Comparative Analysis of Nutritional Advice and a Combined Approach for Addressing Impending Stunting in Infants: A Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
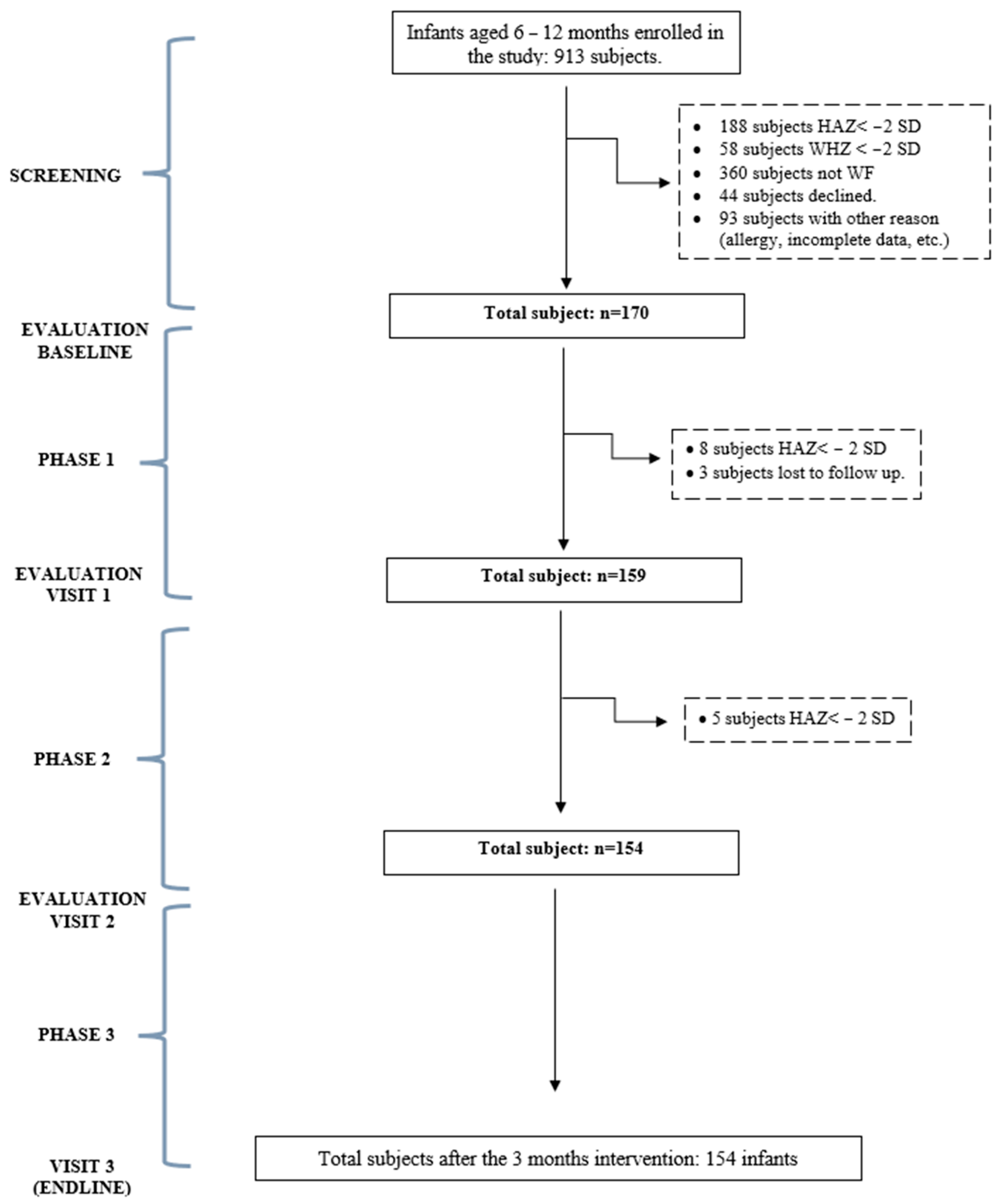
2.1. Subjects
- Infants aged 6–12 months with WF, defined as weight increments < P15th of the WHO weight increment table [10];
- Growth chart available for monitoring (weight, length, and head circumference measured at least once at birth);
- Available height and weight data from both father and mother;
- A parent (either mother or father) agreed to participate in the study and signed their informed consent.
- Subjects with a length-for-age z-score (HAZ) below 2 SD;
- Severe acute malnutrition;
- Presence of cow’s milk allergy;
- Presence of lactose intolerance;
- Presence of galactosemia;
- Major congenital anomaly, severe stunting at birth (newborns whose length-for-gestational age was below 10th percentile), thyroid disorder, major gastrointestinal disease, or other severe diseases, e.g., pneumonia or dehydration;
- Conditions that require special diets, e.g., major renal or hepatic dysfunctions;
- Conditions that influence nutritional status, e.g., moderate to severe dehydration, edema, organomegaly;
- Infants with relative WF but a body weight above the median weight for length (considering that they may become overweight);
- History of a low birth weight (less than 2500 g).
- History of premature birth (born after a period of pregnancy of less than 37 weeks).
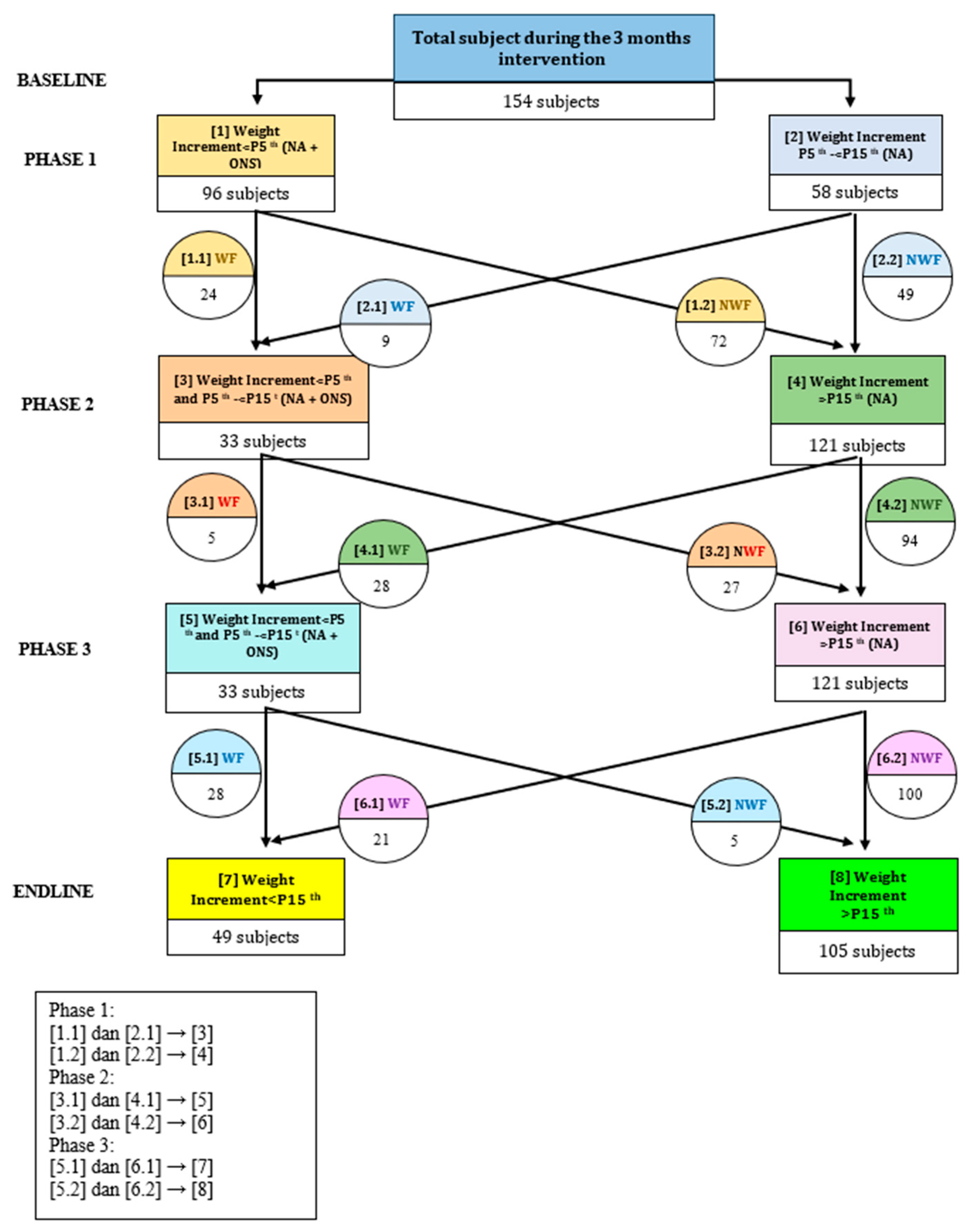
2.2. Study Design
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Isanaka, S.; Hitchings, M.D.T.; Berthé, F.; Briend, A.; Grais, R.F. Linear growth faltering and the role of weight attainment: Prospective analysis of young children recovering from severe wasting in Niger. Matern. Child Nutr. 2019, 15, e12817. [Google Scholar] [CrossRef] [PubMed]
- Onyango, A.W.; Borghi, E.; de Onis, M.; A Frongillo, E.; Victora, C.G.; Dewey, K.G.; Lartey, A.; Bhandari, N.; Baerug, A.; Garza, C. Successive 1-Month Weight Increments in Infancy Can Be Used to Screen for Faltering Linear Growth12. J. Nutr. 2015, 145, 2725–2731. [Google Scholar] [CrossRef] [PubMed]
- Sjarif, D.R.; Nasar, S.S.; Devaera, Y.; Tanjung, C.F. Asuhan Nutrisi Pediatrik (Pediatric Nutrition Care). Rekomendasi IDAI (Indonesian Pediatric Society Recommendation) UKK Nutrisi dan Penyakit Metabolik; IDAI: Jakarta, Indonesia, 2011. [Google Scholar]
- WHO/UNICEF. Global Strategy for Infant and Young Child Feeding; WHO: Geneva, Switzerland, 2003; Available online: https://www.who.int/publications/i/item/9241562218 (accessed on 20 December 2023).
- Cooke, R.; Goulet, O.; Huysentruyt, K.; Joosten, K.; Khadilkar, A.V.; Mao, M.; Meyer, R.; Prentice, A.M.; Singhal, A. Catch-Up Growth in Infants and Young Children with Faltering Growth: Expert Opinion to Guide General Clinicians. J. Pediatr. Gastroenterol. Nutr. 2023, 77, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, N.; Tanaka, Y.; Fukahori, S.; Ishii, S.; Saikusa, N.; Koga, Y.; Higashidate, N.; Masui, D.; Sakamoto, S.; Yagi, M. Adherences to oral nutritional supplementation among hospital outpatients: An online cross-sectional survey in Japan. PLoS ONE 2019, 14, e0222972. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.M. Oral nutritional supplementation: A user’s guide. Paediatr. Child Health 2017, 27, 378–382. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, F.; Hannon, B.A.; Hustead, D.S.; Aw, M.M.; Liu, Z.; Chuah, K.A.; Low, Y.L.; Huynh, D.T.T. Effect of Oral Nutritional Supplementation on Growth in Children with Undernutrition: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3036. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Child Growth Standards: Growth Velocity Based On Weight, Length and Head Circumference: Methods and Development; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- WHO. WHO Guidelines on the Prevention and Management of Wasting and Nutritional Oedema (Acute Malnutrition) in Infants and Children under 5 Years; WHO: Geneva, Switzerland, 2023; Available online: https://www.who.int/publications/i/item/9789240082830 (accessed on 20 January 2024).
- Tang, M.N.; Adolphe, S.; Rogers, S.R.; Frank, D.A. Failure to thrive or growth faltering: Medical, developmental/behavioral, nutritional, and social dimensions. Pediatr. Rev. 2021, 42, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Huynh, D.T.T.; Estorninos, E.; Capeding, R.Z.; Oliver, J.S.; Low, Y.L.; Rosales, F.J. Longitudinal growth and health outcomes in nutritionally at-risk children who received long-term nutritional intervention. J. Hum. Nutr. Diet. 2015, 28, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Lezo, A.; Baldini, L.; Asteggiano, M. Failure to thrive in the outpatient clinic: A new insight. Nutrients 2020, 12, 2202. [Google Scholar] [CrossRef] [PubMed]
- Lebenthal, Y.; Yackobovitch-Gavan, M.; Lazar, L.; Shalitin, S.; Tenenbaum, A.; Shamir, R.; Phillip, M. Effect of a Nutritional Supplement on Growth in Short and Lean Prepubertal Children: A Prospective, Randomized, Double-Blind, Placebo-Controlled Study. J. Pediatr. 2014, 165, 1190–1193.e1. [Google Scholar] [CrossRef] [PubMed]
- Yackobovitch-Gavan, M.; Lebenthal, Y.; Lazar, L.; Shalitin, S.; Demol, S.; Tenenbaum, A.; Shamir, R.; Phillip, M. Effect of Nutritional Supplementation on Growth in Short and Lean Prepubertal Children after 1 Year of Intervention. J. Pediatr. 2016, 179, 154–159.e1. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.O.; Kim, S.; Choe, B.-H.; Seo, J.-H.; Yang, H.R. Effect of nutritional supplement formula on catch-up growth in young children with nonorganic faltering growth: A prospective multicenter study. Nutr. Res. Pract. 2020, 14, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Yalawar, M.; Saibaba, P.V.; Bhatnagar, S.; Ghosh, A.; Jog, P.; Khadilkar, A.V.; Kishore, B.; Paruchuri, A.K.; Pote, P.D.; et al. Oral Nutritional Supplementation Improves Growth in Children at Malnutrition Risk and with Picky Eating Behaviors. Nutrients 2021, 13, 3590. [Google Scholar] [CrossRef] [PubMed]
- Cawood, A.; Smith, C.; Kinnear, F.; Upton, L.; Trace, S.; O’connor, G.; Stratton, R. Effect of oral nutritional supplements on outcomes in children presenting with, or at risk of, faltering growth in clinical settings: A systematic review and meta-analysis. J. Child Health Care 2023, 13674935231185181. [Google Scholar] [CrossRef] [PubMed]
| Phase | Variable | N | NA + ONS (Mean ± SD) | p-Value * | N | NA (Mean ± SD) | p-Value * | ||
|---|---|---|---|---|---|---|---|---|---|
| Screening | Visit 1 | Screening | Visit 1 | ||||||
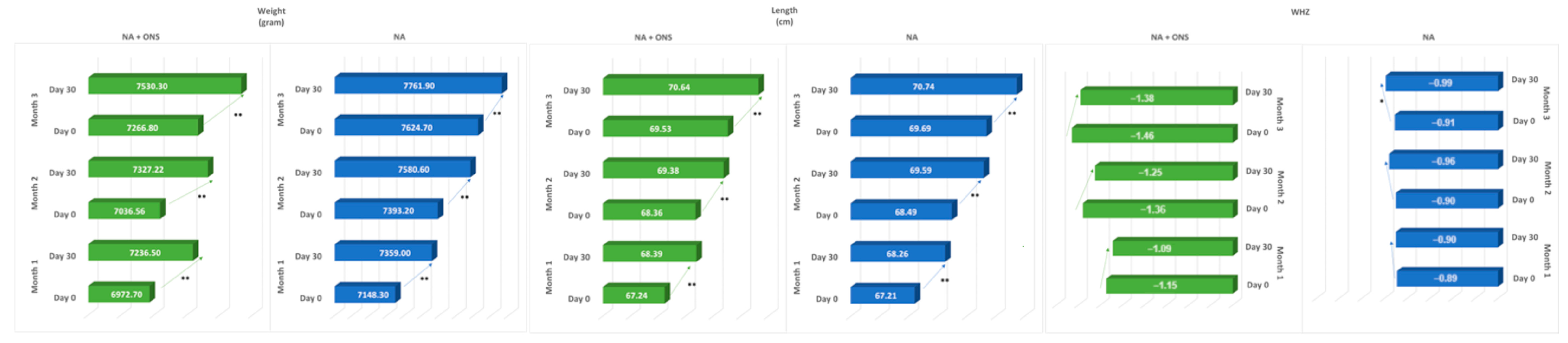
| 1 | Weight (gr) | 105 | 6972.70 ± 641.55 | 7236.50 ± 673.05 | <0.001 | 65 | 7148.30 ± 676.65 | 7359.00 ± 720.63 | <0.001 |
| Length (cm) | 105 | 67.24 ± 2.23 | 68.39 ± 2.23 | <0.001 | 65 | 67.21 ± 2.20 | 68.26 ± 2.21 | <0.001 | |
| BMI (kg/m2) | 105 | 15.40 ± 0.82 | 15.45 ± 0.92 | 0.383 | 65 | 15.80 ± 0.99 | 15.77 ± 1.02 | 0.611 | |
| HAZ | 105 | −0.86 ± 0.66 | −0.98 ± 0.67 | <0.001 | 65 | −0.97 ± 0.64 | −1.11 ± 0.66 | <0.001 | |
| WHZ | 105 | −1.15 ± 0.60 | −1.09 ± 0.67 | 0.198 | 65 | −0.89 ± 0.68 | −0.90 ± 0.69 | 0.974 | |
| WAZ | 105 | −1.39 ± 0.59 | −1.40 ± 0.65 | 0.761 | 65 | −1.21 ± 0.59 | −1.26 ± 0.62 | 0.34 | |
| Weight Increment (gr) | 105 | Not Available | 263.77 + 276.28 | 65 | Not Available | 210.85 ± 216.26 | |||
| Variable | N | NA + ONS (Mean ± SD) | p-Value * | N | NA (Mean ± SD) | p-Value * | |||
| Visit 1 | Visit 2 | Visit 1 | Visit 2 | ||||||
| 2 | Weight (gr) | 35 | 7059.60 ± 539.50 | 7357.60 ± 573.78 | <0.001 | 124 | 7393.20 ± 682.48 | 7580.60 ± 703.32 | <0.001 |
| Length (cm) | 35 | 68.42 ± 1.90 | 69.39 ± 1.86 | <0.001 | 124 | 68.49 ± 2.272 | 69.588 ± 2.27 | <0.001 | |
| BMI (kg/m2) | 35 | 15.06 ± 0.68 | 15.27 ± 0.79 | 0.014 | 124 | 15.74 ± 0.96 | 15.63 ± 0.99 | 0.025 | |
| HAZ | 35 | −1.10 ± 0.68 | −1.17 ± 0.72 | 0.114 | 124 | −0.94 ± 0.62 | −1.02 ± 0.64 | 0.002 | |
| WHZ | 35 | −1.35 ± 0.57 | −1.21 ± 0.60 | 0.072 | 124 | −0.90 ± 0.65 | −0.96 ± 0.69 | 0.094 | |
| WAZ | 35 | −1.59 ± 0.62 | −1.50 ± 0.66 | 0.218 | 124 | −1.21 ± 0.56 | −1.25 ± 0.59 | 0.165 | |
| Weight Increment (gr) | 35 | −36.54 ± 174.24 | 300.00 ± 225.46 | <0.001 | 124 | 340.73 ± 207.58 | 226.62 ± 236.49 | <0.001 | |
| Variable | N | NA + ONS (Mean ± SD) | p-Value * | N | NA (Mean ± SD) | p-Value * | |||
| Visit 2 | Visit 3 | Visit 2 | Visit 3 | ||||||
| 3 | Weight (gr) | 34 | 7266.80 ± 665.97 | 7530.30 ± 693.62 | <0.001 | 120 | 7624.70 ± 671.06 | 7761.90 ± 626.34 | <0.001 |
| Length (cm) | 34 | 69.53 ± 2.43 | 70.64 ± 2.35 | <0.001 | 120 | 69.69 ± 2.01 | 70.74 ± 2.14 | <0.001 | |
| BMI (kg/m2) | 34 | 15.00 ± 0.71 | 15.06 ± 0.66 | 0.523 | 120 | 15.68 ± 1.00 | 15.50 ± 0.93 | 0.002 | |
| HAZ | 34 | −1.13 ± 0.68 | −1.12 ± 0.73 | 0.799 | 120 | −0.97 ± 0.61 | −0.99 ± 0.66 | 0.383 | |
| WHZ | 34 | −1.46 ± 0.53 | −1.38 ± 0.49 | 0.305 | 120 | −0.91 ± 0.69 | −0.99 ± 0.64 | 0.038 | |
| WAZ | 34 | −1.66 ± 0.55 | −1.58 ± 0.53 | 0.227 | 120 | −1.18 ± 0.59 | −1.23 ± 0.59 | 0.088 | |
| Weight Increment (gr) | 34 | 51.32 ± 289.00 | 264.12 ± 271.07 | 0.014 | 120 | 285.55 ± 177.58 | 137.35 ± 278.18 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanjung, C.; Fikri, B.; Prawitasari, T.; Massi, N.; Zainuddin, A.A.; Juliaty, A.; Yullyana, D.S.; Dwitya, S.; Shimojo, N.; Ohno, H.; et al. Comparative Analysis of Nutritional Advice and a Combined Approach for Addressing Impending Stunting in Infants: A Clinical Trial. Nutrients 2024, 16, 2832. https://doi.org/10.3390/nu16172832
Tanjung C, Fikri B, Prawitasari T, Massi N, Zainuddin AA, Juliaty A, Yullyana DS, Dwitya S, Shimojo N, Ohno H, et al. Comparative Analysis of Nutritional Advice and a Combined Approach for Addressing Impending Stunting in Infants: A Clinical Trial. Nutrients. 2024; 16(17):2832. https://doi.org/10.3390/nu16172832
Chicago/Turabian StyleTanjung, Conny, Bahrul Fikri, Titis Prawitasari, Nasrum Massi, Andi Alfian Zainuddin, Aidah Juliaty, Dwi Sora Yullyana, Sarah Dwitya, Naoki Shimojo, Hiroshi Ohno, and et al. 2024. "Comparative Analysis of Nutritional Advice and a Combined Approach for Addressing Impending Stunting in Infants: A Clinical Trial" Nutrients 16, no. 17: 2832. https://doi.org/10.3390/nu16172832
APA StyleTanjung, C., Fikri, B., Prawitasari, T., Massi, N., Zainuddin, A. A., Juliaty, A., Yullyana, D. S., Dwitya, S., Shimojo, N., Ohno, H., & Koletzko, B. (2024). Comparative Analysis of Nutritional Advice and a Combined Approach for Addressing Impending Stunting in Infants: A Clinical Trial. Nutrients, 16(17), 2832. https://doi.org/10.3390/nu16172832






