Exogenous Lactate Treatment Immediately after Exercise Promotes Glycogen Recovery in Type-II Muscle in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Experimental Design
2.3. Exercise Protocol
2.4. Blood Analysis
2.5. Glycogen Concentration Analysis
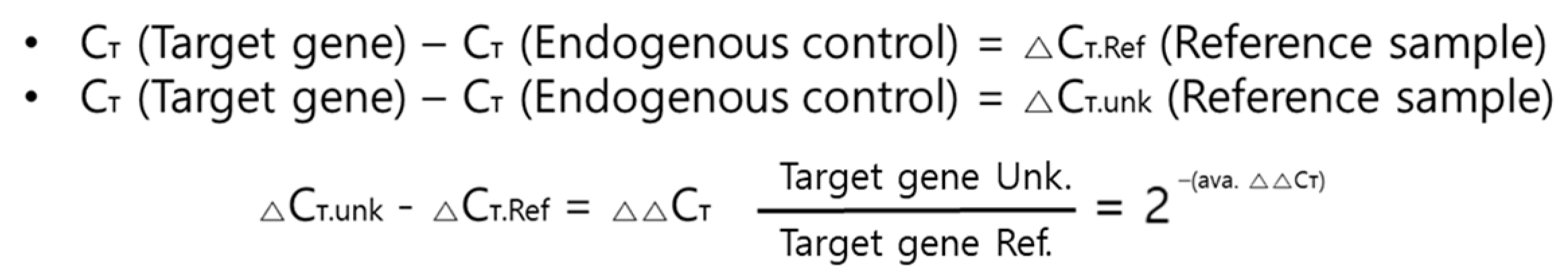
2.6. Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.7. Statistical Analysis
3. Results
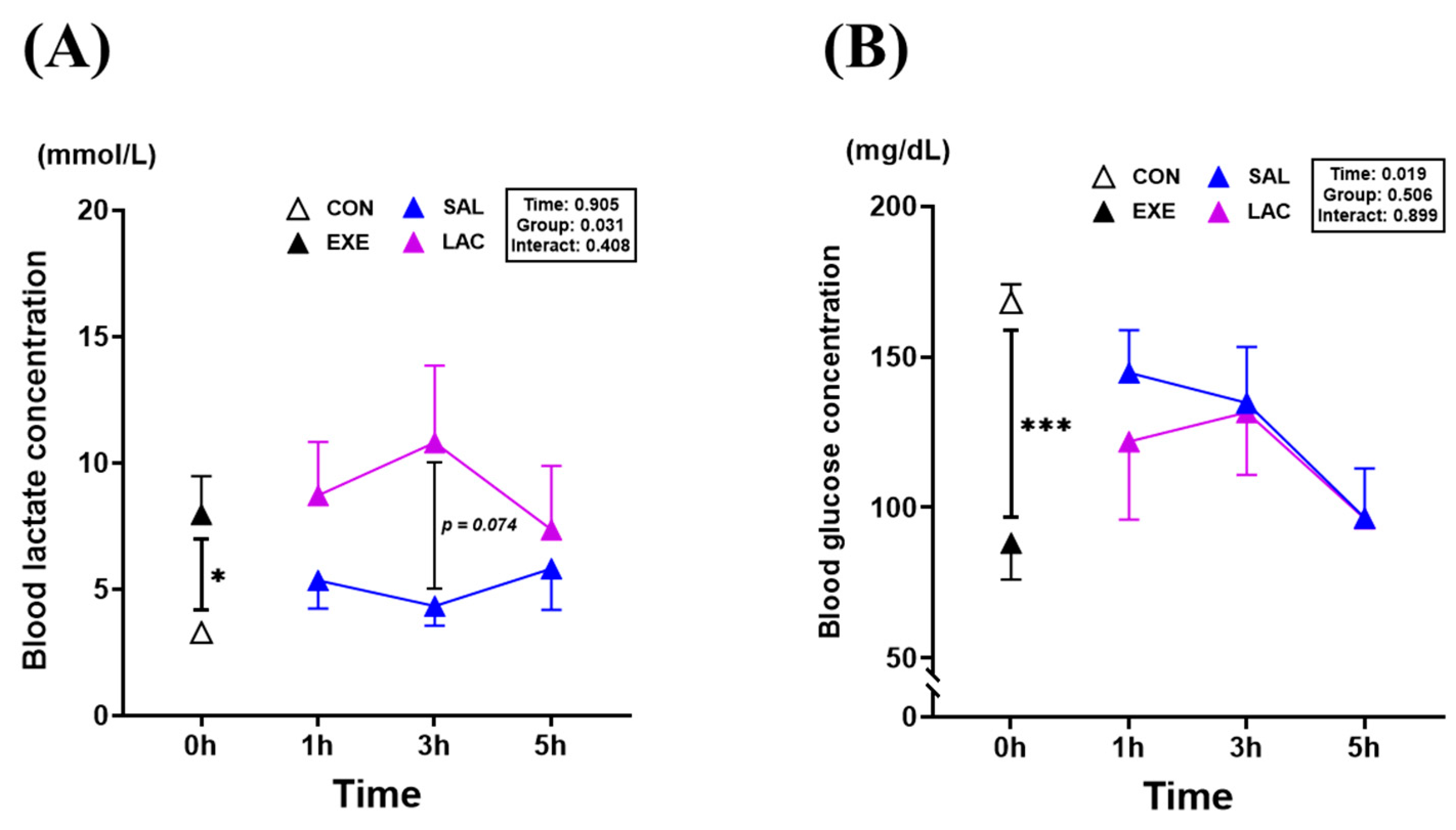
3.1. Blood Lactate and Blood Glucose Level
3.2. Muscle Weight and Muscle Glycogen Concentration
3.3. Glycogen Synthase and MCT-1,4 Gene Expression in Muscle
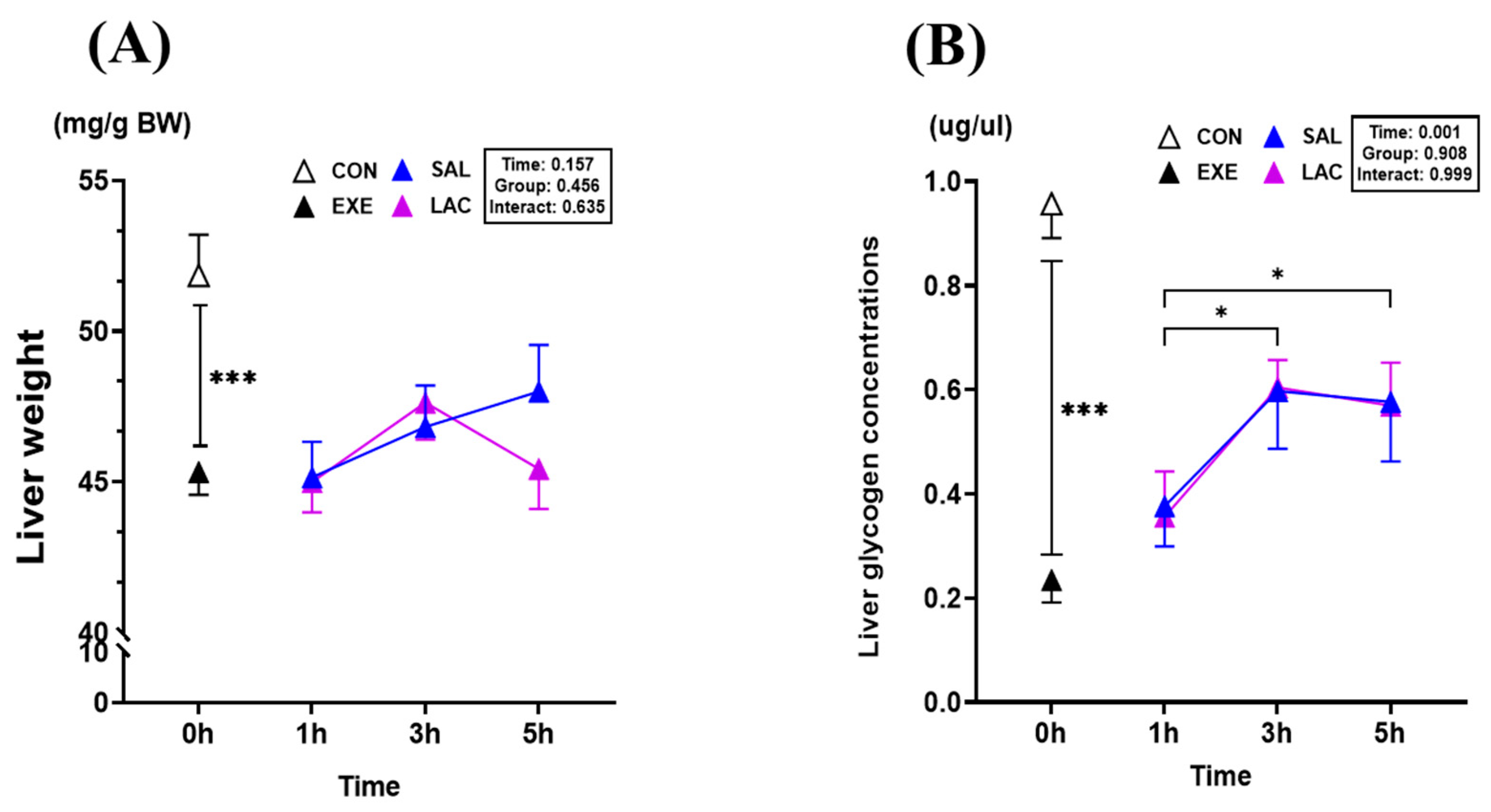
3.4. Liver Weight and Liver Glycogen Concentration
3.5. Gluconeogenesis Gene Expression in Liver
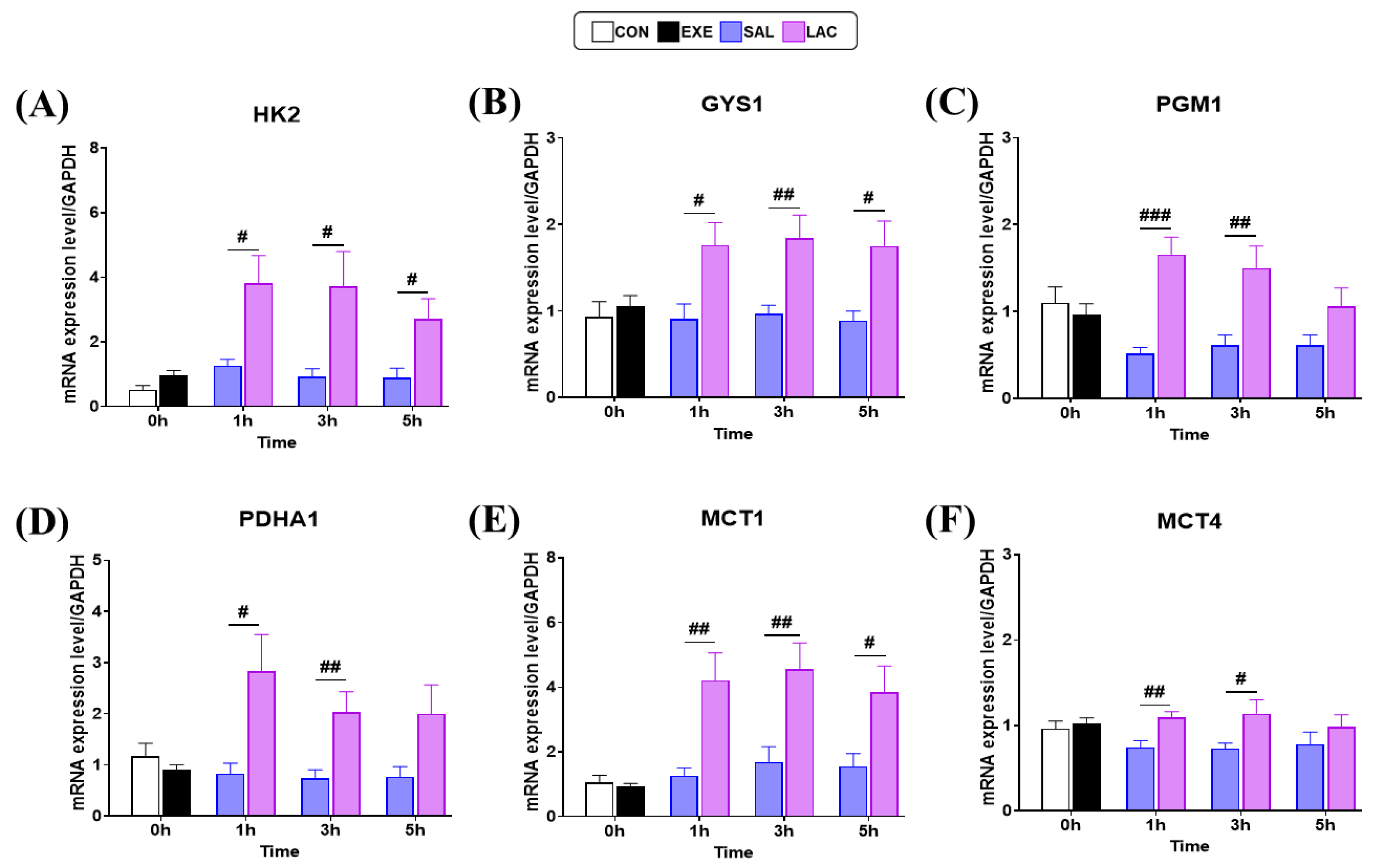
3.6. Glycogen Synthesis and MCT-1,4 Gene Expression in Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Close, G.L.; Hamilton, D.L.; Philp, A.; Burke, L.M.; Morton, J.P. New Strategies in Sport Nutrition to Increase Exercise Performance. Free Radic. Biol. Med. 2016, 98, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal Muscle Energy Metabolism during Exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; González-Lucán, M.; Donapetry-García, C.; Fernández-Fernández, C.; Ameneiros-Rodríguez, E. Glycogen Metabolism in Humans. BBA Clin. 2016, 5, 85–100. [Google Scholar] [CrossRef]
- Ivy, J.L. Muscle glycogen synthesis before and after exercise. Sports Med. 1991, 11, 6–19. [Google Scholar] [CrossRef]
- Takahashi, K.; Kitaoka, Y.; Matsunaga, Y.; Hatta, H. Effect of Post-Exercise Lactate Administration on Glycogen Repletion and Signaling Activation in Different Types of Mouse Skeletal Muscle. Curr. Res. Physiol. 2020, 3, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Ivy, J.L.; Kuo, C.H. Regulation of GLUT4 Protein and Glycogen Synthase during Muscle Glycogen Synthesis after Exercise. Acta Physiol. Scand. 1998, 162, 295–304. [Google Scholar] [CrossRef]
- Hawley, J.A.; Schabort, E.; Noakes, T.D.; Dennis, S.C. Carbohydrate-Loading and Exercise Performance an Update. Sports Med. 1997, 24, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Shulman, R.G.; Rothman, D.L. The “glycogen Shunt” in Exercising Muscle: A Role for Glycogen in Muscle Energetics and Fatigue. Proc. Natl. Acad. Sci. USA 2001, 98, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Thein, L.A.; Thein, J.M.; Landry, G.L. Ergogenic aids. Phys. Ther. 1995, 75, 426–439. [Google Scholar] [CrossRef]
- Sedlock, D.A. The Latest on Carbohydrate Loading: A Practical Approach. Curr. Sports Med. Rep. 2008, 7, 209–213. [Google Scholar] [CrossRef]
- Kondo, E.; Takai, E.; Sagayama, H.; Takahashi, H. Comparison of Three Type of Muscle Glycogen Loading Interventions Using a Very-High-Carbohydrate Diet in an Elite Male Racewalker: A Case Report. Phys. Act. Nutr. 2023, 27, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wiącek, J.; Karolkiewicz, J. Different Approaches to Ergogenic, Pre-, and Probiotic Supplementation in Sports with Different Metabolism Characteristics: A Mini Review. Nutrients 2023, 15, 1541. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Lim, K. Nutrition Supplements to Stimulate Lipolysis: A Review in Relation to Endurance Exercise Capacity. J. Nutr. Sci. Vitaminol. 2016, 62, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moreno, C.; Gutiérrez-Hellín, J.; Amaro-Gahete, F.J.; González-García, J.; Giráldez-Costas, V.; Pérez-García, V.; Del Coso, J. Caffeine Increases Whole-Body Fat Oxidation during 1 h of Cycling at Fatmax. Eur. J. Nutr. 2021, 60, 2077–2085. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC Consensus Statement: Dietary Supplements and the High-Performance Athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef]
- Rawson, E.S.; Miles, M.P.; Larson-Meyer, D.E. Dietary Supplements for Health, Adaptation, and Recovery in Athletes. Int. J. Sport. Nutr. Exerc. Metab. 2018, 28, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, M.; Takeda, M. Lactate as a Signaling Molecule That Regulates Exercise-Induced Adaptations. Biology 2016, 5, 38. [Google Scholar] [CrossRef]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Kitaoka, Y.; Hoshino, D.; Hatta, H. Monocarboxylate Transporter and Lactate Metabolism. J. Phys. Fit. Sports Med. 2012, 1, 247–252. [Google Scholar] [CrossRef]
- Stevenson, R.W.; Mitchell, D.R.; Hendrick, G.K.; Rainey, R.; Cherrington, A.D.; Tyler Frizzell, R. Lactate as Substrate for Glycogen Resynthesis after Exercise. J. Appl. Physiol. 1987, 62, 2237–2240. [Google Scholar] [CrossRef]
- Cori, C.F.; Cori, G.T. Glycogen formation in the liver from d-and l-lactic acid. J. Biol. Chem. 1929, 81, 389–403. [Google Scholar] [CrossRef]
- Hoshino, D.; Hanawa, T.; Takahashi, Y.; Masuda, H.; Kato, M.; Hatta, H. Chronic post-exercise lactate administration with endurance training increases glycogen concentration and monocarboxylate transporter 1 protein in mouse white muscle. J. Nutr. Sci. Vitaminol. 2014, 60, 413–419. [Google Scholar] [CrossRef]
- Hwang, D.; Kim, J.; Kyun, S.; Jang, I.; Kim, T.; Park, H.Y.; Lim, K. Exogenous Lactate Augments Exercise-Induced Improvement in Memory but Not in Hippocampal Neurogenesis. Sci. Rep. 2023, 13, 5838. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Bagby, G.J. Gluconeogenic pathway in liver and muscle glycogen synthesis after exercise. J. Appl. Physiol. 1988, 64, 1591–1599. [Google Scholar] [CrossRef]
- McLane, J.A.; Holloszy, J.O. Glycogen synthesis from lactate in the three types of skeletal muscle. J. Biol. Chem. 1979, 254, 6548–6553. [Google Scholar] [CrossRef]
- Yoo, C.; Kim, J.; Kyun, S.; Hashimoto, T.; Tomi, H.; Lim, K. Synergic Effect of Exogenous Lactate and Caffeine on Fat Oxidation and Hepatic Glycogen Concentration in Resting Rats. Phys. Act. Nutr. 2022, 26, 5–13. [Google Scholar] [CrossRef]
- Jang, I.; Kim, J.; Kyun, S.; Hwang, D.; Lim, K. Acute Administration of Exogenous Lactate Increases Carbohydrate Metabolism during Exercise in Mice. Metabolites 2021, 11, 553. [Google Scholar] [CrossRef] [PubMed]
- E, L.; Lu, J.; Selfridge, J.E.; Burns, J.M.; Swerdlow, R.H. Lactate Administration Reproduces Specific Brain and Liver Exercise-Related Changes. J. Neurochem. 2013, 127, 91–100. [Google Scholar] [CrossRef]
- Kyun, S.; Kim, J.; Hwang, D.; Jang, I.; Choi, J.; Kim, J.; Jung, W.S.; Hwang, H.; Kim, S.W.; Kim, J.; et al. Exogenous Lactate Intake Immediately after Endurance Exercise Increases Time to Exhaustion in VO2max Measurements in Mice. Phys. Act. Nutr. 2023, 27, 13–18. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate as a fulcrum of metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef] [PubMed]
- Shiose, K.; Takahashi, H.; Yamada, Y. Muscle Glycogen Assessment and Relationship with Body Hydration Status: A Narrative Review. Nutrients 2023, 15, 155. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.M.; Plyley, M.J.; Sharp, R.L.; Van Handel, P.J.; McAllister, R.M.; Fink, W.J.; Costill, D.L. Muscle glycogen storage and its relationship with water. Int. J. Sports Med. 1982, 3, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Sarkar, J.; Yamada, J.; Matsunaga, Y.; Nonaka, Y.; Banjo, M.; Sakaguchi, R.; Shinya, T.; Hatta, H. Enhanced Skeletal Muscle Glycogen Repletion after Endurance Exercise Is Associated with Higher Plasma Insulin and Skeletal Muscle Hexokinase 2 Protein Levels in Mice: Comparison of Level Running and Downhill Running Model. J. Physiol. Biochem. 2021, 77, 469–480. [Google Scholar] [CrossRef]
- Irimia, J.M.; Rovira, J.; Nielsen, J.N.; Guerrero, M.; Wojtaszewski, J.F.P.; Cussó, R. Hexokinase 2, Glycogen Synthase and Phosphorylase Play a Key Role in Muscle Glycogen Supercompensation. PLoS ONE 2012, 7, e42453. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.B.; Hay, N. Mitochondrial Hexokinases: Guardians of the Mitochondria. Cell Cycle 2005, 4, 654–658. [Google Scholar] [CrossRef]
- Fueger, P.T.; Shearer, J.; Krueger, T.M.; Posey, K.A.; Bracy, D.P.; Heikkinen, S.; Laakso, M.; Rottman, J.N.; Wasserman, D.H. Hexokinase II Protein Content Is a Determinant of Exercise Endurance Capacity in the Mouse. J. Physiol. 2005, 566, 533–541. [Google Scholar] [CrossRef]
- Leite, T.C.; Coelho, R.G.; Da Silva, D.; Coelho, W.S.; Marinho-Carvalho, M.M.; Sola-Penna, M. Lactate Downregulates the Glycolytic Enzymes Hexokinase and Phosphofructokinase in Diverse Tissues from Mice. FEBS Lett. 2011, 585, 92–98. [Google Scholar] [CrossRef]
- Manchester, J.; Skurat, A.V.; Roach, P.; Hauschka, S.D.; Lawrence, J.C., Jr. Increased glycogen accumulation in transgenic mice overexpressing glycogen synthase in skeletal muscle. Proc. Natl. Acad. Sci. USA 1996, 93, 10707–10711. [Google Scholar] [CrossRef]
- Xirouchaki, C.E.; Mangiafico, S.P.; Bate, K.; Ruan, Z.; Huang, A.M.; Tedjosiswoyo, B.W.; Lamont, B.; Pong, W.; Favaloro, J.; Blair, A.R.; et al. Impaired Glucose Metabolism and Exercise Capacity with Muscle-Specific Glycogen Synthase 1 (Gys1) Deletion in Adult Mice. Mol. Metab. 2016, 5, 221–232. [Google Scholar] [CrossRef]
- Svensson, K.; Dent, J.R.; Tahvilian, S.; Martins, V.F.; Sathe, A.; Ochala, J.; Patel, M.S.; Schenk, S. Defining the Contribution of Skeletal Muscle Pyruvate Dehydrogenase 1 to Exercise Performance and Insulin Action. Am. J. Physiol. Endocrinol. Metab. 2018, 315, 1034–1045. [Google Scholar] [CrossRef]
- Patel, M.S.; Nemeria, N.S.; Furey, W.; Jordan, F. The Pyruvate Dehydrogenase Complexes: Structure-Based Function and Regulation. J. Biol. Chem. 2014, 289, 16615–16623. [Google Scholar] [CrossRef]
- Ward, G.R.; Sutton, J.R.; Jones, N.L.; Toews, C.J. Activation by exercise of human skeletal muscle pyruvate dehydrogenase in vivo. Clin. Sci. 1982, 63, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Dubouchaud, H.; Butterfield, G.E.; Wolfel, E.E.; Bergman, B.C.; Brooks, G.A.; Dubouchaud, G.E.; Butterfield, E.E.; Wolfel, B.C.; Bergman, G.A.B. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am. J. Physiol. -Endocrinol. Metab. 2000, 278, E571–E579. [Google Scholar] [CrossRef] [PubMed]
- Bonen, A. The Expression of Lactate Transporters (MCT1 and MCT4) in Heart and Muscle. Eur. J. Appl. Physiol. 2001, 86, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Coles, L.; Litt, J.; Hatta, H.; Bonen, A. Exercise Rapidly Increases Expression of the Monocarboxylate Transporters MCT1 and MCT4 in Rat Muscle. J. Physiol. 2004, 561, 253–261. [Google Scholar] [CrossRef]
- Kraus-Friedmann, N. Hormonal Regulation of Hepatic Gluconeogenesis. Physiol. Rev. 1984, 64, 170–259. [Google Scholar] [CrossRef]
- Pilkis, S.J.; Granner, D.K. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu. Rev. Physiol. 1992, 54, 885–909. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.G.; Biensø, R.S.; Hassing, H.A.; Jakobsen, A.H.; Pilegaard, H. Exercise-Induced Regulation of Key Factors in Substrate Choice and Gluconeogenesis in Mouse Liver. Mol. Cell. Biochem. 2015, 403, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Wondisford, F.E. Reply to Hernández—Glycolysis and gluconeogenesis: A teaching view. J. Biol. Chem. 2021, 296, 100016. [Google Scholar] [CrossRef]
- Wallace, J.C.; Jitrapakdee, S.; Chapman-Smith, A. Pyruvate carboxylase. Int. J. Biochem. Cell Biol. 1998, 30, 1–5. [Google Scholar] [CrossRef]
- Jitrapakdee, S.; Wallace, J.C. Structure, function and regulation of pyruvate carboxylase. Biochem. J. 1999, 340, 1–16. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Shiota, M.; Shelton, K.D.; Chalkley, R.; Postic, C.; Magnuson, M.A. Phosphoenolpyruvate Carboxykinase Is Necessary for the Integration of Hepatic Energy Metabolism. Mol. Cell. Biol. 2000, 20, 6508–6517. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.C.; He, T.T.; Yan, Z.; Lindner, J.; Sherry, A.D.; Malloy, C.R.; Browning, J.D.D.; Magnuson, M.A. Cytosolic Phosphoenolpyruvate Carboxykinase Does Not Solely Control the Rate of Hepatic Gluconeogenesis in the Intact Mouse Liver. Cell Metab. 2007, 5, 313–320. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Burgess, S.C.; Shiota, M.; Flakoll, P.; Donahue, E.P.; Malloy, C.R.; Sherry, A.D.; Magnuson, M.A. Mechanisms by Which Liver-Specific PEPCK Knockout Mice Preserve Euglycemia During Starvation. Diabetes 2003, 52, 1649–1654. [Google Scholar] [CrossRef]
- Maehlum, S.V.; Felig, P.H.; Wahren, J.O. Splanchnic glucose and muscle glycogen metabolism after glucose feeding during postexercise recovery. Am. J. Physiol.-Endocrinol. Metab. 1978, 235, E255. [Google Scholar] [CrossRef]
- Takahashi, Y.; Matsunaga, Y.; Banjo, M.; Takahashi, K.; Sato, Y.; Seike, K.; Nakano, S.; Hatta, H. Effects of Nutrient Intake Timing on Post-Exercise Glycogen Accumulation and Its Related Signaling Pathways in Mouse Skeletal Muscle. Nutrients 2019, 11, 2555. [Google Scholar] [CrossRef]
- Hamilton, K.S.; Gibbons, F.K.; Bracy, D.P.; Lacy, D.B.; Cherrington, A.D.; Wasserman, D.H. Effect of Prior Exercise on the Partitioning of an Intestinal Glucose Load between Splanchnic Bed and Skeletal Muscle. J. Clin. Investig. 1996, 98, 125–135. [Google Scholar] [CrossRef]
- Gunderson, H.; Wehmeyer, N.; Burnett, D.; Nauman, J.; Hartzell, C.; Savage, S. Exercise and exhaustion effects on glycogen synthesis pathways. J. Appl. Physiol. 1996, 81, 2020–2026. [Google Scholar] [CrossRef]
- Exton, J.H.; Park, C.R. Control of gluconeogenesis in liver: I. General features of gluconeogenesis in the perfused livers of rats. J. Biol. Chem. 1967, 242, 2622–2636. [Google Scholar] [CrossRef] [PubMed]
- Radziuk, J.; Pye, S. Hepatic Glucose Uptake, Gluconeogenesis and the Regulation of Glycogen Synthesis. Diabetes Metab. Res. Rev. 2001, 17, 250–272. [Google Scholar] [CrossRef]
- Bogdanov, P.; Corraliza, L.; Villena, J.A.; Carvalho, A.R.; Garcia-Arumí, J.; Ramos, D.; Ruberte, J.; Simó, R.; Hernández, C. The Db/Db Mouse: A Useful Model for the Study of Diabetic Retinal Neurodegeneration. PLoS ONE 2014, 9, e97302. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Hwang, D.; Kyun, S.; Jang, I.; Kim, S.W.; Park, H.Y.; Lim, K.; Kim, C.; Kim, J. Effects of Post-Exercise Intake of Exogenous Lactate on Energy Substrate Utilization at Rest. Phys. Act. Nutr. 2024, 28, 1–6. [Google Scholar] [CrossRef] [PubMed]



| Tissue. | Primer Name | Sequence of Primers (5′–3′) |
|---|---|---|
| Liver | GAPDH–F | TGG CCT CCA AGG AGT AAG AAA C |
| GAPDH–R | GGG ATA GGG CCT CTC TTG CT | |
| G6Pase–F | GCC TCC GGA AGT ATT GTC TCA T | |
| G6Pase–R | CAC CCC TAG CCC TTT TAG TAG CA | |
| PC–F | GCC CTA TGT TGC CCA CAA CT | |
| PC–R | GAA CGG GAT GTT CGG GAT AA | |
| PCK1–F | TGT TCG GGC GGA TTG AAG | |
| PCK1–R | TCA GGT TCA AGG CGT TTT CC | |
| FBP1–F | TCT GCA CCG CGA TCA AAG | |
| FBP1–R | TGG TTG AGC CAG CGA TAC C | |
| GK–F | TGT GAG GTC GGC ATG ATT GT | |
| GK–R | CCT TCC ACC AGC TCC ACA TT | |
| GYS2–F | GAG ACA GTC TTT GCC TCC TGT GA | |
| GYS2–R | CCT TGA CTC TGT CTG CAC GAT T | |
| MCT1–F | GTG ACC ATT GTG GAA TGC TG | |
| MCT1–R | CTC CGC TTT CTG TTC TTT GG | |
| MCT4–F | CAAAGTGGATCTGCGGTGAA | |
| MCT4–R | GGCTGGGTCCCTGGTTTAG | |
| Muscle | GAPDH–F | TGG CCT CCA AGG AGT AAG AAA C |
| GAPDH–R | GGG ATA GGG CCT CTC TTG CT | |
| HK2–F | GGG AAG AAG AGA GAC TCG GAA TC | |
| HK2–R | CAT CCC TGC CTC GCA TAC A | |
| GYS1–F | CCA GCA CTC GGT AGG TAG AGG TA | |
| GYS1–R | GTG TCT CAT GTT GCC CAG TTT G | |
| PGM1–F | GAC GGC CGC TTC TAC ATG A | |
| PGM1–R | CCA ATA ACC AGG CGA CCA AT | |
| PDHA1–F | GTG ACC TTC ATC GGC TAG AAG AG | |
| PDHA1–R | GCA CAG TCT GCA TCA TCC TGT AG | |
| MCT1–F | GTG ACC ATT GTG GAA TGC TG | |
| MCT1–R | CTC CGC TTT CTG TTC TTT GG | |
| MCT4–F | CAAAGTGGATCTGCGGTGAA | |
| MCT4–R | GGCTGGGTCCCTGGTTTAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.; Hwang, D.; Kyun, S.; Jang, I.; Kim, S.-W.; Park, H.-Y.; Hwang, H.; Lim, K.; Kim, J. Exogenous Lactate Treatment Immediately after Exercise Promotes Glycogen Recovery in Type-II Muscle in Mice. Nutrients 2024, 16, 2831. https://doi.org/10.3390/nu16172831
Kim T, Hwang D, Kyun S, Jang I, Kim S-W, Park H-Y, Hwang H, Lim K, Kim J. Exogenous Lactate Treatment Immediately after Exercise Promotes Glycogen Recovery in Type-II Muscle in Mice. Nutrients. 2024; 16(17):2831. https://doi.org/10.3390/nu16172831
Chicago/Turabian StyleKim, Taeho, Deunsol Hwang, Sunghwan Kyun, Inkwon Jang, Sung-Woo Kim, Hun-Young Park, Hyejung Hwang, Kiwon Lim, and Jisu Kim. 2024. "Exogenous Lactate Treatment Immediately after Exercise Promotes Glycogen Recovery in Type-II Muscle in Mice" Nutrients 16, no. 17: 2831. https://doi.org/10.3390/nu16172831
APA StyleKim, T., Hwang, D., Kyun, S., Jang, I., Kim, S.-W., Park, H.-Y., Hwang, H., Lim, K., & Kim, J. (2024). Exogenous Lactate Treatment Immediately after Exercise Promotes Glycogen Recovery in Type-II Muscle in Mice. Nutrients, 16(17), 2831. https://doi.org/10.3390/nu16172831







