Research on the Anti-Fatigue Effects and Mechanisms of Arecoline in Sleep-Deprived Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ethical Statement
2.3. Animals and Experimental Design
2.4. SD Procedure
2.5. Behavioral Tests
2.5.1. Forelimb Grip Strength Test
2.5.2. Rotary Latency Experiment
2.5.3. Exhaustive Swimming Test
2.6. Sample Collection
2.7. Biochemical Parameter Assays
2.8. Western Blotting Analysis
2.9. Statistical Analysis
3. Results
3.1. Effect of Arecoline on Fatigue-Related Behaviors in SD-Induced Mice
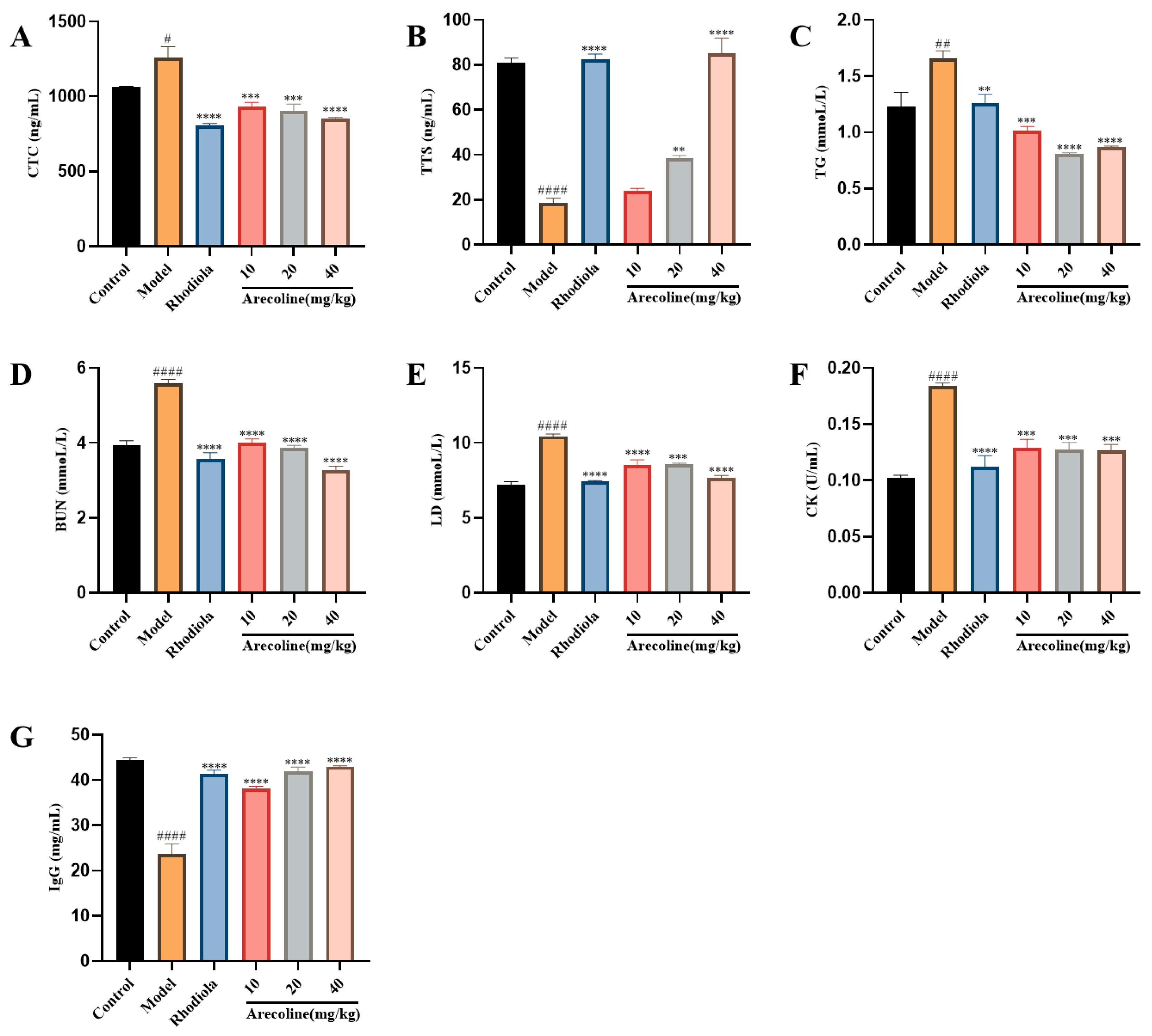
3.2. Effects of Arecoline on Serum Biochemical Indexes in Mice
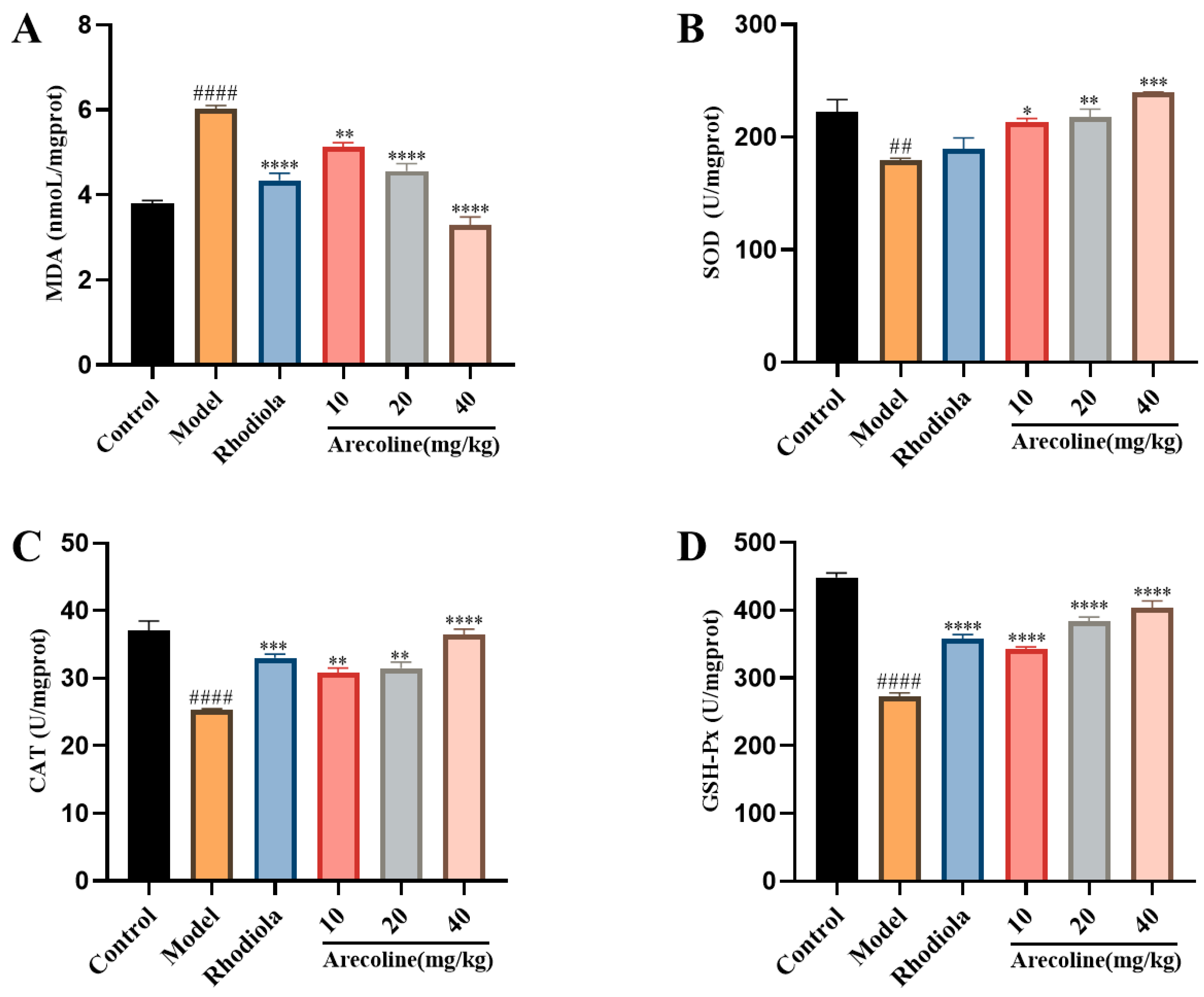
3.3. Effects of Arecoline on the Oxidative Stress Indexes of Gastrocnemius Muscle in Mice
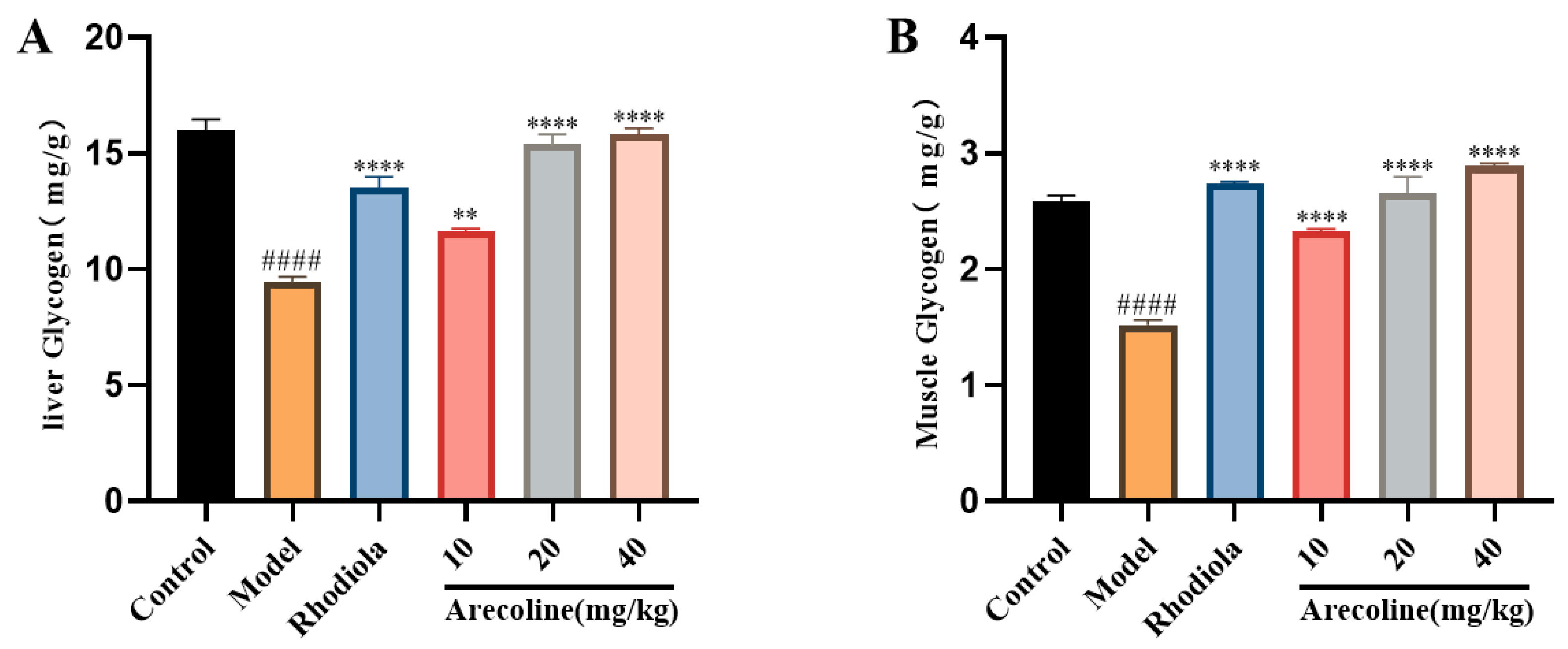
3.4. Effects of Arecoline on the Glycolipid Metabolism Indexes in Mice
3.5. Effects of Arecoline on Biochemical Indices of Mouse Hippocampus
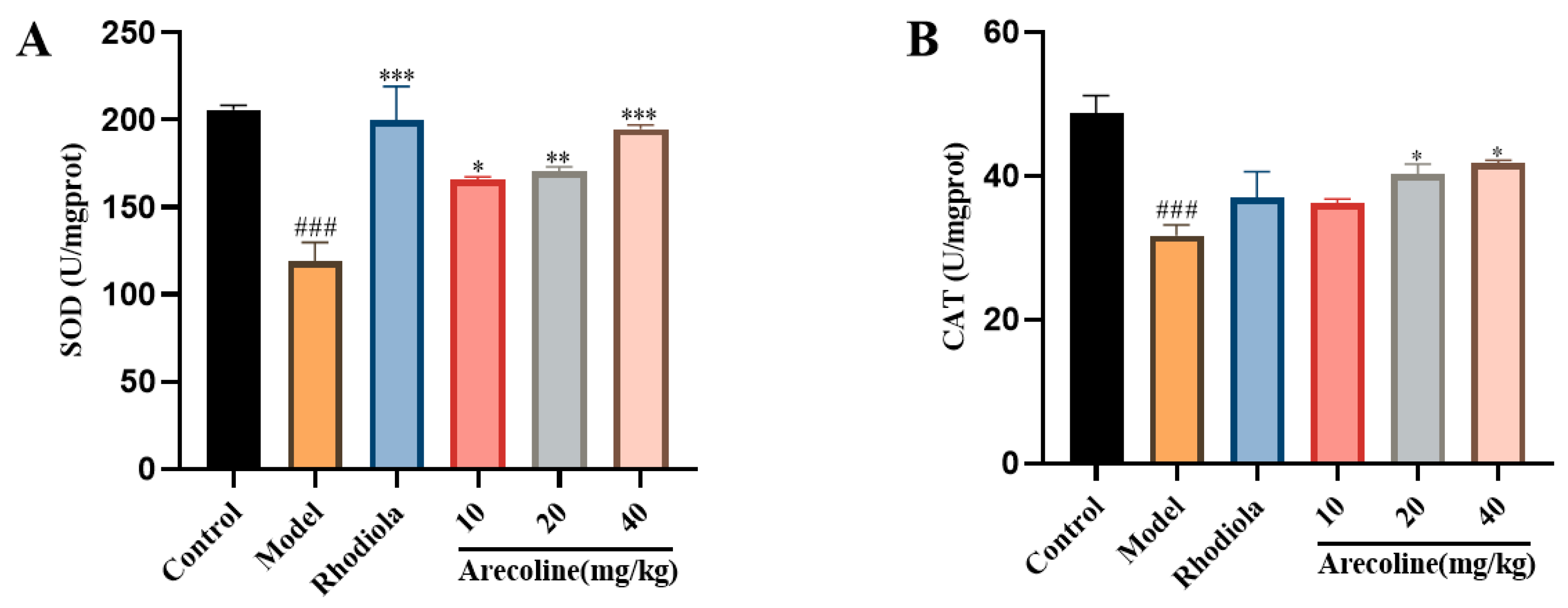
3.5.1. Effects of Arecoline on the Oxidative Stress Indexes in the Hippocampus of Mice
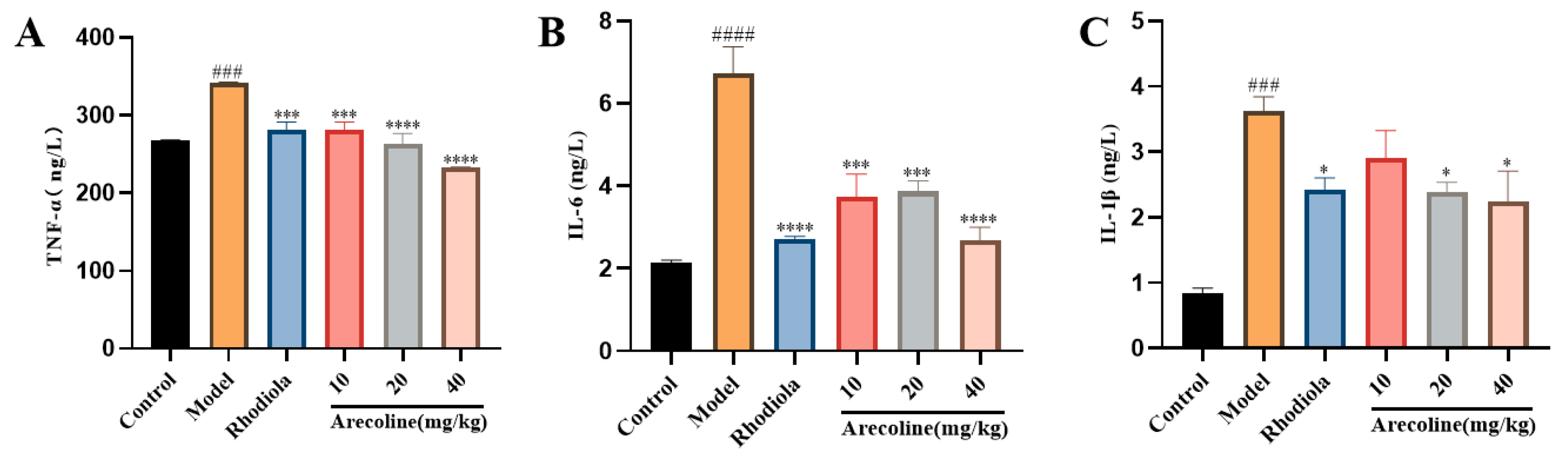
3.5.2. Effects of Arecoline on Cytokines TNF-α, IL-6 and IL-1β in Mouse Hippocampus
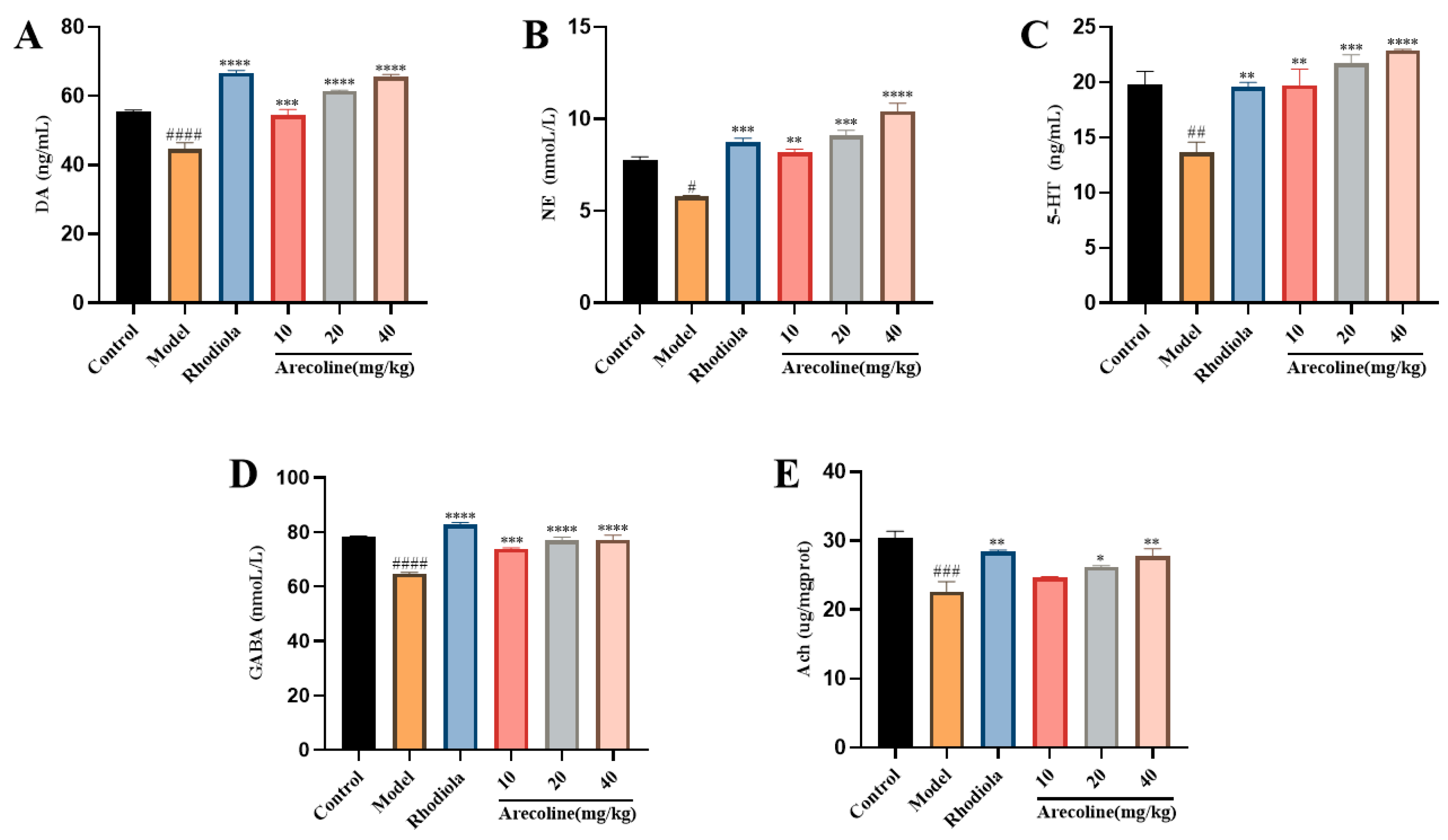
3.5.3. Effects of Arecoline on the Content of 5-HT, DA, NE, GABA, and Ach in the Hippocampus of Mice
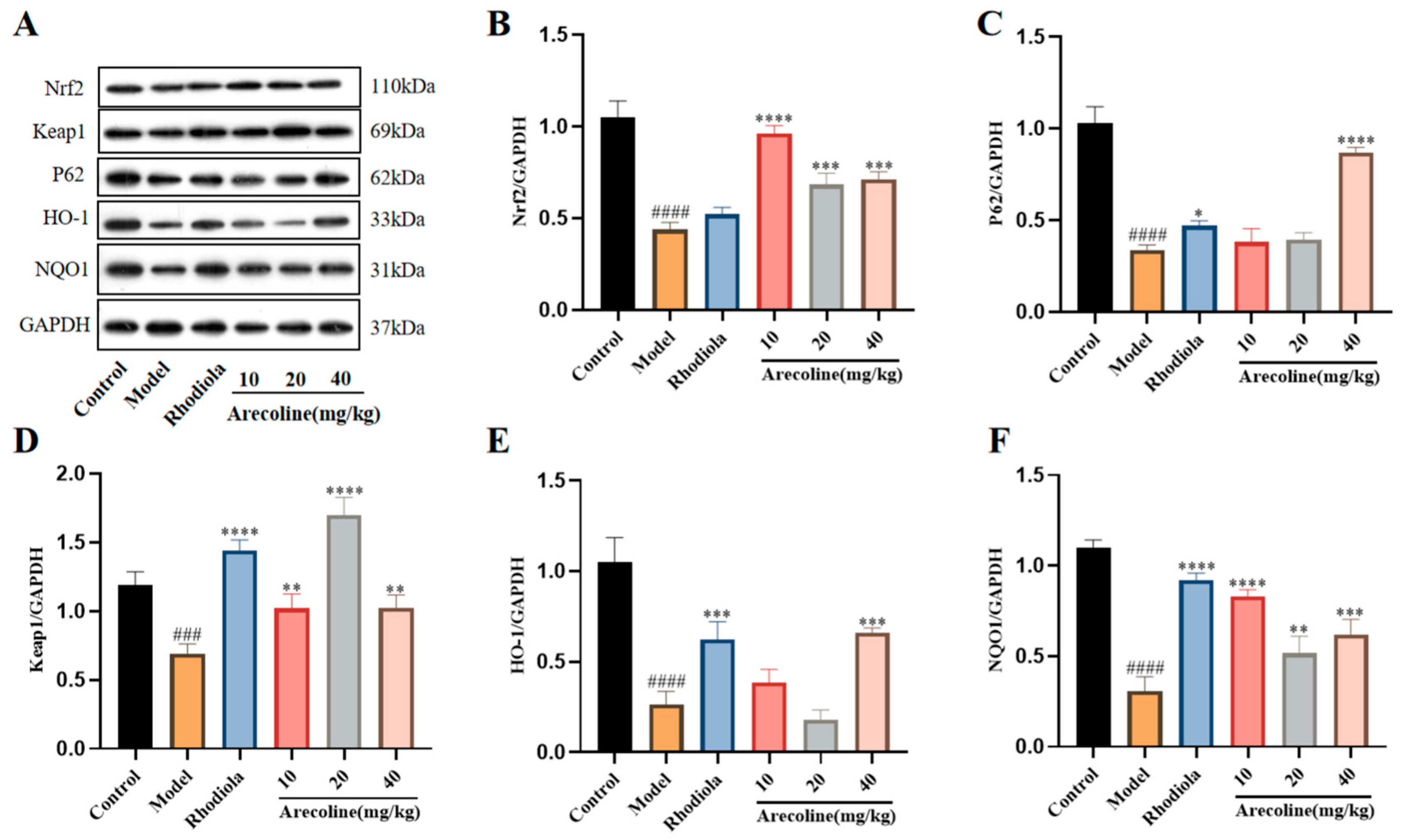
3.6. Effects of Arecoline on the Keap1/Nrf2/HO-1 Signaling Pathway in the Gastrocnemius of Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matura, L.A.; Malone, S.; Jaime-Lara, R.; Riegel, B. A Systematic Review of Biological Mechanisms of Fatigue in Chronic Illness. Biol. Res. Nurs. 2018, 20, 410–421. [Google Scholar] [PubMed]
- Hsiao, C.Y.; Hsu, Y.J.; Tung, Y.T.; Lee, M.C.; Huang, C.C.; Hsieh, C.C. Effects of Antrodia camphorata and Panax ginseng supplementation on anti-fatigue properties in mice. J. Vet. Med. Sci. 2018, 80, 284–291. [Google Scholar] [PubMed]
- Shi, J.; Shen, J.; Xie, J.; Zhi, J.; Xu, Y. Chronic fatigue syndrome in Chinese middle-school students. Medicine 2018, 97, e9716. [Google Scholar] [PubMed]
- Peng, W.; Liu, Y.J.; Wu, N.; Sun, T.; He, X.Y.; Gao, Y.X.; Wu, C.J. Areca catechu L. (Arecaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Ethnopharmacol. 2015, 164, 340–356. [Google Scholar] [PubMed]
- Papke, R.L.; Horenstein, N.A.; Stokes, C. Nicotinic Activity of Arecoline, the Psychoactive Element of “Betel Nuts”, Suggests a Basis for Habitual Use and Anti-Inflammatory Activity. PLoS ONE 2015, 10, e0140907. [Google Scholar]
- Ko, A.M.; Tu, H.P.; Ko, Y.C. Systematic Review of Roles of Arecoline and Arecoline N-Oxide in Oral Cancer and Strategies to Block Carcinogenesis. Cells 2023, 12, 1208. [Google Scholar] [CrossRef]
- Huang, L.W.; Hsieh, B.S.; Cheng, H.L.; Hu, Y.C.; Chang, W.T.; Chang, K.L. Arecoline decreases interleukin-6 production and induces apoptosis and cell cycle arrest in human basal cell carcinoma cells. Toxicol. Appl. Pharmacol. 2012, 258, 199–207. [Google Scholar]
- Yao, C.; Zhang, Y.; Sun, X.; Pei, H.; Wei, S.; Wang, M.; Chang, Q.; Liu, X.; Jiang, N. Areca catechu L. ameliorates chronic unpredictable mild stress-induced depression behavior in rats by the promotion of the BDNF signaling pathway. Biomed. Pharmacother. 2023, 164, 114459. [Google Scholar]
- Ma, J.; DU, X.; Zhao, A.; Wang, Z.; Guo, Q.; Qin, N.; Wang, R. Anti-hypoxic pharmacological effects of betelnut polyphenols. Zhong Nan Da Xue Xue Bao. Yi Xue Ban J. Cent. S. Univ. Med. Sci. 2022, 47, 512–520. [Google Scholar]
- Ling, H.Y.; Yao, Q.X.; Qi, Z.Q.; Yang, S.S.; He, J.Q.; Zhang, K.F.; Hu, B. Effects of arecoline on hepatic insulin resistance in type 2 diabetic rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi Chin. J. Appl. Physiol. 2014, 30, 208–212. [Google Scholar]
- Saha, I.; Das, J.; Maiti, B.; Chatterji, U. A protective role of arecoline hydrobromide in experimentally induced male diabetic rats. BioMed Res. Int. 2015, 2015, 136738. [Google Scholar]
- Li, C.B.; Yang, X.; Tang, W.B.; Liu, C.Y.; Xie, D.P. Arecoline excites the contraction of distal colonic smooth muscle strips in rats via the M3 receptor-extracellular Ca2+ influx–Ca2+ store release pathway. Can. J. Physiol. Pharmacol. 2010, 88, 439–447. [Google Scholar]
- Yi, P.; Tang, Y.R.; Zhou, F. Research progress on chemical constituents and pharmacological activities of Areca catechu. China J. Chin. Mater. Medica 2019, 50, 2498–2504. [Google Scholar]
- Zhang, C.J.; Lv, F.J.; Tao, H.T. Research progress on the active components of areca nut and their functions. Food Nutr. China 2008, 6, 50–53. [Google Scholar]
- Montoro, P.; Maldini, M.; Piacente, S.; Macchia, M.; Pizza, C. Metabolite fingerprinting of Camptotheca acuminata and the HPLC-ESI-MS/MS analysis of camptothecin and related alkaloids. J. Pharm. Biomed. Anal. 2010, 51, 405–415. [Google Scholar]
- Chavan, Y.V.; Singhal, R.S. Separation of polyphenols and arecoline from areca nut (Areca catechu L.) by solvent extraction, its antioxidant activity, and identification of polyphenols. J. Sci. Food Agric. 2013, 93, 2580–2589. [Google Scholar]
- Yi, M.S.; Pan, F.B.; Guo, J.H.; Ji, X.L.; Liu, Y.Q. Research into Chemical Constituents and Pharmacological Activities in Areca catechu L. Food Res. Dev. 2021, 42, 219–224. [Google Scholar]
- Liu, Y.L.; Xv, W.W.; Zhou, D. Anti-ageing effects of betel nut extract grown in Hainan. China Trop. Med. 2017, 17, 123–125. [Google Scholar]
- Sun, Y.; Wang, D.Y.; Sun, J.; Fan, B.; Song, H.B.; Liu, X.M.; Lu, C.; Wang, F.Z. Arecoline improves neuroinflammation of BV2 cells induced by lipopolysaccharide and its mechanism. Drugs Clin. 2024, 39, 541–554. [Google Scholar]
- Sun, Y.; Wang, D.Y.; Sun, J.; Bai, Y.J.; Fan, B.; Song, H.B.; Ji, J.B.; Lu, C.; Wang, F.Z. Neuroprotective effect and mechanism of arecoline on H2O2-induced damage in SH-SY5Y cell. Chin. J. Trop. Crops 2024, 45, 1252–1261. [Google Scholar]
- Lu, C.; Wei, Z.; Jiang, N.; Chen, Y.; Wang, Y.Q.; Li, S.Y.; Wang, Q.; Fan, B.; Liu, X.M.; Wang, F.Z. Soy isoflavones protects against cognitive deficits induced by chronic sleep deprivation via alleviating oxidative stress and suppressing neuroinflammation. Phytother. Res. 2022, 36, 2072–2080. [Google Scholar] [PubMed]
- Durmer, J.S.; Dinges, D.F. Neurocognitive consequences of sleep deprivation. Semin. Neurol. 2005, 25, 117–129. [Google Scholar] [PubMed]
- Research Institute of Medicine Committee on Sleep Medicine. The National Academies Collection: Reports funded by National Institutes of Health. In Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem; Colten, H.R., Altevogt, B.M., Eds.; National Academies Press (US): Washington, DC, USA, 2006. [Google Scholar]
- Nedeltcheva, A.V.; Scheer, F.A. Metabolic effects of sleep disruption, links to obesity and diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 293–298. [Google Scholar]
- Liu, Y.; Liu, C. Antifatigue and increasing exercise performance of Actinidia arguta crude alkaloids in mice. J. Food Drug Anal. 2016, 24, 738–745. [Google Scholar]
- Liu, C.; Shao, C.; Du, Q.; He, C.; Sun, X.; Lou, A.; Ma, Z.; Yu, J. Mechanism and effects of fructose diphosphate on anti-hypoxia fatigue and learning memory ability. Can. J. Physiol. Pharmacol. 2020, 98, 733–740. [Google Scholar]
- Ma, L.-L.; Lin, J.-Z.; Liu, H.-Y.; Liu, H.-M.; Huang, W.; Tan, P.; Han, L.; Xu, R.-C.; Zhang, D.-K. Accurate positioning and analysis on health function of high frequency anti-fatigue herbal medicines. China J. Chin. Mater. Med. 2020, 45, 3608–3616. [Google Scholar]
- Pei, H.Y.; Jiang, N.; Wang, M.D.; Wang, F.Z.; Ao, D.M.; Wang, Q. Study on the antidepressant effect and mechanism of betel nut on mice. Chin. J. Comp. Med. 2022, 32, 24–32. [Google Scholar]
- Chai, X.; Pan, M.; Wang, J.; Feng, M.; Wang, Y.; Zhang, Q.; Sun, Y. Cordycepin exhibits anti-fatigue effect via activating TIGAR/SIRT1/PGC-1α signaling pathway. Biochem. Biophys. Res. Commun. 2022, 637, 127–135. [Google Scholar]
- Prats, C.; Graham, T.E.; Shearer, J. The dynamic life of the glycogen granule. J. Biol. Chem. 2018, 293, 7089–7098. [Google Scholar]
- Vigh-Larsen, J.F.; Ørtenblad, N.; Spriet, L.L.; Overgaard, K.; Mohr, M. Muscle Glycogen Metabolism and High-Intensity Exercise Performance: A Narrative Review. Sports Med. 2021, 51, 1855–1874. [Google Scholar] [PubMed]
- Park, S.H.; Jang, S.; Lee, S.W.; Park, S.D.; Sung, Y.Y.; Kim, H.K. Akebia quinata Decaisne aqueous extract acts as a novel anti-fatigue agent in mice exposed to chronic restraint stress. J. Ethnopharmacol. 2018, 222, 270–279. [Google Scholar]
- Xu, M.; Su, S.; Jiang, S.; Li, W.; Zhang, Z.; Zhang, J.; Hu, X. Short-term arecoline exposure affected the systemic health state of mice, in which gut microbes played an important role. Ecotoxicol. Environ. Saf. 2023, 259, 115055. [Google Scholar] [PubMed]
- Yao, H.; Zhang, D.; Yu, H.; Shen, H.; Lan, X.; Liu, H.; Chen, X.; Wu, X.; Zhang, G.; Wang, X. Chronic ethanol exposure induced anxiety-like behaviour by altering gut microbiota and GABA system. Addict. Biol. 2022, 27, e13203. [Google Scholar]
- Rong, M.; Jia, J.J.; Lin, M.Q.; He, X.L.; Xie, Z.Y.; Wang, N.; Zhang, Z.H.; Dong, Y.J.; Xu, W.F.; Huang, J.H.; et al. The effect of modified Qiyuan paste on mice with low immunity and sleep deprivation by regulating GABA nerve and immune system. Chin. Med. 2024, 19, 84. [Google Scholar] [PubMed]
- Johnston, G.; Krogsgaard-Larsen, P.; Stephanson, A. Betel nut constituents as inhibitors of γ-aminobutyric acid uptake. Nature 1975, 258, 627–628. [Google Scholar]
- Chu, N.-S. Effects of betel chewing on the central and autonomic nervous systems. J. Biomed. Sci. 2001, 8, 229–236. [Google Scholar] [PubMed]
- Free, R.; Clark, J.; Amara, S.; Sibley, D.R. Neurotransmission in the Central Nervous System. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13th ed.; Brunton, L.L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Winger, G. Nicotinic aspects of the discriminative stimulus effects of arecoline. Behav. Pharmacol. 2021, 32, 581–589. [Google Scholar]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar]
- Tzanavari, T.; Giannogonas, P.; Karalis, K.P. TNF-alpha and obesity. Curr. Dir. Autoimmun. 2010, 11, 145–156. [Google Scholar]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J. Cell Biochem. 2018, 119, 105–110. [Google Scholar]
- Moelants, E.A.; Mortier, A.; Van Damme, J.; Proost, P. Regulation of TNF-α with a focus on rheumatoid arthritis. Immunol. Cell Biol. 2013, 91, 393–401. [Google Scholar] [PubMed]
- Ma, K.; Zhang, H.; Baloch, Z. Pathogenetic and Therapeutic Applications of Tumor Necrosis Factor-α (TNF-α) in Major Depressive Disorder: A Systematic Review. Int. J. Mol. Sci. 2016, 17, 733. [Google Scholar] [CrossRef]
- Bae, S.H.; Sung, S.H.; Oh, S.Y.; Lim, J.M.; Lee, S.K.; Park, Y.N.; Lee, H.E.; Kang, D.; Rhee, S.G. Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab. 2013, 17, 73–84. [Google Scholar] [PubMed]
- Ahmed, A.; Misrani, A.; Tabassum, S.; Yang, L.; Long, C. Minocycline inhibits sleep deprivation-induced aberrant microglial activation and Keap1-Nrf2 expression in mouse hippocampus. Brain Res. Bull. 2021, 174, 41–52. [Google Scholar] [PubMed]
- Zheng, J.L.; Zeng, L.; Shen, B.; Xu, M.Y.; Zhu, A.Y.; Wu, C.W. Antioxidant defenses at transcriptional and enzymatic levels and gene expression of Nrf2-Keap1 signaling molecules in response to acute zinc exposure in the spleen of the large yellow croaker Pseudosciaena crocea. Fish Shellfish Immunol. 2016, 52, 1–8. [Google Scholar]
- Fourtounis, J.; Wang, I.M.; Mathieu, M.C.; Claveau, D.; Loo, T.; Jackson, A.L.; Crackower, M.A. Gene expression profiling following NRF2 and KEAP1 siRNA knockdown in human lung fibroblasts identifies CCL11/Eotaxin-1 as a novel NRF2 regulated gene. Respir. Res. 2012, 13, 92. [Google Scholar]
- Jiang, T.; Harder, B.; Rojo de la Vega, M.; Wong, P.K.; Chapman, E.; Zhang, D.D. p62 links autophagy and Nrf2 signaling. Free Radic. Biol. Med. 2015, 88 Pt B, 199–204. [Google Scholar]
- Kerr, F.; Sofola-Adesakin, O.; Ivanov, D.K.; Gatliff, J.; Gomez Perez-Nievas, B.; Bertrand, H.C.; Martinez, P.; Callard, R.; Snoeren, I.; Cochemé, H.M.; et al. Direct Keap1-Nrf2 disruption as a potential therapeutic target for Alzheimer’s disease. PLoS Genet. 2017, 13, e1006593. [Google Scholar]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Sun, Y.; Liu, J.; Sun, J.; Fan, B.; Lu, C.; Wang, F. Research on the Anti-Fatigue Effects and Mechanisms of Arecoline in Sleep-Deprived Mice. Nutrients 2024, 16, 2783. https://doi.org/10.3390/nu16162783
Wang D, Sun Y, Liu J, Sun J, Fan B, Lu C, Wang F. Research on the Anti-Fatigue Effects and Mechanisms of Arecoline in Sleep-Deprived Mice. Nutrients. 2024; 16(16):2783. https://doi.org/10.3390/nu16162783
Chicago/Turabian StyleWang, Danyang, Yuan Sun, Jiameng Liu, Jing Sun, Bei Fan, Cong Lu, and Fengzhong Wang. 2024. "Research on the Anti-Fatigue Effects and Mechanisms of Arecoline in Sleep-Deprived Mice" Nutrients 16, no. 16: 2783. https://doi.org/10.3390/nu16162783
APA StyleWang, D., Sun, Y., Liu, J., Sun, J., Fan, B., Lu, C., & Wang, F. (2024). Research on the Anti-Fatigue Effects and Mechanisms of Arecoline in Sleep-Deprived Mice. Nutrients, 16(16), 2783. https://doi.org/10.3390/nu16162783






