Maternal Docosahexaenoic Acid Supplementation Alters Maternal and Fetal Docosahexaenoic Acid Status and Placenta Phospholipids in Pregnancies Complicated by High Body Mass Index
Abstract
1. Introduction
2. Methods
2.1. Sample Collection
2.2. Total DHA Quantification
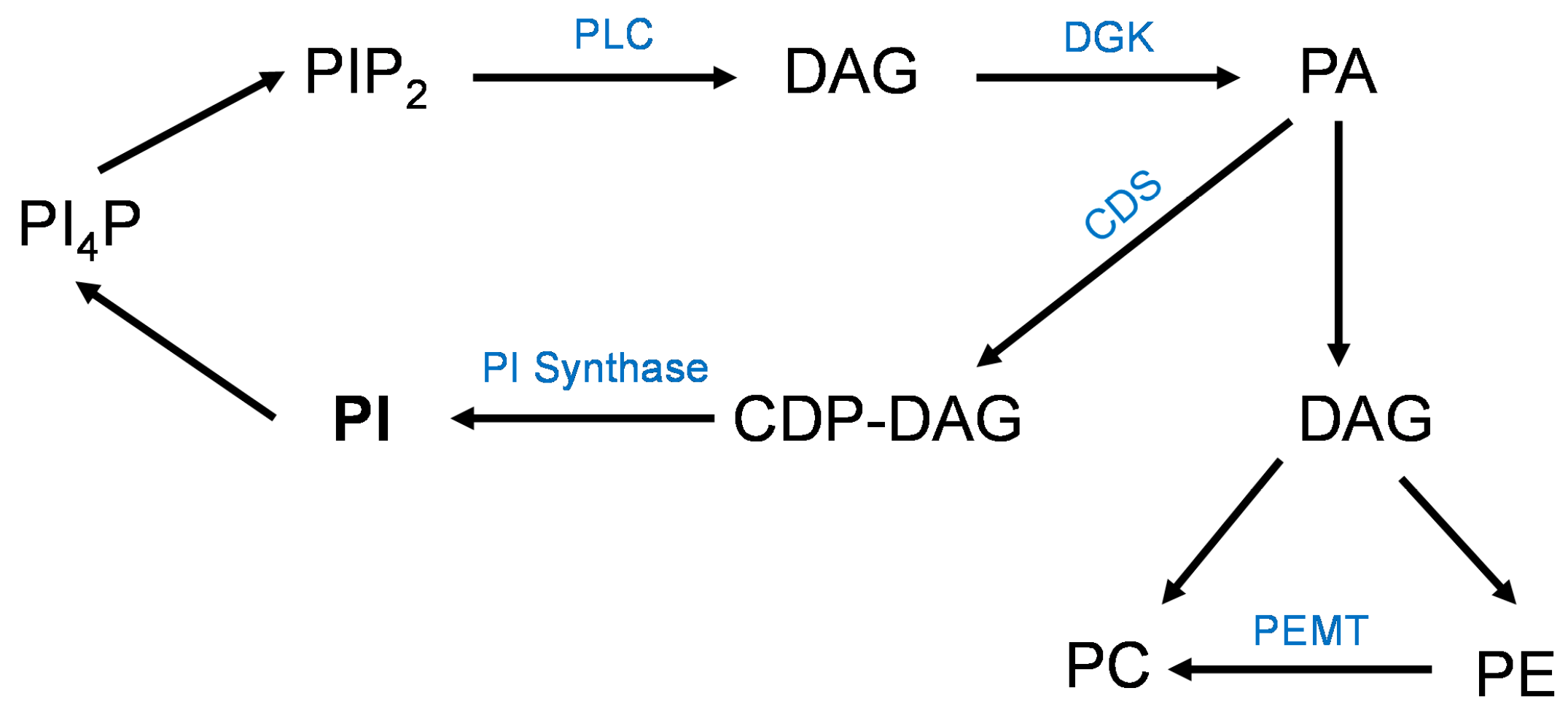
2.3. Phospholipid Analysis
2.4. Choline Analysis
2.5. Statistics
3. Results
3.1. Clinical Characteristics
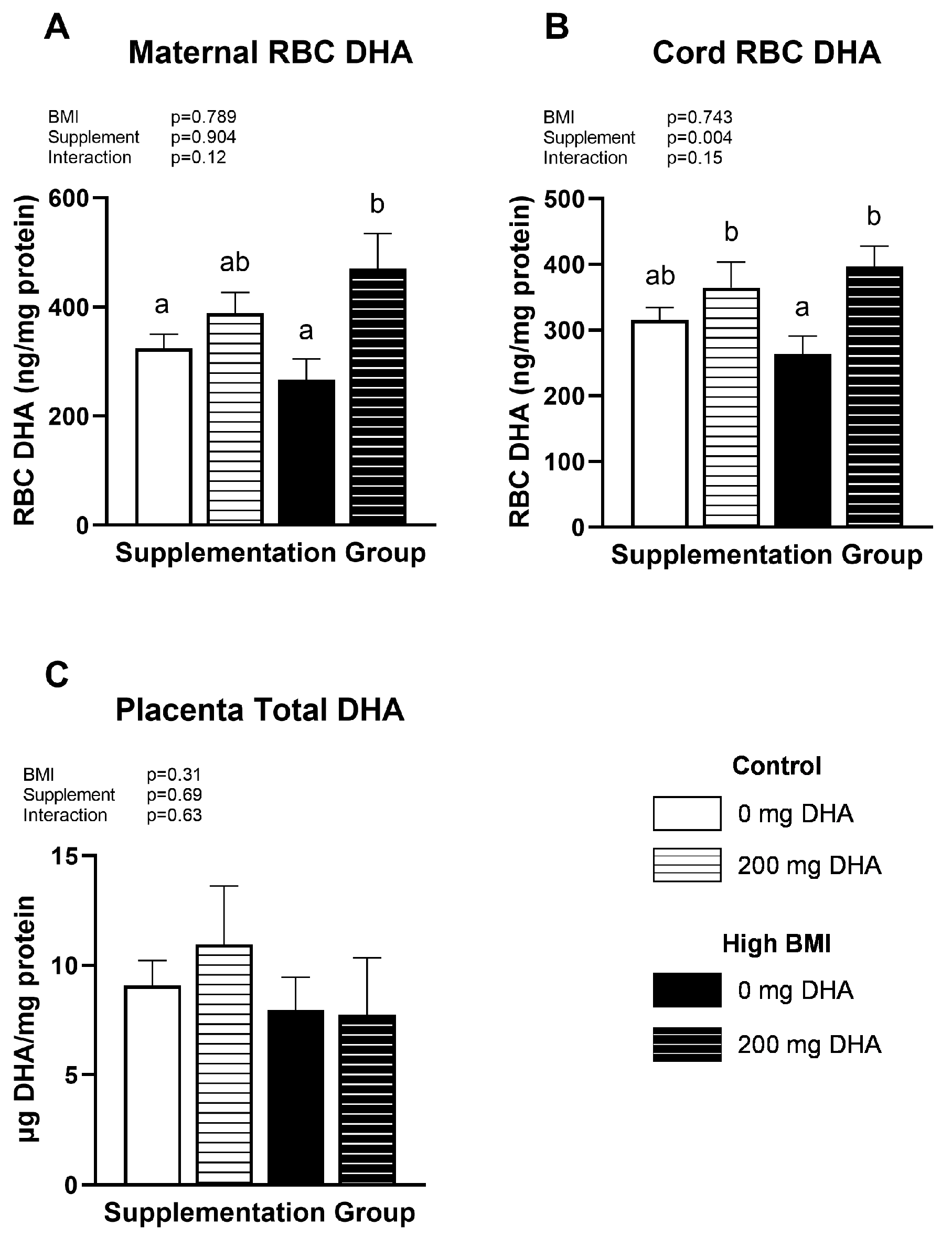
3.2. Maternal, Fetal, Placental DHA Status
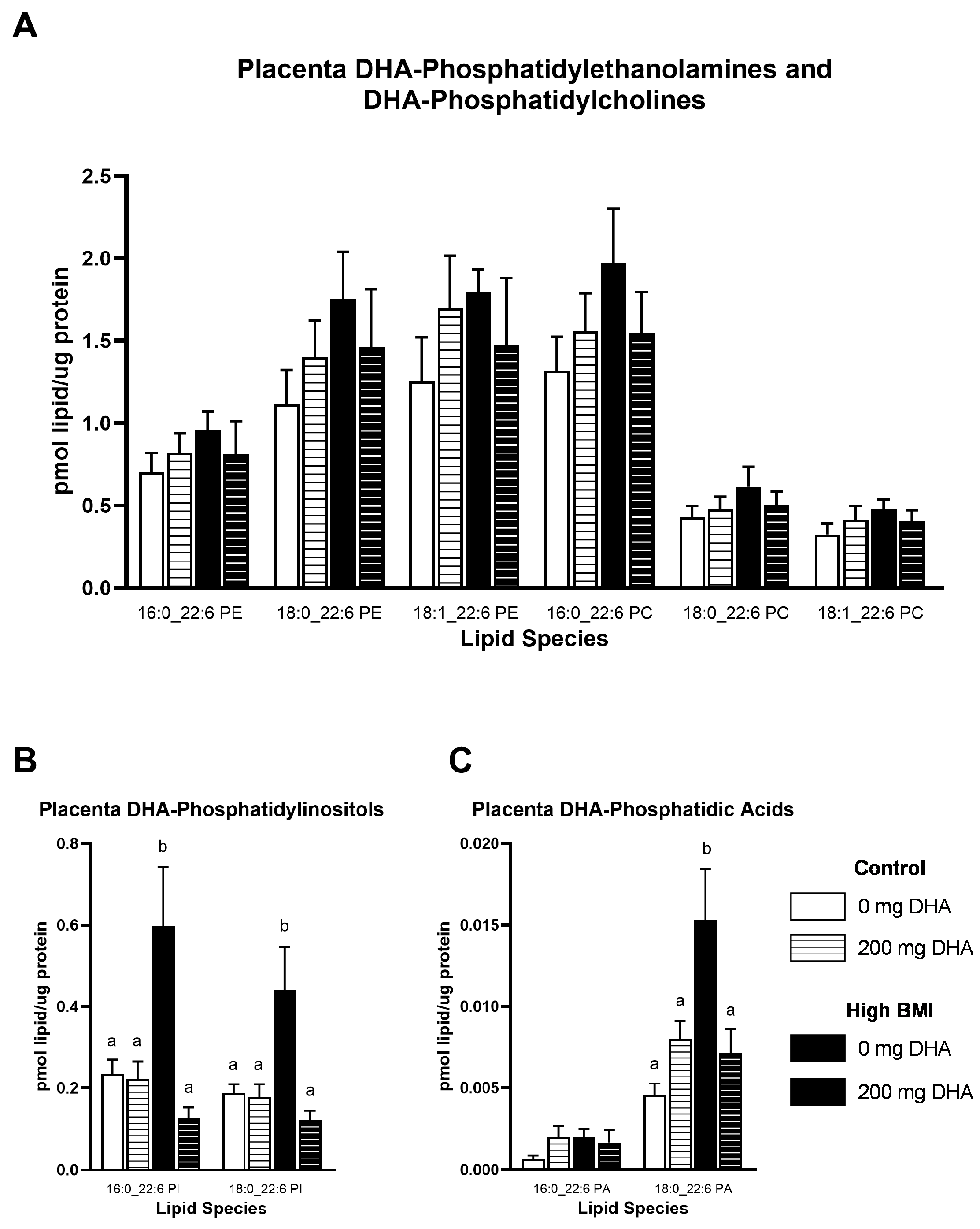
3.3. Placental DHA-Phospholipid Concentrations
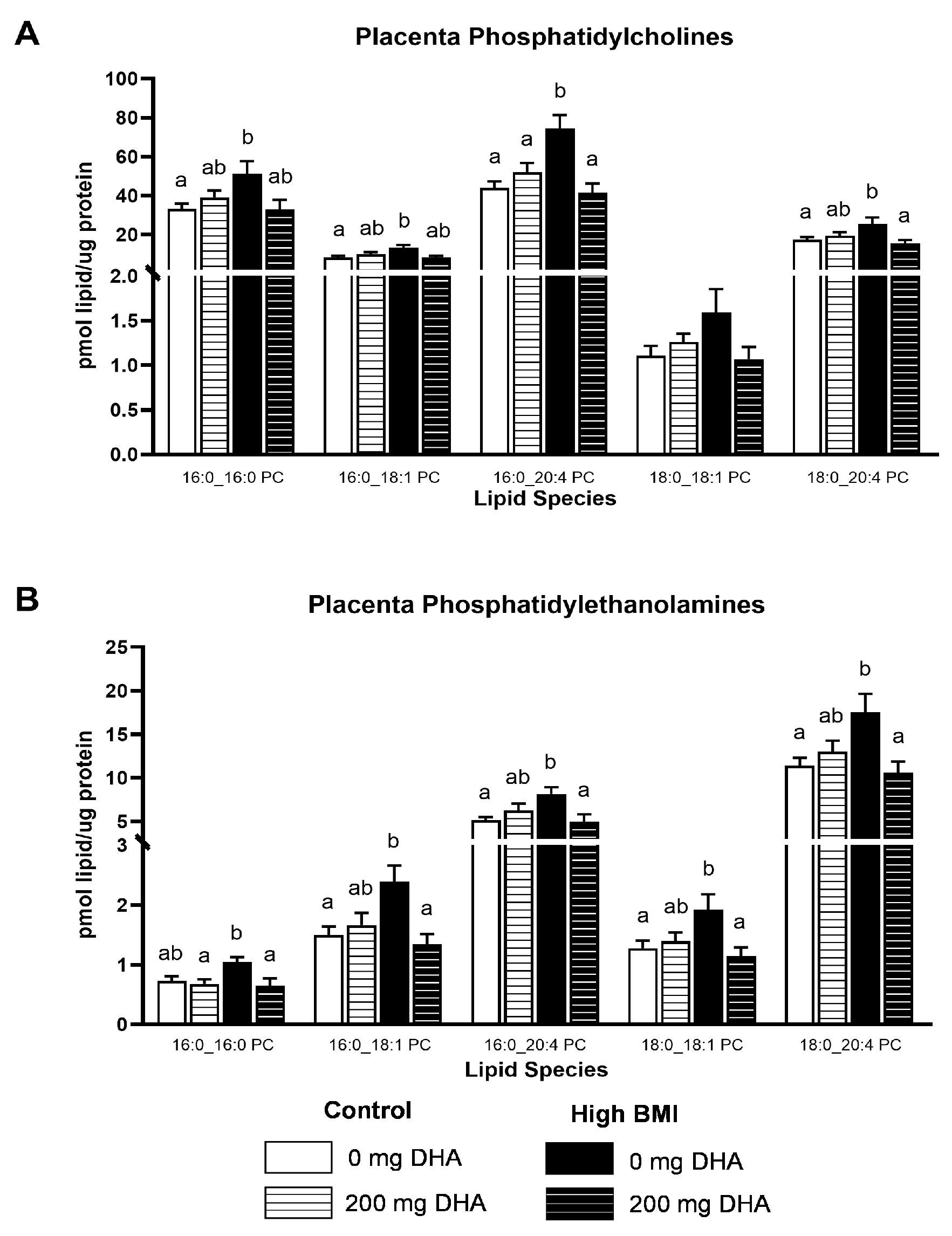
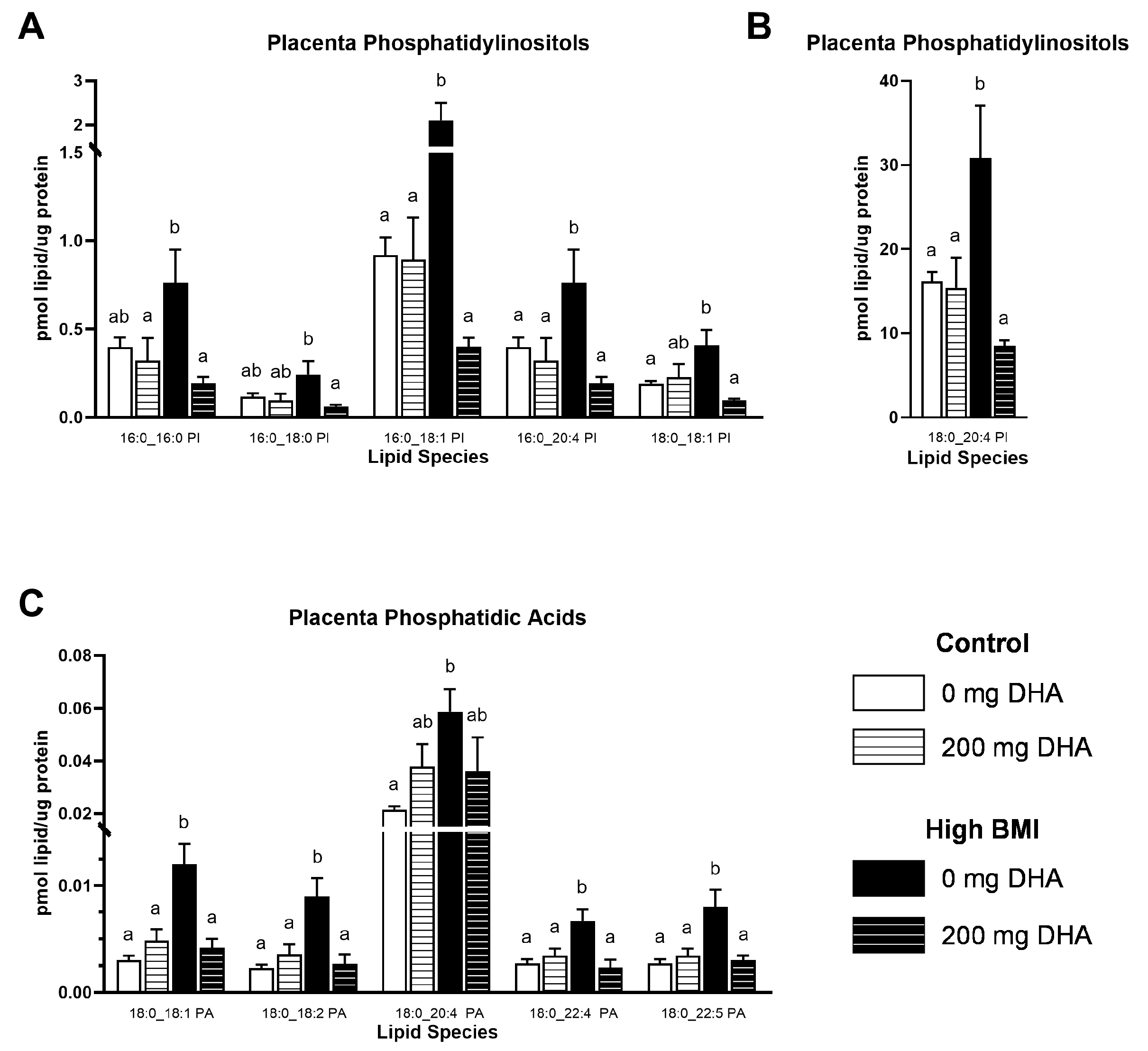
3.4. Placental Phospholipids
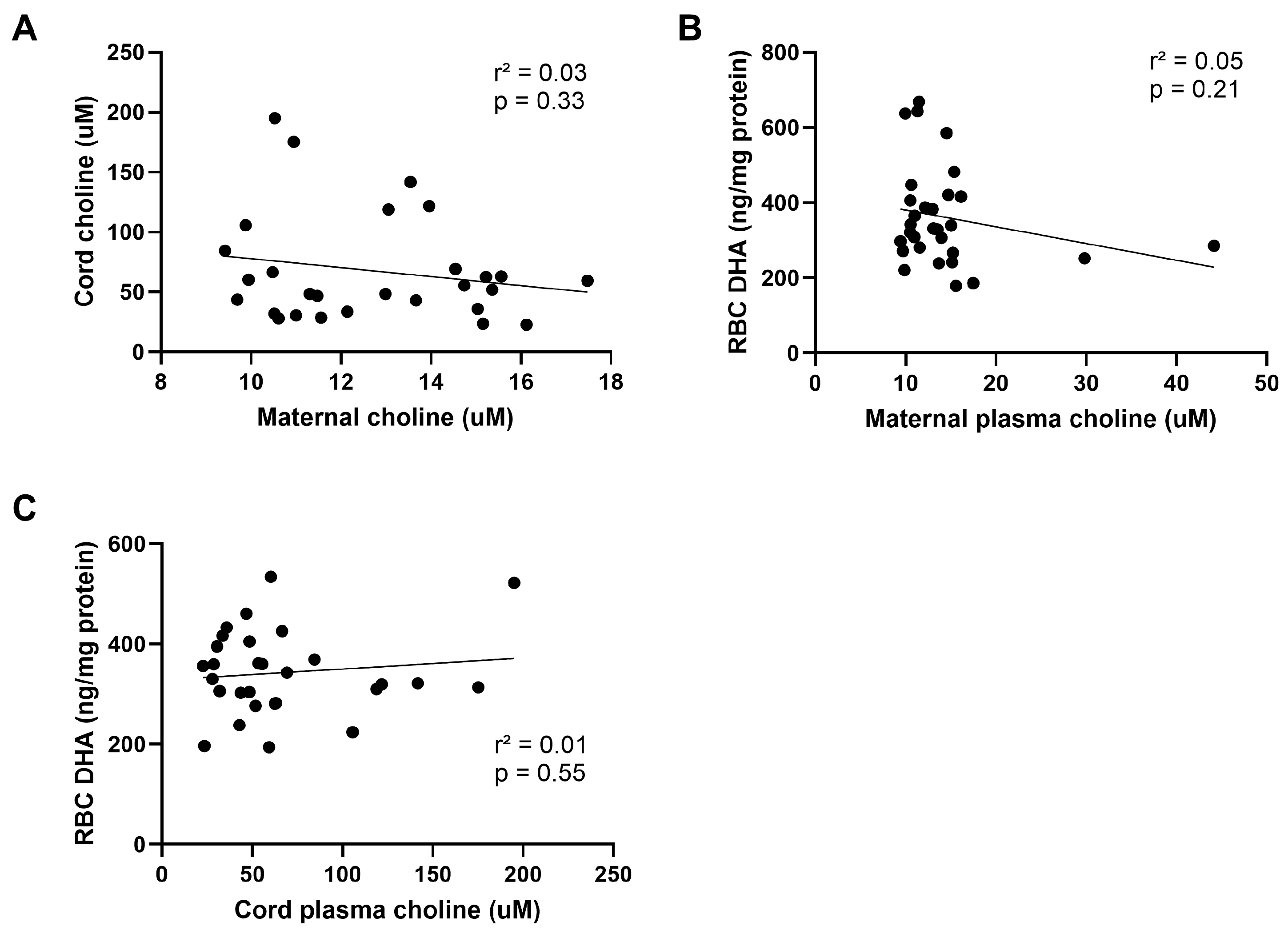
3.5. Choline Status
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carlson, S.E.; Colombo, J.; Gajewski, B.J.; Gustafson, K.M.; Mundy, D.; Yeast, J.; Georgieff, M.K.; Markley, L.A.; Kerling, E.H.; Shaddy, D.J. DHA supplementation and pregnancy outcomes. Am. J. Clin. Nutr. 2013, 97, 808–815. [Google Scholar] [CrossRef]
- Guesnet, P.; Alessandri, J.M. Docosahexaenoic acid (DHA) and the developing central nervous system (CNS)—Implications for dietary recommendations. Biochimie 2011, 93, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, U.; Gonzalez-Casanova, I.; Schnaas, L.; DiGirolamo, A.; Quezada, A.D.; Pallo, B.C.; Hao, W.; Neufeld, L.M.; Rivera, J.A.; Stein, A.D.; et al. Prenatal supplementation with DHA improves attention at 5 y of age: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Calder, P.C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fulgoni, V.L.; Kris-Etherton, P.M.; Mitmesser, S.H. Dietary Intakes of EPA and DHA Omega-3 Fatty Acids among US Childbearing-Age and Pregnant Women: An Analysis of NHANES 2001–2014. Nutrients 2018, 10, 416. [Google Scholar] [CrossRef]
- Thompson, M.; Hein, N.; Hanson, C.; Smith, L.M.; Anderson-Berry, A.; Richter, C.K.; Stessy Bisselou, K.; Kusi Appiah, A.; Kris-Etherton, P.; Skulas-Ray, A.C.; et al. Omega-3 Fatty Acid Intake by Age, Gender, and Pregnancy Status in the United States: National Health and Nutrition Examination Survey 2003–2014. Nutrients 2019, 11, 177. [Google Scholar] [CrossRef]
- Leyrolle, Q.; Decoeur, F.; Dejean, C.; Briere, G.; Leon, S.; Bakoyiannis, I.; Baroux, E.; Sterley, T.L.; Bosch-Bouju, C.; Morel, L.; et al. N-3 PUFA deficiency disrupts oligodendrocyte maturation and myelin integrity during brain development. Glia 2022, 70, 50–70. [Google Scholar] [CrossRef]
- Basak, S.; Mallick, R.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Maternal Supply of Both Arachidonic and Docosahexaenoic Acids Is Required for Optimal Neurodevelopment. Nutrients 2021, 13, 2061. [Google Scholar] [CrossRef]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Yelland, L.; Quinlivan, J.; Ryan, P.; Team, D.O.I. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA 2010, 304, 1675–1683. [Google Scholar] [CrossRef]
- Kuratko, C.N.; Barrett, E.C.; Nelson, E.B.; Salem, N., Jr. The relationship of docosahexaenoic acid (DHA) with learning and behavior in healthy children: A review. Nutrients 2013, 5, 2777–2810. [Google Scholar] [CrossRef]
- Gustafson, K.M.; Christifano, D.N.; Hoyer, D.; Schmidt, A.; Carlson, S.E.; Colombo, J.; Mathis, N.B.; Sands, S.A.; Chollet-Hinton, L.; Brown, A.R.; et al. Prenatal docosahexaenoic acid effect on maternal-infant DHA-equilibrium and fetal neurodevelopment: A randomized clinical trial. Pediatr. Res. 2022, 92, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Calabuig-Navarro, V.; Puchowicz, M.; Glazebrook, P.; Haghiac, M.; Minium, J.; Catalano, P.; Hauguel deMouzon, S.; O’Tierney-Ginn, P. Effect of omega-3 supplementation on placental lipid metabolism in overweight and obese women. Am. J. Clin. Nutr. 2016, 103, 1064–1072. [Google Scholar] [CrossRef]
- Rasool, A.; Mahmoud, T.; Mathyk, B.; Kaneko-Tarui, T.; Roncari, D.; White, K.O.; O'Tierney-Ginn, P. Obesity downregulates lipid metabolism genes in first trimester placenta. Sci. Rep. 2022, 12, 19368. [Google Scholar] [CrossRef] [PubMed]
- Klatt, K.C.; McDougall, M.Q.; Malysheva, O.V.; Taesuwan, S.; Loinard-Gonzalez, A.A.P.; Nevins, J.E.H.; Beckman, K.; Bhawal, R.; Anderson, E.; Zhang, S.; et al. Prenatal choline supplementation improves biomarkers of maternal docosahexaenoic acid (DHA) status among pregnant participants consuming supplemental DHA: A randomized controlled trial. Am. J. Clin. Nutr. 2022, 116, 820–832. [Google Scholar] [CrossRef]
- Larque, E.; Demmelmair, H.; Gil-Sanchez, A.; Prieto-Sanchez, M.T.; Blanco, J.E.; Pagan, A.; Faber, F.L.; Zamora, S.; Parrilla, J.J.; Koletzko, B. Placental transfer of fatty acids and fetal implications. Am. J. Clin. Nutr. 2011, 94, 1908S–1913S. [Google Scholar] [CrossRef]
- Ferchaud-Roucher, V.; Kramer, A.; Silva, E.; Pantham, P.; Weintraub, S.T.; Jansson, T.; Powell, T.L. A potential role for lysophosphatidylcholine in the delivery of long chain polyunsaturated fatty acids to the fetal circulation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Powell, T.L.; Barentsen, K.; Vaughan, O.; Uhlson, C.; Zemski Berry, K.; Erickson, K.; Faer, K.; Chassen, S.S.; Jansson, T. Knockdown of Placental Major Facilitator Superfamily Domain Containing 2a in Pregnant Mice Reduces Fetal Brain Growth and Phospholipid Docosahexaenoic Acid Content. Nutrients 2023, 15, 4956. [Google Scholar] [CrossRef]
- Lager, S.; Ramirez, V.I.; Acosta, O.; Meireles, C.; Miller, E.; Gaccioli, F.; Rosario, F.J.; Gelfond, J.A.L.; Hakala, K.; Weintraub, S.T.; et al. Docosahexaenoic Acid Supplementation in Pregnancy Modulates Placental Cellular Signaling and Nutrient Transport Capacity in Obese Women. J. Clin. Endocrinol. Metab. 2017, 102, 4557–4567. [Google Scholar] [CrossRef]
- Lager, S.; Jansson, T.; Powell, T.L. Differential regulation of placental amino acid transport by saturated and unsaturated fatty acids. Am. J. Physiol. Cell Physiol. 2014, 307, C738–C744. [Google Scholar] [CrossRef]
- Ramsay, J.E.; Ferrell, W.R.; Crawford, L.; Wallace, A.M.; Greer, I.A.; Sattar, N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J. Clin. Endocrinol. Metab. 2002, 87, 4231–4237. [Google Scholar] [CrossRef]
- Saben, J.; Lindsey, F.; Zhong, Y.; Thakali, K.; Badger, T.M.; Andres, A.; Gomez-Acevedo, H.; Shankar, K. Maternal obesity is associated with a lipotoxic placental environment. Placenta 2014, 35, 171–177. [Google Scholar] [CrossRef]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef]
- Nemkov, T.; D’Alessandro, A.; Hansen, K.C. Three-minute method for amino acid analysis by UHPLC and high-resolution quadrupole orbitrap mass spectrometry. Amino Acids 2015, 47, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- Young, I.E.; Parker, H.M.; Cook, R.L.; O’Dwyer, N.J.; Garg, M.L.; Steinbeck, K.S.; Cheng, H.L.; Donges, C.; Franklin, J.L.; O’Connor, H.T. Association between Obesity and Omega-3 Status in Healthy Young Women. Nutrients 2020, 12, 1480. [Google Scholar] [CrossRef]
- Epand, R.M. Features of the Phosphatidylinositol Cycle and its Role in Signal Transduction. J. Membr. Biol. 2017, 250, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.R.; Schiavo, G.; Irvine, R.F. Immunocytochemical techniques reveal multiple, distinct cellular pools of PtdIns4P and PtdIns(4,5)P(2). Biochem. J. 2009, 422, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Shulga, Y.V.; Topham, M.K.; Epand, R.M. Substrate specificity of diacylglycerol kinase-epsilon and the phosphatidylinositol cycle. FEBS Lett. 2011, 585, 4025–4028. [Google Scholar] [CrossRef]
- Carman, G.M.; Han, G.S. Phosphatidic acid phosphatase, a key enzyme in the regulation of lipid synthesis. J. Biol. Chem. 2009, 284, 2593–2597. [Google Scholar] [CrossRef]
- Henneberry, A.L.; Wright, M.M.; McMaster, C.R. The major sites of cellular phospholipid synthesis and molecular determinants of Fatty Acid and lipid head group specificity. Mol. Biol. Cell 2002, 13, 3148–3161. [Google Scholar] [CrossRef]
- Plociennikowska, A.; Hromada-Judycka, A.; Dembinska, J.; Roszczenko, P.; Ciesielska, A.; Kwiatkowska, K. Contribution of CD14 and TLR4 to changes of the PI(4,5)P2 level in LPS-stimulated cells. J. Leukoc. Biol. 2016, 100, 1363–1373. [Google Scholar] [CrossRef]
- Plociennikowska, A.; Zdioruk, M.I.; Traczyk, G.; Swiatkowska, A.; Kwiatkowska, K. LPS-induced clustering of CD14 triggers generation of PI(4,5)P2. J. Cell Sci. 2015, 128, 4096–4111. [Google Scholar] [CrossRef]
- Kwiatkowska, K.; Ciesielska, A. Lipid-mediated regulation of pro-inflammatory responses induced by lipopolysaccharide. Postepy Biochem. 2018, 64, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, T.O.; Lass, A. DAG tales: The multiple faces of diacylglycerol--stereochemistry, metabolism, and signaling. Cell. Mol. Life Sci. 2015, 72, 3931–3952. [Google Scholar] [CrossRef] [PubMed]
- Arner, P.; Ryden, M. Fatty Acids, Obesity and Insulin Resistance. Obes. Facts 2015, 8, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.A.; Riley, S.C.; Reynolds, R.M.; Barr, S.; Evans, M.; Statham, A.; Hor, K.; Jabbour, H.N.; Norman, J.E.; Denison, F.C. Placental structure and inflammation in pregnancies associated with obesity. Placenta 2011, 32, 247–254. [Google Scholar] [CrossRef]
- Yang, X.; Haghiac, M.; Glazebrook, P.; Minium, J.; Catalano, P.M.; Hauguel-de Mouzon, S. Saturated fatty acids enhance TLR4 immune pathways in human trophoblasts. Hum. Reprod. 2015, 30, 2152–2159. [Google Scholar] [CrossRef]
- Lager, S.; Gaccioli, F.; Ramirez, V.I.; Jones, H.N.; Jansson, T.; Powell, T.L. Oleic acid stimulates system A amino acid transport in primary human trophoblast cells mediated by toll-like receptor 4. J. Lipid Res. 2013, 54, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Le, O.T.; Nguyen, T.T.; Lee, S.Y. Phosphoinositide turnover in Toll-like receptor signaling and trafficking. BMB Rep. 2014, 47, 361–368. [Google Scholar] [CrossRef]
- Chiang, C.Y.; Veckman, V.; Limmer, K.; David, M. Phospholipase Cgamma-2 and intracellular calcium are required for lipopolysaccharide-induced Toll-like receptor 4 (TLR4) endocytosis and interferon regulatory factor 3 (IRF3) activation. J. Biol. Chem. 2012, 287, 3704–3709. [Google Scholar] [CrossRef]
- Silva, E.; Ferchaud-Roucher, V.; Kramer, A.; Madi, L.; Pantham, P.; Chassen, S.; Jansson, T.; Powell, T.L. Oleic acid stimulation of amino acid uptake in primary human trophoblast cells is mediated by phosphatidic acid and mTOR signaling. FASEB Bioadv. 2024, 6, 1–11. [Google Scholar] [CrossRef]
- Hwang, D.H.; Kim, J.A.; Lee, J.Y. Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid. Eur. J. Pharmacol. 2016, 785, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Plakidas, A.; Lee, W.H.; Heikkinen, A.; Chanmugam, P.; Bray, G.; Hwang, D.H. Differential modulation of Toll-like receptors by fatty acids: Preferential inhibition by n-3 polyunsaturated fatty acids. J. Lipid Res. 2003, 44, 479–486. [Google Scholar] [CrossRef]
- Menon, D.; Salloum, D.; Bernfeld, E.; Gorodetsky, E.; Akselrod, A.; Frias, M.A.; Sudderth, J.; Chen, P.H.; DeBerardinis, R.; Foster, D.A. Lipid sensing by mTOR complexes via de novo synthesis of phosphatidic acid. J. Biol. Chem. 2017, 292, 6303–6311. [Google Scholar] [CrossRef] [PubMed]

| Control | High BMI | p Values | |||||
|---|---|---|---|---|---|---|---|
| DHA Supplementation (mg/d) | 0 | 200 | 0 | 200 | BMI | Supp | B*S |
| N | 10 | 7 | 6 | 7 | |||
| Maternal Age | 30.9 ± 1.97 | 34.0 ± 2.12 | 30.0 ± 2.19 | 35.5 ± 1.23 | 0.88 | 0.04 | 0.56 |
| BMI | 22.24 ± 0.57 | 22.1 ± 0.61 | 31.93 ± 2.33 | 30.1 ± 0.92 | <0.0001 | 0.38 | 0.45 |
| Fetal Sex (M/F) | 5/5 | 2/6 | 2/4 | 5/1 | |||
| Correlation with Corresponding Phosphatidic Acid Species | ||
|---|---|---|
| Species | R2 | p Value |
| 18:0_18:1 PC | 0.39 | 0.0003 |
| 18:0_18:2 PC | 0.67 | <0.0001 |
| 18:0_20:4 PC | 0.26 | 0.004 |
| 18:0_22:4 PC | 0.14 | 0.04 |
| 18:0_22:6 PC | 0.32 | 0.0013 |
| 18:0_18:1 PE | 0.38 | 0.0004 |
| 18:0_18:2 PE | 0.53 | <0.0001 |
| 18:0_20:4 PE | 0.15 | 0.04 |
| 18:0_22:4 PE | 0.24 | 0.008 |
| 18:0_22:5 PE | 0.33 | 0.0014 |
| 18:0_22:6 PE | 0.33 | 0.0011 |
| 18:0_18:1 PI | 0.62 | <0.0001 |
| 18:0_18:2 PI | 0.75 | <0.0001 |
| 18:0_20:4 PI | 0.22 | 0.01 |
| 18:0_22:4 PI | 0.34 | 0.001 |
| 18:0_22:5 PI | 0.22 | 0.01 |
| 18:0_22:6 PI | 0.56 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bidne, K.L.; Zemski Berry, K.; Dillon, M.; Jansson, T.; Powell, T.L. Maternal Docosahexaenoic Acid Supplementation Alters Maternal and Fetal Docosahexaenoic Acid Status and Placenta Phospholipids in Pregnancies Complicated by High Body Mass Index. Nutrients 2024, 16, 2934. https://doi.org/10.3390/nu16172934
Bidne KL, Zemski Berry K, Dillon M, Jansson T, Powell TL. Maternal Docosahexaenoic Acid Supplementation Alters Maternal and Fetal Docosahexaenoic Acid Status and Placenta Phospholipids in Pregnancies Complicated by High Body Mass Index. Nutrients. 2024; 16(17):2934. https://doi.org/10.3390/nu16172934
Chicago/Turabian StyleBidne, Katie L., Karin Zemski Berry, Mairead Dillon, Thomas Jansson, and Theresa L. Powell. 2024. "Maternal Docosahexaenoic Acid Supplementation Alters Maternal and Fetal Docosahexaenoic Acid Status and Placenta Phospholipids in Pregnancies Complicated by High Body Mass Index" Nutrients 16, no. 17: 2934. https://doi.org/10.3390/nu16172934
APA StyleBidne, K. L., Zemski Berry, K., Dillon, M., Jansson, T., & Powell, T. L. (2024). Maternal Docosahexaenoic Acid Supplementation Alters Maternal and Fetal Docosahexaenoic Acid Status and Placenta Phospholipids in Pregnancies Complicated by High Body Mass Index. Nutrients, 16(17), 2934. https://doi.org/10.3390/nu16172934





