The Prevalence and Prognosis of Cachexia in Patients with Non-Sarcopenic Dysphagia: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Diagnosis of Sarcopenic Dysphagia
2.3. Cachexia Diagnostic Criteria
- Subjective symptom: loss of appetite;
- Objective measure: reduced grip strength (less than 28 kg in men and less than 18 kg in women);
- Biomarker: increased C-reactive protein (CRP) levels (greater than 0.5 mg/dL).
2.4. Outcomes and Other Data
2.5. Statistical Analysis
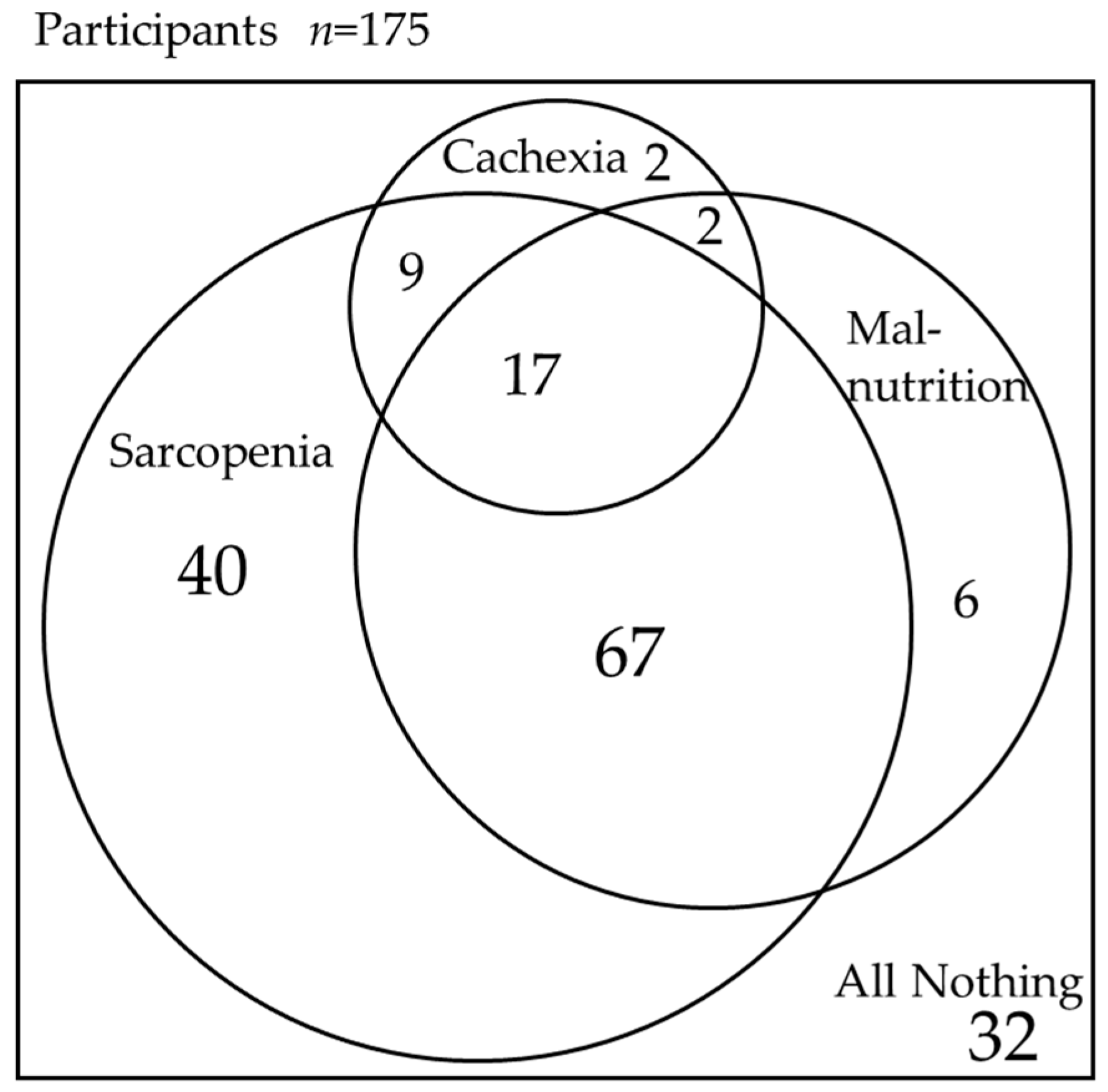
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rech, R.S.; de Goulart, B.N.G.; Dos Santos, K.W.; Marcolino, M.A.Z.; Hilgert, J.B. Frequency and associated factors for swallowing impairment in community-dwelling older persons: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2022, 34, 2945–2961. [Google Scholar] [CrossRef] [PubMed]
- Rajati, F.; Ahmadi, N.; Naghibzadeh, Z.A.; Kazeminia, M. The global prevalence of oropharyngeal dysphagia in different populations: A systematic review and meta-analysis. J. Transl. Med. 2022, 20, 175. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Miquilussi, P.A.; Gonçalves, F.M.; Taveira, K.V.M.; Stechman-Neto, J.; Nascimento, W.V.; de Araujo, C.M.; Schroder, A.G.D.; Massi, G.; Santos, R.S. The Prevalence of Oropharyngeal Dysphagia in Adults: A Systematic Review and Meta-analysis. Dysphagia 2024, 39, 163–176. [Google Scholar] [CrossRef]
- Blanař, V.; Hödl, M.; Lohrmann, C.; Amir, Y.; Eglseer, D. Dysphagia and factors associated with malnutrition risk: A 5-year multicentre study. J. Adv. Nurs. 2019, 75, 3566–3576. [Google Scholar] [CrossRef]
- Xue, W.; He, X.; Su, J.; Li, S.; Zhang, H. Association between dysphagia and activities of daily living in older adults: A systematic review and meta-analysis. Eur. Geriatr. Med. 2024, 1–17. [Google Scholar] [CrossRef]
- Roberts, H.; Lambert, K.; Walton, K. The Prevalence of Dysphagia in Individuals Living in Residential Aged Care Facilities: A Systematic Review and Meta-Analysis. Healthcare 2024, 12, 649. [Google Scholar] [CrossRef] [PubMed]
- Almirall, J.; Rofes, L.; Serra-Prat, M.; Icart, R.; Palomera, E.; Arreola, V.; Clavé, P. Oropharyngeal dysphagia is a risk factor for community-acquired pneumonia in the elderly. Eur. Respir. J. 2013, 41, 923–928. [Google Scholar] [CrossRef]
- Patel, D.A.; Krishnaswami, S.; Steger, E.; Conover, E.; Vaezi, M.F.; Ciucci, M.R.; Francis, D.O. Economic and survival burden of dysphagia among inpatients in the United States. Dis. Esophagus 2018, 31, dox131. [Google Scholar] [CrossRef]
- Márquez-Sixto, A.; Navarro-Esteva, J.; Batista-Guerra, L.Y.; Simón-Bautista, D.; Rodríguez-de Castro, F. Prevalence of Oropharyngeal Dysphagia and Its Value as a Prognostic Factor in Community-Acquired Pneumonia: A Prospective Case-Control Study. Cureus 2024, 16, e55310. [Google Scholar] [CrossRef]
- Kakehi, S.; Isono, E.; Wakabayashi, H.; Shioya, M.; Ninomiya, J.; Aoyama, Y.; Murai, R.; Sato, Y.; Takemura, R.; Mori, A.; et al. Sarcopenic Dysphagia and Simplified Rehabilitation Nutrition Care Process: An Update. Ann. Rehabil. Med. 2023, 47, 337–347. [Google Scholar] [CrossRef]
- Arai, H.; Maeda, K.; Wakabayashi, H.; Naito, T.; Konishi, M.; Assantachai, P.; Auyeung, W.T.; Chalermsri, C.; Chen, W.; Chew, J.; et al. Diagnosis and outcomes of cachexia in Asia: Working Consensus Report from the Asian Working Group for Cachexia. J. Cachexia Sarcopenia Muscle 2023, 14, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Bradley, L.; Del Fabbro, E. Updates in Cancer Cachexia: Clinical Management and Pharmacologic Interventions. Cancers 2024, 16, 1696. [Google Scholar] [CrossRef] [PubMed]
- Bertocchi, E.; Frigo, F.; Buonaccorso, L.; Venturelli, F.; Bassi, M.C.; Tanzi, S. Cancer cachexia: A scoping review on non-pharmacological interventions. Asia Pac. J. Oncol. Nurs. 2024, 11, 100438. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, T.; Maeda, K.; Takeuchi, T.; Mizuno, A.; Kato, R.; Ishida, Y.; Ueshima, J.; Shimizu, A.; Amano, K.; Mori, N. Validity of the diagnostic criteria from the Asian Working Group for Cachexia in advanced cancer. J. Cachexia Sarcopenia Muscle 2024, 15, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.M.; Zhuang, C.L.; Dong, Q.T.; Yu, Z.; Cheng, J.; Shen, X.; Wang, S.L. Characteristics and prognostic impact of cancer cachexia defined by the Asian Working Group for Cachexia consensus in patients with curable gastric cancer. Clin. Nutr. 2024, 43, 1524–1531. [Google Scholar] [CrossRef]
- Ida, S.; Imataka, K.; Morii, S.; Katsuki, K.; Murata, K. Frequency and Overlap of Cachexia, Malnutrition, and Sarcopenia in Elderly Patients with Diabetes Mellitus: A Study Using AWGC, GLIM, and AWGS2019. Nutrients 2024, 16, 236. [Google Scholar] [CrossRef]
- Yoshikoshi, S.; Imamura, K.; Yamamoto, S.; Suzuki, Y.; Harada, M.; Osada, S.; Matsuzawa, R.; Matsunaga, A. Prevalence and relevance of cachexia as diagnosed by two different definitions in patients undergoing hemodialysis: A retrospective and exploratory study. Arch. Gerontol. Geriatr. 2024, 124, 105447. [Google Scholar] [CrossRef]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.S.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.A.E.; Baldwin, C.; Chasen, M.; et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open 2021, 6, 100092. [Google Scholar] [CrossRef]
- Buccheri, E.; Dell’Aquila, D.; Russo, M.; Chiaramonte, R.; Vecchio, M. Appendicular Skeletal Muscle Mass in Older Adults Can Be Estimated with a Simple Equation Using a Few Zero-Cost Variables. J. Geriatr. Phys. Ther. 2024. [Google Scholar] [CrossRef]
- Huang, L.; Shu, X.; Ge, N.; Gao, L.; Xu, P.; Zhang, Y.; Chen, Y.; Yue, J.; Wu, C. The accuracy of screening instruments for sarcopenia: A diagnostic systematic review and meta-analysis. Age Ageing 2023, 52, afad152. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Kakehi, S.; Mizuno, S.; Kinoshita, T.; Toga, S.; Ohtsu, M.; Nishioka, S.; Momosaki, R. Prevalence and prognosis of cachexia according to the Asian Working Group for Cachexia criteria in sarcopenic dysphagia: A retrospective cohort study. Nutrition 2024, 122, 112385. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Wakabayashi, H.; Nishioka, S.; Momosaki, R. Malnutrition and cachexia may affect death but not functional improvement in patients with sarcopenic dysphagia. Eur. Geriatr. Med. 2024, 15, 777–785. [Google Scholar] [CrossRef]
- Mizuno, S.; Wakabayashi, H.; Fujishima, I.; Kishima, M.; Itoda, M.; Yamakawa, M.; Wada, F.; Kato, R.; Furiya, Y.; Nishioka, S.; et al. Construction and Quality Evaluation of the Japanese Sarcopenic Dysphagia Database. J. Nutr. Health Aging 2021, 25, 926–932. [Google Scholar] [CrossRef]
- Miyai, I.; Sonoda, S.; Nagai, S.; Takayama, Y.; Inoue, Y.; Kakehi, A.; Kurihara, M.; Ishikawa, M. Results of new policies for inpatient rehabilitation coverage in Japan. Neurorehabil. Neural Repair 2011, 25, 540–547. [Google Scholar] [CrossRef]
- Fujishima, I.; Fujiu-Kurachi, M.; Arai, H.; Hyodo, M.; Kagaya, H.; Maeda, K.; Mori, T.; Nishioka, S.; Oshima, F.; Ogawa, S.; et al. Sarcopenia and dysphagia: Position paper by four professional organizations. Geriatr. Gerontol. Int. 2019, 19, 91–97. [Google Scholar] [CrossRef]
- Mori, T.; Fujishima, I.; Wakabayashi, H.; Oshima, F.; Itoda, M.; Kunieda, K.; Kayashita, J.; Nishioka, S.; Sonoda, A.; Kuroda, Y.; et al. Development, reliability, and validity of a diagnostic algorithm for sarcopenic dysphagia. JCSM Clin. Rep. 2017, 2, 1–10. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Akagi, J. Decreased tongue pressure is associated with sarcopenia and sarcopenic dysphagia in the elderly. Dysphagia 2015, 30, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Kunieda, K.; Ohno, T.; Fujishima, I.; Hojo, K.; Morita, T. Reliability and validity of a tool to measure the severity of dysphagia: The Food Intake LEVEL Scale. J. Pain Symptom Manag. 2013, 46, 201–206. [Google Scholar] [CrossRef]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Ueshima, J.; Inoue, T.; Saino, Y.; Kobayashi, H.; Murotani, K.; Mori, N.; Maeda, K. Diagnosis and prevalence of cachexia in Asians: A scoping review. Nutrition 2024, 119, 112301. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Zimmers, T.A. Sex Differences in Cancer Cachexia. Curr. Osteoporos. Rep. 2020, 18, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Geppert, J.; Rohm, M. Cancer cachexia: Biomarkers and the influence of age. Mol. Oncol. 2024, 1–17. [Google Scholar] [CrossRef]
- Permuth, J.B.; Park, M.A.; Chen, D.T.; Basinski, T.; Powers, B.D.; Gwede, C.K.; Dezsi, K.B.; Gomez, M.; Vyas, S.L.; Biachi, T.; et al. Leveraging real-world data to predict cancer cachexia stage, quality of life, and survival in a racially and ethnically diverse multi-institutional cohort of treatment-naïve patients with pancreatic ductal adenocarcinoma. Front. Oncol. 2024, 14, 1362244. [Google Scholar] [CrossRef]
- Han, J.; Liu, X.; Wang, J.; Tang, M.; Xu, J.; Tan, S.; Wu, G. Prognostic value of body composition in patients with digestive tract cancers: A prospective cohort study of 8267 adults from China. Clin. Nutr. ESPEN 2024, 62, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Caldwell, M.E.; Greene, N.P. Muscle metabolism and atrophy: Let’s talk about sex. Biol. Sex Differ. 2019, 10, 43. [Google Scholar] [CrossRef]
- Zhong, X.; Narasimhan, A.; Silverman, L.M.; Young, A.R.; Shahda, S.; Liu, S.; Wan, J.; Liu, Y.; Koniaris, L.G.; Zimmers, T.A. Sex specificity of pancreatic cancer cachexia phenotypes, mechanisms, and treatment in mice and humans: Role of Activin. J. Cachexia Sarcopenia Muscle 2022, 13, 2146–2161. [Google Scholar] [CrossRef]
- Bonomi, P.D.; Crawford, J.; Dunne, R.F.; Roeland, E.J.; Smoyer, K.E.; Siddiqui, M.K.; McRae, T.D.; Rossulek, M.I.; Revkin, J.H.; Tarasenko, L.C. Mortality burden of pre-treatment weight loss in patients with non-small-cell lung cancer: A systematic literature review and meta-analysis. J. Cachexia Sarcopenia Muscle 2024, 15, 1226–1239. [Google Scholar] [CrossRef]
- Amano, K.; Okamura, S.; Baracos, V.E.; Mori, N.; Sakaguchi, T.; Uneno, Y.; Hiratsuka, Y.; Hamano, J.; Miura, T.; Ishiki, H.; et al. Impacts of fluid retention on prognostic abilities of cachexia diagnostic criteria in cancer patients with refractory cachexia. Clin. Nutr. ESPEN 2024, 60, 373–381. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Tsintavis, P.; Potsaki, P.; Papandreou, D. Differences in the Prevalence of Sarcopenia in Community-Dwelling, Nursing Home and Hospitalized Individuals. A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2020, 24, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Churilov, I.; Churilov, L.; MacIsaac, R.J.; Ekinci, E.I. Systematic review and meta-analysis of prevalence of sarcopenia in post acute inpatient rehabilitation. Osteoporos. Int. 2018, 29, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.N.; Thiam, C.N.; Ang, Q.X.; Engkasan, J.; Ong, T. Incident sarcopenia in hospitalized older people: A systematic review. PLoS ONE 2023, 18, e0289379. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Pell, J.P.; Celis-Morales, C.; Ho, F.K. Frailty, sarcopenia, cachexia and malnutrition as comorbid conditions and their associations with mortality: A prospective study from UK Biobank. J. Public Health 2022, 44, e172–e180. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H. Hospital-associated sarcopenia, acute sarcopenia, and iatrogenic sarcopenia: Prevention of sarcopenia during hospitalization. J. Gen. Fam. Med. 2023, 24, 146–147. [Google Scholar] [CrossRef]
- Liposits, G.; Singhal, S.; Krok-Schoen, J.L. Interventions to improve nutritional status for older patients with cancer—A holistic approach is needed. Curr. Opin. Support. Palliat. Care 2023, 17, 15–21. [Google Scholar] [CrossRef]
- Agostini, D.; Gervasi, M.; Ferrini, F.; Bartolacci, A.; Stranieri, A.; Piccoli, G.; Barbieri, E.; Sestili, P.; Patti, A.; Stocchi, V.; et al. An Integrated Approach to Skeletal Muscle Health in Aging. Nutrients 2023, 15, 1802. [Google Scholar] [CrossRef]
- Mititelu, M.; Licu, M.; Neacșu, S.M.; Călin, M.F.; Matei, S.R.; Scafa-Udriște, A.; Stanciu, T.I.; Busnatu, Ș.; Olteanu, G.; Măru, N.; et al. An Assessment of Behavioral Risk Factors in Oncology Patients. Nutrients 2024, 16, 2527. [Google Scholar] [CrossRef] [PubMed]
- Mangano, G.R.A.; Avola, M.; Blatti, C.; Caldaci, A.; Sapienza, M.; Chiaramonte, R.; Vecchio, M.; Pavone, V.; Testa, G. Non-Adherence to Anti-Osteoporosis Medication: Factors Influencing and Strategies to Overcome It. A Narrative Review. J. Clin. Med. 2022, 12, 14. [Google Scholar] [CrossRef]
- Finch, A.; Benham, A. Patient attitudes and experiences towards exercise during oncological treatment. A qualitative systematic review. Support. Care Cancer 2024, 32, 509. [Google Scholar] [CrossRef]
- Gautam, P.; Shankar, A. Management of cancer cachexia towards optimizing care delivery and patient outcomes. Asia Pac. J. Oncol. Nurs. 2023, 10, 100322. [Google Scholar] [CrossRef] [PubMed]
- Park, M.A.; Whelan, C.J.; Ahmed, S.; Boeringer, T.; Brown, J.; Crowder, S.L.; Gage, K.; Gregg, C.; Jeong, D.K.; Jim, H.S.L.; et al. Defining and Addressing Research Priorities in Cancer Cachexia through Transdisciplinary Collaboration. Cancers 2024, 16, 2364. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.; Boocock, E.; Grande, A.J.; Maddocks, M. Exercise-based interventions for cancer cachexia: A systematic review of randomised and non-randomised controlled trials. Asia Pac. J. Oncol. Nurs. 2023, 10, 100335. [Google Scholar] [CrossRef]
- Slee, A.; Reid, J. Exercise and nutrition interventions for renal cachexia. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 219–225. [Google Scholar] [CrossRef]
- Meza-Valderrama, D.; Marco, E.; Dávalos-Yerovi, V.; Muns, M.D.; Tejero-Sánchez, M.; Duarte, E.; Sánchez-Rodríguez, D. Sarcopenia, Malnutrition, and Cachexia: Adapting Definitions and Terminology of Nutritional Disorders in Older People with Cancer. Nutrients 2021, 13, 761. [Google Scholar] [CrossRef] [PubMed]
- Aprile, G.; Basile, D.; Giaretta, R.; Schiavo, G.; La Verde, N.; Corradi, E.; Monge, T.; Agustoni, F.; Stragliotto, S. The Clinical Value of Nutritional Care before and during Active Cancer Treatment. Nutrients 2021, 13, 1196. [Google Scholar] [CrossRef]

| Total n = 175 | Cachexia (+) n = 30 | Cachexia (−) n = 145 | p-Value | |
|---|---|---|---|---|
| Age, years, mean ± SD * | 77 ± 11 | 78 ± 9 | 77 ± 11 | 0.437 a |
| Sex, n (%) | 0.103 b | |||
| Men | 103 (59%) | 22 (73%) | 81 (56%) | |
| Women | 72 (41%) | 8 (27%) | 64 (44%) | |
| Setting, n (%) | 0.001 b | |||
| Acute care hospitals | 78 (45%) | 23 (77%) | 55 (38%) | |
| Rehabilitation hospitals | 76 (43%) | 4 (13%) | 72 (50%) | |
| Others | 21 (12%) | 3 (10%) | 18 (12%) | |
| Main causative diseases of dysphagia, n (%) | ||||
| Cerebral infarction | 77 (44%) | 9 (30%) | 68 (47%) | 0.113 b |
| Cerebral hemorrhage | 19 (11%) | 2 (7%) | 17 (12%) | 0.536 b |
| Subarachnoid hemorrhage | 9 (5%) | 0 (0%) | 9 (6%) | 0.361 b |
| Parkinsonism | 15 (9%) | 5 (17%) | 10 (7%) | 0.142 b |
| Dementia | 12 (7%) | 3 (10%) | 9 (6%) | 0.435 b |
| Cancer | 8 (5%) | 7 (23%) | 1 (1%) | <0.001 b |
| Others | 35 (20%) | 4 (13%) | 31 (21%) | 0.453 b |
| Comorbidities, n (%) | ||||
| Chronic heart failure | 21 (12%) | 9 (30%) | 12 (8%) | <0.001 b |
| Cancer | 13 (7%) | 15 (50%) | 8 (6%) | <0.001 b |
| Chronic renal failure | 8 (5%) | 3 (10%) | 5 (3%) | 0.139 b |
| Chronic obstructive pulmonary disease | 8 (5%) | 4 (13%) | 4 (3%) | 0.030 |
| Chronic respiratory failure | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Progressive worsening or uncontrolled chronic infections | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Chronic liver failure | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Rheumatoid arthritis and other collagen diseases | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Sarcopenia, n (%) | 133 (76%) | 26 (87%) | 107 (74%) | 0.133 |
| Malnutrition, n (%) | 92 (53%) | 19 (63%) | 73 (50%) | 0.181 |
| Body mass index, kg/m2, mean ± SD * | 21.0 ± 4.0 | 18.9 ± 3.0 | 21.4 ± 4.0 | <0.001 a |
| Body weight change in 6 months, %, median (IQR **) | 3.3 (−1.9, 12.7) | 11.0 (3.3, 19.7) | 1.5 (−3.5, 10.5) | 0.074 c |
| Handgrip strength, kg, mean ± SD * | 16.5 ± 10.9 | 15.2 ± 10.2 | 16.8 ± 11.1 | 0.467 a |
| C-reactive protein, mg/dL, median (IQR **) | 0.5 (0.1, 1.9) | 3.4 (0.8, 11.6) | 0.3 (0.1, 1.0) | <0.001 c |
| FILS *** initial, median (IQR **) | 7 (1, 7) | 2 (1, 7) | 7 (1, 7) | 0.160 c |
| FILS follow-up, median (IQR **) | 8 (7, 8) | 7 (5.5, 8) | 8 (7, 8) | 0.585 c |
| Barthel Index initial, median (IQR **) | 25 (10, 50) | 25 (5, 67.5) | 30 (10, 50) | 0.406 c |
| Barthel Index follow-up, median (IQR **) | 50 (20, 85) | 35 (12.5, 75) | 50 (20, 85) | 0.469 c |
| Death, n (%) | 7 (4%) | 5 (17%) | 2 (1%) | 0.002 b |
| No | Sex | Causative Disease of Admission | Cause of Death | Sarcopenia | Malnutrition | Cachexia |
|---|---|---|---|---|---|---|
| 1 | Woman | Urinary tract infection | Senility | + | + | + |
| 2 | Man | Gastric cancer | Systemic metastatic cancer | + | − | + |
| 3 | Man | Colorectal cancer | Colorectal cancer | + | + | + |
| 4 | Man | Urinary tract infection | Pyothorax | + | + | + |
| 5 | Man | Cardiogenic cerebral embolism | Unknown | + | + | + |
| 6 | Woman | Cerebral infarction | Stroke | + | + | − |
| 7 | Woman | Multiple system atrophy | Septic shock | + | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakehi, S.; Wakabayashi, H.; Nagai, T.; Nishioka, S.; Isono, E.; Otsuka, Y.; Ninomiya, J.; Momosaki, R. The Prevalence and Prognosis of Cachexia in Patients with Non-Sarcopenic Dysphagia: A Retrospective Cohort Study. Nutrients 2024, 16, 2917. https://doi.org/10.3390/nu16172917
Kakehi S, Wakabayashi H, Nagai T, Nishioka S, Isono E, Otsuka Y, Ninomiya J, Momosaki R. The Prevalence and Prognosis of Cachexia in Patients with Non-Sarcopenic Dysphagia: A Retrospective Cohort Study. Nutrients. 2024; 16(17):2917. https://doi.org/10.3390/nu16172917
Chicago/Turabian StyleKakehi, Shingo, Hidetaka Wakabayashi, Takako Nagai, Shinta Nishioka, Eri Isono, Yukiko Otsuka, Junki Ninomiya, and Ryo Momosaki. 2024. "The Prevalence and Prognosis of Cachexia in Patients with Non-Sarcopenic Dysphagia: A Retrospective Cohort Study" Nutrients 16, no. 17: 2917. https://doi.org/10.3390/nu16172917
APA StyleKakehi, S., Wakabayashi, H., Nagai, T., Nishioka, S., Isono, E., Otsuka, Y., Ninomiya, J., & Momosaki, R. (2024). The Prevalence and Prognosis of Cachexia in Patients with Non-Sarcopenic Dysphagia: A Retrospective Cohort Study. Nutrients, 16(17), 2917. https://doi.org/10.3390/nu16172917







