, E.; Narici, M.V.; Cenci, L.; Piccini, F.; Pincella, M.; Grigoletto, D.; Marcolin, G. Effects of 30 Days of Ketogenic Diet on Body Composition, Muscle Strength, Muscle Area, Metabolism, and Performance in Semi-Professional Soccer Players. J. Int. Soc. Sports Nutr. 2021, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Ashtary-Larky, D.; Bagheri, R.; Asbaghi, O.; Tinsley, G.M.; Kooti, W.; Abbasnezhad, A.; Afrisham, R.; Wong, A. Effects of Resistance Training Combined with a Ketogenic Diet on Body Composition: A Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 5717–5732. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M. Ketogenic low-CHO, High-fat Diet: The Future of Elite Endurance Sport? J. Physiol. 2021, 599, 819–843. [Google Scholar] [CrossRef] [PubMed]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef]
- Cheng, L.; Jin, H.; Qiang, Y.; Wu, S.; Yan, C.; Han, M.; Xiao, T.; Yan, N.; An, H.; Zhou, X.; et al. High Fat Diet Exacerbates Dextran Sulfate Sodium Induced Colitis through Disturbing Mucosal Dendritic Cell Homeostasis. Int. Immunopharmacol. 2016, 40, 1–10. [Google Scholar] [CrossRef]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-Fat-Induced Taurocholic Acid Promotes Pathobiont Expansion and Colitis in Il10-/- Mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Wolters, M.; Ahrens, J.; Romaní-Pérez, M.; Watkins, C.; Sanz, Y.; Benítez-Páez, A.; Stanton, C.; Günther, K. Dietary Fat, the Gut Microbiota, and Metabolic Health—A Systematic Review Conducted within the MyNewGut Project. Clin. Nutr. 2019, 38, 2504–2520. [Google Scholar] [CrossRef] [PubMed]
- Rl, H. A Review of the Role of the Gut Microbiome in Personalized Sports Nutrition. Front. Nutr. 2020, 6, 504337. [Google Scholar]
- Bueno, N.B.; de Melo, I.S.V.; de Oliveira, S.L.; Ataide, T.d.R. Very-Low-Carbohydrate Ketogenic Diet v. Low-Fat Diet for Long-Term Weight Loss: A Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Grimaldi, K.; Bianco, A.; Lodi, A.; Cenci, L.; Parmagnani, A. Medium Term Effects of a Ketogenic Diet and a Mediterranean Diet on Resting Energy Expenditure and Respiratory Ratio. BMC Proc. 2012, 6, P37. [Google Scholar] [CrossRef]
- Zambrano, A.K.; Cadena-Ullauri, S.; Guevara-Ramírez, P.; Frias-Toral, E.; Ruiz-Pozo, V.A.; Paz-Cruz, E.; Tamayo-Trujillo, R.; Chapela, S.; Montalván, M.; Sarno, G.; et al. The Impact of a Very-Low-Calorie Ketogenic Diet in the Gut Microbiota Composition in Obesity. Nutrients 2023, 15, 2728. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Pugliese, G.; Salzano, C.; Savastano, S.; Colao, A. The Management of Very Low-Calorie Ketogenic Diet in Obesity Outpatient Clinic: A Practical Guide. J. Transl. Med. 2019, 17, 356. [Google Scholar] [CrossRef] [PubMed]
- Sundgot-Borgen, J.; Garthe, I. Elite Athletes in Aesthetic and Olympic Weight-Class Sports and the Challenge of Body Weight and Body Compositions. J. Sports Sci. 2011, 29 (Suppl. S1), S101–S114. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.E.; Jakicic, J.; Gunderson, S. Diet and Body Composition. Effect of Very Low Calorie Diets and Exercise. Sports Med. 1991, 12, 237–249. [Google Scholar] [CrossRef]
- Rogerson, D. Vegan Diets: Practical Advice for Athletes and Exercisers. J. Int. Soc. Sports Nutr. 2017, 14, 36. [Google Scholar] [CrossRef]
- Thomas, M.S.; Calle, M.; Fernandez, M.L. Healthy Plant-Based Diets Improve Dyslipidemias, Insulin Resistance, and Inflammation in Metabolic Syndrome. A Narrative Review. Adv. Nutr. 2023, 14, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Aidoo, R.; Abe-Inge, V.; Kwofie, E.M.; Baum, J.I.; Kubow, S. Sustainable Healthy Diet Modeling for a Plant-Based Dietary Transitioning in the United States. NPJ Sci. Food 2023, 7, 61. [Google Scholar] [CrossRef]
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and Cardiovascular Health. Int. J. Mol. Sci. 2018, 19, 3988. [Google Scholar] [CrossRef] [PubMed]
- Dawczynski, C.; Weidauer, T.; Richert, C.; Schlattmann, P.; Dawczynski, K.; Kiehntopf, M. Corrigendum: Nutrient Intake and Nutrition Status in Vegetarians and Vegans in Comparison to Omnivores-the Nutritional Evaluation (NuEva) Study. Front. Nutr. 2022, 9, 975159. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J.; Appleby, P.N.; Rosell, M.S. Health Effects of Vegetarian and Vegan Diets. Proc. Nutr. Soc. 2006, 65, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J. Nutrition Concerns and Health Effects of Vegetarian Diets. Nutr. Clin. Pract. 2010, 25, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.; Dawczynski, C.; Schwarz, M.; Maares, M.; Kipp, K.; Haase, H.; Kipp, A.P. Selenium, Zinc, and Copper Status of Vegetarians and Vegans in Comparison to Omnivores in the Nutritional Evaluation (NuEva) Study. Nutrients 2023, 15, 3538. [Google Scholar] [CrossRef] [PubMed]
- Meehan, C.J.; Beiko, R.G. A Phylogenomic View of Ecological Specialization in the Lachnospiraceae, a Family of Digestive Tract-Associated Bacteria. Genome Biol. Evol. 2014, 6, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Pan, H.; Li, X.; Gao, Y.; Liu, G.; Xia, L.C. Identifying Gut Microbiota Associated with Colorectal Cancer Using a Zero-Inflated Lognormal Model. Front. Microbiol. 2019, 10, 826. [Google Scholar] [CrossRef]
- Barnard, N.D.; Goldman, D.M.; Loomis, J.F.; Kahleova, H.; Levin, S.M.; Neabore, S.; Batts, T.C. Plant-Based Diets for Cardiovascular Safety and Performance in Endurance Sports. Nutrients 2019, 11, 130. [Google Scholar] [CrossRef]
- Tipton, K.D.; Wolfe, R.R. Protein and Amino Acids for Athletes. J. Sports Sci. 2004, 22, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient Metabolism by the Human Gut Microbiome: Major Fermentation by-Products and Their Impact on Host Health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Blachier, F.; Beaumont, M.; Portune, K.J.; Steuer, N.; Lan, A.; Audebert, M.; Khodorova, N.; Andriamihaja, M.; Airinei, G.; Benamouzig, R.; et al. High-Protein Diets for Weight Management: Interactions with the Intestinal Microbiota and Consequences for Gut Health. A Position Paper by the My New Gut Study Group. Clin. Nutr. 2019, 38, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Lowery, L.; Forsythe, C.E. Protein and Overtraining: Potential Applications for Free-Living Athletes. J. Int. Soc. Sports Nutr. 2006, 3, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Effects of the Mediterranean Diet on Health and Gut Microbiota. Nutrients 2023, 15, 2150. [Google Scholar] [CrossRef] [PubMed]
- Bellastella, G.; Scappaticcio, L.; Caiazzo, F.; Tomasuolo, M.; Carotenuto, R.; Caputo, M.; Arena, S.; Caruso, P.; Maiorino, M.I.; Esposito, K. Mediterranean Diet and Thyroid: An Interesting Alliance. Nutrients 2022, 14, 4130. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Tumolo, M.R.; Garbarino, S. Mediterranean Diet on Sleep: A Health Alliance. Nutrients 2022, 14, 2998. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, K.; Saneei, P.; Hajhashemy, Z.; Esmaillzadeh, A. Adherence to the Mediterranean Diet, Five-Year Weight Change, and Risk of Overweight and Obesity: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2022, 13, 152–166. [Google Scholar] [CrossRef]
- Jimenez-Torres, J.; Alcalá-Diaz, J.F.; Torres-Peña, J.D.; Gutierrez-Mariscal, F.M.; Leon-Acuña, A.; Gómez-Luna, P.; Fernández-Gandara, C.; Quintana-Navarro, G.M.; Fernandez-Garcia, J.C.; Perez-Martinez, P.; et al. Mediterranean Diet Reduces Atherosclerosis Progression in Coronary Heart Disease: An Analysis of the CORDIOPREV Randomized Controlled Trial. Stroke 2021, 52, 3440–3449. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Wang, S.; Han, R.; Cao, Y.; Hua, W.; Mao, Y.; Zhang, X.; Pang, X.; Wei, C.; et al. Interactions between Gut Microbiota, Host Genetics and Diet Relevant to Development of Metabolic Syndromes in Mice. ISME J. 2010, 4, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Djuric, Z.; Bassis, C.M.; Plegue, M.A.; Ren, J.; Chan, R.; Sidahmed, E.; Turgeon, D.K.; Ruffin, M.T.; Kato, I.; Sen, A. Colonic Mucosal Bacteria Are Associated with Inter-Individual Variability in Serum Carotenoid Concentrations. J. Acad. Nutr. Diet. 2018, 118, 606–616.e3. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M. Skeletal Muscle Metabolism during Exercise in Humans. Clin. Exp. Pharmacol. Physiol. 2000, 27, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. Exercise-Induced Stress Behavior, Gut-Microbiota-Brain Axis and Diet: A Systematic Review for Athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A. A Step Towards Personalized Sports Nutrition: Carbohydrate Intake During Exercise. Sports Med. 2014, 44, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E. Carbohydrate Intake during Exercise and Performance. Nutrition 2004, 20, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Spriet, L.L. Diet and Nutraceuticals for Mental and Physical Performance in Athletes. Sports Med. 2022, 52, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Rollo, I.; Williams, C. Carbohydrate Nutrition and Skill Performance in Soccer. Sports Med. 2023, 53, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Faits, T.; Walker, M.E.; Rodriguez-Morato, J.; Meng, H.; Gervis, J.E.; Galluccio, J.M.; Lichtenstein, A.H.; Johnson, W.E.; Matthan, N.R. Exploring Changes in the Human Gut Microbiota and Microbial-Derived Metabolites in Response to Diets Enriched in Simple, Refined, or Unrefined Carbohydrate-Containing Foods: A Post Hoc Analysis of a Randomized Clinical Trial. Am. J. Clin. Nutr. 2020, 112, 1631–1641. [Google Scholar] [CrossRef]
- Holscher, H.D.; Chumpitazi, B.P.; Dahl, W.J.; Fahey, G.C.; Liska, D.J.; Slavin, J.L.; Verbeke, K. Perspective: Assessing Tolerance to Nondigestible Carbohydrate Consumption. Adv. Nutr. 2022, 13, 2084–2097. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-Induced Extinctions in the Gut Microbiota Compound over Generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, X.; Chen, D.; Yu, B.; He, J.; Luo, Y.; Zheng, P.; Chen, H.; Yan, H.; Huang, Z. Effects of Protocatechuic Acid on Antioxidant Capacity, Mitochondrial Biogenesis and Skeletal Muscle Fiber Transformation. J. Nutr. Biochem. 2023, 116, 109327. [Google Scholar] [CrossRef] [PubMed]
- Caesar, R.; Tremaroli, V.; Kovatcheva-Datchary, P.; Cani, P.D.; Bäckhed, F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015, 22, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Repiso, C.; Hernández-García, C.; García-Almeida, J.M.; Bellido, D.; Martín-Núñez, G.M.; Sánchez-Alcoholado, L.; Alcaide-Torres, J.; Sajoux, I.; Tinahones, F.J.; Moreno-Indias, I. Effect of Synbiotic Supplementation in a Very-Low-Calorie Ketogenic Diet on Weight Loss Achievement and Gut Microbiota: A Randomized Controlled Pilot Study. Mol. Nutr. Food Res. 2019, 63, e1900167. [Google Scholar]
- Kimmel, M.; Keller, D.; Farmer, S.; Warrino, D.E. A Controlled Clinical Trial to Evaluate the Effect of GanedenBC(30) on Immunological Markers. Methods Find Exp. Clin. Pharmacol. 2010, 32, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Minevich, J.; Olson, M.A.; Mannion, J.P.; Boublik, J.H.; McPherson, J.O.; Lowery, R.P.; Shields, K.; Sharp, M.; De Souza, E.O.; Wilson, J.M.; et al. Digestive Enzymes Reduce Quality Differences between Plant and Animal Proteins: A Double-Blind Crossover Study. J. Int. Soc. Sports Nutr. 2015, 12, P26. [Google Scholar] [CrossRef]
- Jäger, R.; Purpura, M.; Farmer, S.; Cash, H.A.; Keller, D. Probiotic Bacillus Coagulans GBI-30, 6086 Improves Protein Absorption and Utilization. Probiotics Antimicrob. Proteins 2018, 10, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Albracht-Schulte, K.; Islam, T.; Johnson, P.; Moustaid-Moussa, N. Systematic Review of Beef Protein Effects on Gut Microbiota: Implications for Health. Adv. Nutr. 2020, 12, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shi, X.; Lin, X.; Ye, K.; Xu, X.; Li, C.; Zhou, G. Beef, Chicken, and Soy Proteins in Diets Induce Different Gut Microbiota and Metabolites in Rats. Front. Microbiol. 2017, 8, 1395. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lin, X.; Zhao, F.; Shi, X.; Li, H.; Li, Y.; Zhu, W.; Xu, X.; Li, C.; Zhou, G. Meat, Dairy and Plant Proteins Alter Bacterial Composition of Rat Gut Bacteria. Sci. Rep. 2015, 5, 15220. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, X.; Li, H.; Li, Y.; Shi, X.; Zhao, F.; Xu, X.; Li, C.; Zhou, G. Intake of Meat Proteins Substantially Increased the Relative Abundance of Genus Lactobacillus in Rat Feces. PLoS ONE 2016, 11, e0152678. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.M.; Pan, C.; Cantor, R.M.; Tang, W.H.W.; Garcia-Garcia, J.C.; Kurtz, I.; Hazen, S.L.; Bergeron, N.; Krauss, R.M.; Lusis, A.J. Impact of Individual Traits, Saturated Fat, and Protein Source on the Gut Microbiome. mBio 2018, 9, e01604-18. [Google Scholar] [CrossRef] [PubMed]
- Losasso, C.; Eckert, E.M.; Mastrorilli, E.; Villiger, J.; Mancin, M.; Patuzzi, I.; Di Cesare, A.; Cibin, V.; Barrucci, F.; Pernthaler, J.; et al. Assessing the Influence of Vegan, Vegetarian and Omnivore Oriented Westernized Dietary Styles on Human Gut Microbiota: A Cross Sectional Study. Front. Microbiol. 2018, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Lauretani, F.; Tana, C.; Nouvenne, A.; Ridolo, E.; Meschi, T. Exercise and Immune System as Modulators of Intestinal Microbiome: Implications for the Gut-Muscle Axis Hypothesis. Exerc. Immunol. Rev. 2019, 25, 84–95. [Google Scholar] [PubMed]
- Casati, M.; Ferri, E.; Azzolino, D.; Cesari, M.; Arosio, B. Gut Microbiota and Physical Frailty through the Mediation of Sarcopenia. Exp. Gerontol. 2019, 124, 110639. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.-H.; Wang, M.; Yuan, Y.; Yan, J.-K.; Peng, Y.; Xu, G.; Weng, X. Konjac Glucomannan Counteracted the Side Effects of Excessive Exercise on Gut Microbiome, Endurance, and Strength in an Overtraining Mice Model. Nutrients 2023, 15, 4206. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Fu, J.; Qiao, Y.; Cao, J.; Deehan, E.C.; Li, Z.; Jin, M.; Wang, X.; Wang, Y. Higher Intake of Microbiota-Accessible Carbohydrates and Improved Cardiometabolic Risk Factors: A Meta-Analysis and Umbrella Review of Dietary Management in Patients with Type 2 Diabetes. Am. J. Clin. Nutr. 2021, 113, 1515–1530. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Yamashita, H.; Fujisawa, K.; Ito, E.; Idei, S.; Kawaguchi, N.; Kimoto, M.; Hiemori, M.; Tsuji, H. Improvement of Obesity and Glucose Tolerance by Acetate in Type 2 Diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Biosci. Biotechnol. Biochem. 2007, 71, 1236–1243. [Google Scholar] [CrossRef]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-Activated Protein Kinase (AMPK) Action in Skeletal Muscle via Direct Phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic Control through the PGC-1 Family of Transcription Coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzi, S.C.; Britton, R.A. Adaptation of the Gut Microbiota to Modern Dietary Sugars and Sweeteners. Adv. Nutr. 2020, 11, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.N.; Chassard, C.; Lacroix, C. Gut Microbial Adaptation to Dietary Consumption of Fructose, Artificial Sweeteners and Sugar Alcohols: Implications for Host-Microbe Interactions Contributing to Obesity. Obes. Rev. 2012, 13, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 Richest Dietary Sources of Polyphenols: An Application of the Phenol-Explorer Database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef]
- Bowtell, J.; Kelly, V. Fruit-Derived Polyphenol Supplementation for Athlete Recovery and Performance. Sports Med. 2019, 49, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Pereira Panza, V.S.; Diefenthaeler, F.; da Silva, E.L. Benefits of Dietary Phytochemical Supplementation on Eccentric Exercise-Induced Muscle Damage: Is Including Antioxidants Enough? Nutrition 2015, 31, 1072–1082. [Google Scholar] [CrossRef]
- Carey, C.C.; Lucey, A.; Doyle, L. Flavonoid Containing Polyphenol Consumption and Recovery from Exercise-Induced Muscle Damage: A Systematic Review and Meta-Analysis. Sports Med. 2021, 51, 1293–1316. [Google Scholar] [CrossRef]
- Somerville, V.; Bringans, C.; Braakhuis, A. Polyphenols and Performance: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.; Leonard, W.; Beasley, J.T.; Fang, Z.; Zhang, P.; Ranadheera, C.S. Anthocyanins-Gut Microbiota-Health Axis: A Review. Crit. Rev. Food Sci. Nutr. 2023, 15, 2367. [Google Scholar] [CrossRef] [PubMed]
- Eker, M.E.; Aaby, K.; Budic-Leto, I.; Brnčić, S.R.; El, S.N.; Karakaya, S.; Simsek, S.; Manach, C.; Wiczkowski, W.; de Pascual-Teresa, S. A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability. Foods 2019, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Varillas-Delgado, D.; Morencos, E.; Gutiérrez-Hellín, J.; Aguilar-Navarro, M.; Muñoz, A.; Mendoza Láiz, N.; Perucho, T.; Maestro, A.; Tellería-Orriols, J.J. Genetic Profiles to Identify Talents in Elite Endurance Athletes and Professional Football Players. PLoS ONE 2022, 17, e0274880. [Google Scholar] [CrossRef]
- Mayta-Apaza, A.C.; Pottgen, E.; De Bodt, J.; Papp, N.; Marasini, D.; Howard, L.; Abranko, L.; Van de Wiele, T.; Lee, S.-O.; Carbonero, F. Impact of Tart Cherries Polyphenols on the Human Gut Microbiota and Phenolic Metabolites in Vitro and in Vivo. J. Nutr. Biochem. 2018, 59, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, L.; Angelino, D.; Vivas, E.I.; Kerby, R.L.; García-Viguera, C.; Del Rio, D.; Rey, F.E.; Mena, P. Differential Catabolism of an Anthocyanin-Rich Elderberry Extract by Three Gut Microbiota Bacterial Species. J. Agric. Food Chem. 2020, 68, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Koerich, A.C.C.; Borszcz, F.K.; Thives Mello, A.; de Lucas, R.D.; Hansen, F. Effects of the Ketogenic Diet on Performance and Body Composition in Athletes and Trained Adults: A Systematic Review and Bayesian Multivariate Multilevel Meta-Analysis and Meta-Regression. Crit. Rev. Food Sci. Nutr. 2023, 63, 11399–11424. [Google Scholar] [CrossRef] [PubMed]
- West, S.; Monteyne, A.J.; van der Heijden, I.; Stephens, F.B.; Wall, B.T. Nutritional Considerations for the Vegan Athlete. Adv. Nutr. 2023, 14, 774–795. [Google Scholar] [CrossRef]
- Phillips, S.M.; Van Loon, L.J.C. Dietary Protein for Athletes: From Requirements to Optimum Adaptation. J. Sports Sci. 2011, 29 (Suppl. S1), S29–S38. [Google Scholar] [CrossRef]
| Author, Year | Country | Sample Size, Sex and Age | Main Findings on Gut Microbial Composition | |
|---|---|---|---|---|
| Athletes/Physically Active Population | Non-Athletes/Sedentary Population | |||
| Xu et al., 2022 [53] | China | n = 66 (males = 36, females = 30), Age: 18–25 years | Bacteroidetes (52.53%) Firmicutes (43.99%) Prevotella (20.88%) Bacteroides (24.96%) Faecalibacterium (6.86%) Megamonas (11.67%) | Bacteroidetes (62.81%) Firmicutes (32.14%) Prevotella (26.81%) Bacteroides (25.01%) Faecalibacterium (10.57%) Megamonas (5.15%) |
| Humińska-Lisowska et al., 2024 [55] | Poland | n = 52, males Age: 19–24 years | Enterotype: Endurance group: Bacteroides-driven (46.70%) Strength group: Prevotella-driven (50.00%) | Enterotype: Control group: Bacteroides-driven (40.90%) Ruminococcus-driven (40.90%) |
| Hintikka et al., 2022 [54] | Finland | n = 54 (males = 28, females = 26) Age: Athlete group: 27.1 ± 5.1 years Control group: 27.4 ± 5.6 years | Bacteroidetes (50.40%) Firmicutes (46.00%) Proteobacteria (2.30%) Actinobacteria (0.79%) | Firmicutes (48.30%) Bacteroidetes (46.20%) Proteobacteria (3.36%) Actinobacteria (1.57%) |
| Author, Year | Dietary Pattern | Substance | Subjects | Pathway | Most Important Findings |
|---|---|---|---|---|---|
| (Caesar et al., 2015 [115]) | Ketogenic diet | Saturated fat | Male mice | LPS/TLR4 pathway | Increases inflammatory indices in WAT |
| (Minevich et al., 2015 [119]) | High-protein diet | Bacillus coagulans GBI-30, 6086 Protein | Males (n = 11) | Promote the absorb and utilize of protein | Produces proteases which can increase amino acid absorption in humans |
| (Zhu et al., 2017 [121]) | High-protein diet | Animal protein | Male rats (n = 32) | Decrease the binding of CD14 and LPS-binding protein | Higher abundance of Lactobacilli Higher ratio of Firmicutes to Bacteroidetes Lower butyrate Lower SCFAs-producing bacteria Lower LPS-binding protein Lower transcription factor CD14 receptor Lower inflammation |
| (Jäger et al., 2007 [81]) | Plant-based diet/ Mediterranean diet | Dietary fiber | C2C12 myotubes Female mice | AMPK/PGC-1α pathway | Enhances fatty acid oxidation of muscle |
| (Yang et al., 2023 [132]) | Plant-based diet/ Mediterranean diet | Anthocyanins | C2C12 myotubes Male mice (n = 60) | AMPK signaling pathway | Reduces oxidative stress Promotes mitochondrial biogenesis Converse skeletal muscle fiber |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yang, K.; Xu, M.; Zhang, Y.; Weng, X.; Luo, J.; Li, Y.; Mao, Y.-H. Dietary Patterns, Gut Microbiota and Sports Performance in Athletes: A Narrative Review. Nutrients 2024, 16, 1634. https://doi.org/10.3390/nu16111634
Chen Y, Yang K, Xu M, Zhang Y, Weng X, Luo J, Li Y, Mao Y-H. Dietary Patterns, Gut Microbiota and Sports Performance in Athletes: A Narrative Review. Nutrients. 2024; 16(11):1634. https://doi.org/10.3390/nu16111634
Chicago/Turabian StyleChen, Yonglin, Keer Yang, Mingxin Xu, Yishuo Zhang, Xiquan Weng, Jiaji Luo, Yanshuo Li, and Yu-Heng Mao. 2024. "Dietary Patterns, Gut Microbiota and Sports Performance in Athletes: A Narrative Review" Nutrients 16, no. 11: 1634. https://doi.org/10.3390/nu16111634
APA StyleChen, Y., Yang, K., Xu, M., Zhang, Y., Weng, X., Luo, J., Li, Y., & Mao, Y.-H. (2024). Dietary Patterns, Gut Microbiota and Sports Performance in Athletes: A Narrative Review. Nutrients, 16(11), 1634. https://doi.org/10.3390/nu16111634






