The Association between Individual Food Groups, Limbic System White Matter Tracts, and Episodic Memory: Initial Data from the Aiginition Longitudinal Biomarker Investigation of Neurodegeneration (ALBION) Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Neuropsychological Evaluation of Memory Function
2.3. Neuroimaging Acquisition and Analysis
2.4. Dietary Intake Assessment
2.5. Statistical Analysis
3. Results
3.1. Descriptive Values for the Main Variables of the Study
3.2. Association between Memory Performance and Integrity of Limbic WM Tracts
3.3. Food Groups and Integrity of Memory-Related (Limbic) WM Tracts
3.4. Food Groups and Performance on Memory-Related Neuropsychological Tests
4. Discussion
4.1. Food Groups and Integrity of Limbic WM Tracts
4.1.1. Food Groups with a Potential Beneficial Role on Limbic WM Integrity: Non-Refined Cereals, Fruits, Vegetables, Legumes, and Fish
4.1.2. Food Groups with a Potential Harmful Role on Limbic WM Integrity: Dairy, Red Meat, and Cold Cuts
4.1.3. Food Groups with a Dose- and Frequency-Specific Role on Limbic WM Integrity: Alcohol
4.1.4. Potential Mechanisms Underlying the Association between Individual Food Groups and Integrity of Limbic WM Tracts
4.2. Food Groups and Episodic Memory
4.2.1. Food Groups with a Potential Beneficial Role on Episodic Memory: Non-Refined Cereals, Fruits, Vegetables, Legumes, and Fish
4.2.2. Food Groups with a Potential Harmful Role on Episodic Memory: Dairy, Red Meat, and Cold Cuts
4.2.3. Food Groups with a Dose- and Frequency-Specific Role on Episodic Memory: Alcohol
4.3. Research and Clinical Considerations
4.4. Strengths, Limitations, and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morrison, J.H.; Baxter, M.G. The ageing cortical synapse: Hallmarks and implications for cognitive decline. Nat. Rev. Neurosci. 2012, 13, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef] [PubMed]
- Squire, L.R. Memory and the Hippocampus—A Synthesis from Findings with Rats, Monkeys, and Humans. Psychol. Rev. 1992, 99, 195–231. [Google Scholar] [CrossRef]
- Banwinkler, M.; Theis, H.; Prange, S.; van Eimeren, T. Imaging the Limbic System in Parkinson’s Disease—A Review of Limbic Pathology and Clinical Symptoms. Brain Sci. 2022, 12, 1248. [Google Scholar] [CrossRef]
- Rolls, E.T. Limbic systems for emotion and for memory, but no single limbic system. Cortex 2015, 62, 119–157. [Google Scholar] [CrossRef]
- Catani, M.; Dell’acqua, F.; Thiebaut de Schotten, M. A revised limbic system model for memory, emotion and behaviour. Neurosci. Biobehav. Rev. 2013, 37, 1724–1737. [Google Scholar] [CrossRef]
- Hyman, B.T.; Van Hoesen, G.W.; Kromer, L.J.; Damasio, A.R. Perforant pathway changes and the memory impairment of Alzheimer’s disease. Ann. Neurol. 1986, 20, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Aggarwal, M. In vivo magnetic resonance imaging of the human limbic white matter. Front. Aging Neurosci. 2014, 6, 321. [Google Scholar] [CrossRef] [PubMed]
- Pascalau, R.; Popa Stanila, R.; Sfrangeu, S.; Szabo, B. Anatomy of the Limbic White Matter Tracts as Revealed by Fiber Dissection and Tractography. World Neurosurg. 2018, 113, e672–e689. [Google Scholar] [CrossRef] [PubMed]
- Augustinack, J.C.; Helmer, K.; Huber, K.E.; Kakunoori, S.; Zollei, L.; Fischl, B. Direct visualization of the perforant pathway in the human brain with ex vivo diffusion tensor imaging. Front. Hum. Neurosci. 2010, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, M.; Snyder, H.M.; Carrillo, M.C.; Fazio, S.; Kim, H.; Johns, H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015, 11, 718–726. [Google Scholar] [CrossRef] [PubMed]
- McFall, G.P.; McDermott, K.L.; Dixon, R.A. Modifiable Risk Factors Discriminate Memory Trajectories in Non-Demented Aging: Precision Factors and Targets for Promoting Healthier Brain Aging and Preventing Dementia. J. Alzheimers Dis. 2019, 70, S101–S118. [Google Scholar] [CrossRef]
- Konwar, S.; Manca, R.; De Marco, M.; Soininen, H.; Venneri, A. The effect of physical activity on white matter integrity in aging and prodromal to mild Alzheimer’s disease with vascular comorbidity. Front. Aging Neurosci. 2023, 15, 1096798. [Google Scholar] [CrossRef] [PubMed]
- de Groot, M.; Ikram, M.A.; Akoudad, S.; Krestin, G.P.; Hofman, A.; van der Lugt, A.; Niessen, W.J.; Vernooij, M.W. Tract-specific white matter degeneration in aging: The Rotterdam Study. Alzheimers Dement. 2015, 11, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, L.Y.; Marzo, A.; Munoz-Ruiz, M.; Ikram, M.A.; Kivipelto, M.; Ruefenacht, D.; Venneri, A.; Soininen, H.; Wanke, I.; Ventikos, Y.A.; et al. Modifiable lifestyle factors in dementia: A systematic review of longitudinal observational cohort studies. J. Alzheimers Dis. 2014, 42, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018, 17, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Peneau, S.; Galan, P.; Jeandel, C.; Ferry, M.; Andreeva, V.; Hercberg, S.; Kesse-Guyot, E.; Group, S.V.M.R. Fruit and vegetable intake and cognitive function in the SU.VI.MAX 2 prospective study. Am. J. Clin. Nutr. 2011, 94, 1295–1303. [Google Scholar] [CrossRef]
- Xu, X.; Xiao, S.; Rahardjo, T.B.; Hogervorst, E. Tofu intake is associated with poor cognitive performance among community-dwelling elderly in China. J. Alzheimers Dis. 2015, 43, 669–675. [Google Scholar] [CrossRef]
- Samieri, C.; Morris, M.C.; Bennett, D.A.; Berr, C.; Amouyel, P.; Dartigues, J.F.; Tzourio, C.; Chasman, D.I.; Grodstein, F. Fish Intake, Genetic Predisposition to Alzheimer Disease, and Decline in Global Cognition and Memory in 5 Cohorts of Older Persons. Am. J. Epidemiol. 2018, 187, 933–940. [Google Scholar] [CrossRef]
- Park, K.M.; Fulgoni, V.L., 3rd. The association between dairy product consumption and cognitive function in the National Health and Nutrition Examination Survey. Br. J. Nutr. 2013, 109, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Elias, M.F.; Dore, G.A.; Robbins, M.A. Relation between dairy food intake and cognitive function: The Maine-Syracuse Longitudinal Study. Int. Dairy J. 2012, 22, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Ohara, T.; Ninomiya, T.; Hata, J.; Yoshida, D.; Mukai, N.; Nagata, M.; Uchida, K.; Shirota, T.; Kitazono, T.; et al. Milk and dairy consumption and risk of dementia in an elderly Japanese population: The Hisayama Study. J. Am. Geriatr. Soc. 2014, 62, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Grodstein, F.; Rosner, B.A.; Kang, J.H.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Willett, W.C.; Okereke, O.I. Mediterranean diet and cognitive function in older age. Epidemiology 2013, 24, 490–499. [Google Scholar] [CrossRef]
- Wengreen, H.; Munger, R.G.; Cutler, A.; Quach, A.; Bowles, A.; Corcoran, C.; Tschanz, J.T.; Norton, M.C.; Welsh-Bohmer, K.A. Prospective study of Dietary Approaches to Stop Hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: The Cache County Study on Memory, Health and Aging. Am. J. Clin. Nutr. 2013, 98, 1263–1271. [Google Scholar] [CrossRef]
- Fischer, K.; Melo van Lent, D.; Wolfsgruber, S.; Weinhold, L.; Kleineidam, L.; Bickel, H.; Scherer, M.; Eisele, M.; van den Bussche, H.; Wiese, B.; et al. Prospective Associations between Single Foods, Alzheimer’s Dementia and Memory Decline in the Elderly. Nutrients 2018, 10, 852. [Google Scholar] [CrossRef] [PubMed]
- Fortune, N.C.; Harville, E.W.; Guralnik, J.M.; Gustat, J.; Chen, W.; Qi, L.; Bazzano, L.A. Dietary intake and cognitive function: Evidence from the Bogalusa Heart Study. Am. J. Clin. Nutr. 2019, 109, 1656–1663. [Google Scholar] [CrossRef]
- Zhu, J.; Xiang, Y.B.; Cai, H.; Li, H.; Gao, Y.T.; Zheng, W.; Shu, X.O. A Prospective Investigation of Dietary Intake and Functional Impairments Among the Elderly. Am. J. Epidemiol. 2018, 187, 2372–2386. [Google Scholar] [CrossRef]
- Zhang, H.; Cade, J.; Hadie, L. Consumption of Red Meat Is Negatively Associated with Cognitive Function: A Cross-Sectional Analysis of UK Biobank. Curr. Dev. Nutr. 2020, 4, 1510. [Google Scholar] [CrossRef]
- Stampfer, M.J.; Kang, J.H.; Chen, J.; Cherry, R.; Grodstein, F. Effects of moderate alcohol consumption on cognitive function in women. N. Engl. J. Med. 2005, 352, 245–253. [Google Scholar] [CrossRef]
- Gregory, S.; Pullen, H.; Ritchie, C.W.; Shannon, O.M.; Stevenson, E.J.; Muniz-Terrera, G. Mediterranean diet and structural neuroimaging biomarkers of Alzheimer’s and cerebrovascular disease: A systematic review. Exp. Gerontol. 2023, 172, 112065. [Google Scholar] [CrossRef]
- Drouka, A.; Mamalaki, E.; Karavasilis, E.; Scarmeas, N.; Yannakoulia, M. Dietary and Nutrient Patterns and Brain MRI Biomarkers in Dementia-Free Adults. Nutrients 2022, 14, 2345. [Google Scholar] [CrossRef]
- Croll, P.H.; Voortman, T.; Ikram, M.A.; Franco, O.H.; Schoufour, J.D.; Bos, D.; Vernooij, M.W. Better diet quality relates to larger brain tissue volumes: The Rotterdam Study. Neurology 2018, 90, e2166–e2173. [Google Scholar] [CrossRef] [PubMed]
- Staubo, S.C.; Aakre, J.A.; Vemuri, P.; Syrjanen, J.A.; Mielke, M.M.; Geda, Y.E.; Kremers, W.K.; Machulda, M.M.; Knopman, D.S.; Petersen, R.C.; et al. Mediterranean diet, micronutrients and macronutrients, and MRI measures of cortical thickness. Alzheimers Dement. 2017, 13, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, E.Y.; Shin, C. Changes in Brain Volume Associated with Vegetable Intake in a General Population. J. Am. Coll. Nutr. 2019, 38, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Brickman, A.M.; Stern, Y.; Habeck, C.G.; Razlighi, Q.R.; Luchsinger, J.A.; Manly, J.J.; Schupf, N.; Mayeux, R.; Scarmeas, N. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology 2015, 85, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Raji, C.A.; Erickson, K.I.; Lopez, O.L.; Kuller, L.H.; Gach, H.M.; Thompson, P.M.; Riverol, M.; Becker, J.T. Regular fish consumption and age-related brain gray matter loss. Am. J. Prev. Med. 2014, 47, 444–451. [Google Scholar] [CrossRef]
- Titova, O.E.; Ax, E.; Brooks, S.J.; Sjogren, P.; Cederholm, T.; Kilander, L.; Kullberg, J.; Larsson, E.M.; Johansson, L.; Ahlstrom, H.; et al. Mediterranean diet habits in older individuals: Associations with cognitive functioning and brain volumes. Exp. Gerontol. 2013, 48, 1443–1448. [Google Scholar] [CrossRef]
- Paul, C.A.; Au, R.; Fredman, L.; Massaro, J.M.; Seshadri, S.; Decarli, C.; Wolf, P.A. Association of alcohol consumption with brain volume in the Framingham study. Arch. Neurol. 2008, 65, 1363–1367. [Google Scholar] [CrossRef]
- Downer, B.; Jiang, Y.; Zanjani, F.; Fardo, D. Effects of alcohol consumption on cognition and regional brain volumes among older adults. Am. J. Alzheimers Dis. Other Dement. 2015, 30, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Scarmeas, N.; Short, E.E.; Luchsinger, J.A.; DeCarli, C.; Stern, Y.; Manly, J.J.; Schupf, N.; Mayeux, R.; Brickman, A.M. Alcohol intake and brain structure in a multiethnic elderly cohort. Clin. Nutr. 2014, 33, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Topiwala, A.; Allan, C.L.; Valkanova, V.; Zsoldos, E.; Filippini, N.; Sexton, C.; Mahmood, A.; Fooks, P.; Singh-Manoux, A.; Mackay, C.E.; et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: Longitudinal cohort study. BMJ 2017, 357, j2353. [Google Scholar] [CrossRef]
- Akbaraly, T.; Sexton, C.; Zsoldos, E.; Mahmood, A.; Filippini, N.; Kerleau, C.; Verdier, J.M.; Virtanen, M.; Gabelle, A.; Ebmeier, K.P.; et al. Association of Long-Term Diet Quality with Hippocampal Volume: Longitudinal Cohort Study. Am. J. Med. 2018, 131, 1372–1381.e4. [Google Scholar] [CrossRef] [PubMed]
- Immonen, S.; Launes, J.; Jarvinen, I.; Virta, M.; Vanninen, R.; Schiavone, N.; Lehto, E.; Tuulio-Henriksson, A.; Lipsanen, J.; Michelsson, K.; et al. Moderate alcohol use is associated with decreased brain volume in early middle age in both sexes. Sci. Rep. 2020, 10, 13998. [Google Scholar] [CrossRef]
- Sasaki, H.; Abe, O.; Yamasue, H.; Fukuda, R.; Yamada, H.; Takei, K.; Suga, M.; Takao, H.; Kasai, K.; Aoki, S.; et al. Structural and diffusional brain abnormality related to relatively low level alcohol consumption. Neuroimage 2009, 46, 505–510. [Google Scholar] [CrossRef]
- McEvoy, L.K.; Fennema-Notestine, C.; Elman, J.A.; Eyler, L.T.; Franz, C.E.; Hagler, D.J., Jr.; Hatton, S.N.; Lyons, M.J.; Panizzon, M.S.; Dale, A.M.; et al. Alcohol intake and brain white matter in middle aged men: Microscopic and macroscopic differences. Neuroimage Clin. 2018, 18, 390–398. [Google Scholar] [CrossRef]
- Gu, Y.; Vorburger, R.S.; Gazes, Y.; Habeck, C.G.; Stern, Y.; Luchsinger, J.A.; Manly, J.J.; Schupf, N.; Mayeux, R.; Brickman, A.M. White matter integrity as a mediator in the relationship between dietary nutrients and cognition in the elderly. Ann. Neurol. 2016, 79, 1014–1025. [Google Scholar] [CrossRef]
- Kalligerou, F.; Ntanasi, E.; Voskou, P.; Velonakis, G.; Karavasilis, E.; Mamalaki, E.; Kyrozis, A.; Sigala, E.; Economou, N.T.; Patas, K.; et al. Aiginition Longitudinal Biomarker Investigation of Neurodegeneration (ALBION): Study design, cohort description, and preliminary data. Postgrad. Med. 2019, 131, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Daskalaki, A.; Kalligerou, F.; Ntanasi, E.; Mamalaki, E.; Gargalionis, A.N.; Patas, K.; Chatzipanagiotou, S.; Yannakoulia, M.; Constantinides, V.C. Initial Data and a Clinical Diagnosis Transition for the Aiginition Longitudinal Biomarker Investigation of Neurodegeneration (ALBION) Study. Medicina 2022, 58, 1179. [Google Scholar] [CrossRef]
- Christidi, F.; Karavasilis, E.; Samiotis, K.; Bisdas, S.; Papanikolaou, N. Fiber tracking: A qualitative and quantitative comparison between four different software tools on the reconstruction of major white matter tracts. Eur. J. Radiol. Open 2016, 3, 153–161. [Google Scholar] [CrossRef]
- Moshfegh, A.J.; Rhodes, D.G.; Baer, D.J.; Murayi, T.; Clemens, J.C.; Rumpler, W.V.; Paul, D.R.; Sebastian, R.S.; Kuczynski, K.J.; Ingwersen, L.A.; et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008, 88, 324–332. [Google Scholar] [CrossRef]
- Conway, J.M.; Ingwersen, L.A.; Moshfegh, A.J. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: An observational validation study. J. Am. Diet. Assoc. 2004, 104, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.M.; Ingwersen, L.A.; Vinyard, B.T.; Moshfegh, A.J. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am. J. Clin. Nutr. 2003, 77, 1171–1178. [Google Scholar] [CrossRef]
- Nien, S.W.; Lin, I.H.; Wu, H.C.; Chen, Y.H.; Yang, S.C. Evaluation of Dietary Intake in Individuals with Mild Cognitive Impairment. Nutrients 2023, 15, 3694. [Google Scholar] [CrossRef]
- Zamroziewicz, M.K.; Barbey, A.K. Nutritional Cognitive Neuroscience: Innovations for Healthy Brain Aging. Front. Neurosci. 2016, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.E.A.; Leoni, V.; Klein-Flugge, M.C.; Ebmeier, K.P.; Suri, S. Associations of dietary markers with brain volume and connectivity: A systematic review of MRI studies. Ageing Res. Rev. 2021, 70, 101360. [Google Scholar] [CrossRef]
- Booth, T.; Mottus, R.; Corley, J.; Gow, A.J.; Henderson, R.D.; Maniega, S.M.; Murray, C.; Royle, N.A.; Sprooten, E.; Hernandez, M.C.; et al. Personality, health, and brain integrity: The Lothian birth cohort study 1936. Health Psychol 2014, 33, 1477–1486. [Google Scholar] [CrossRef]
- Pelletier, A.; Barul, C.; Feart, C.; Helmer, C.; Bernard, C.; Periot, O.; Dilharreguy, B.; Dartigues, J.F.; Allard, M.; Barberger-Gateau, P.; et al. Mediterranean diet and preserved brain structural connectivity in older subjects. Alzheimers Dement. 2015, 11, 1023–1031. [Google Scholar] [CrossRef]
- Beaulieu, C. The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed. 2002, 15, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Winklewski, P.J.; Sabisz, A.; Naumczyk, P.; Jodzio, K.; Szurowska, E.; Szarmach, A. Understanding the Physiopathology Behind Axial and Radial Diffusivity Changes-What Do We Know? Front. Neurol. 2018, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.H.; Argyelan, M.; Aggarwal, M.; Chandon, T.S.; Karlsgodt, K.H.; Mori, S.; Malhotra, A.K. The role of myelination in measures of white matter integrity: Combination of diffusion tensor imaging and two-photon microscopy of CLARITY intact brains. Neuroimage 2017, 147, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Klawiter, E.C.; Schmidt, R.E.; Trinkaus, K.; Liang, H.F.; Budde, M.D.; Naismith, R.T.; Song, S.K.; Cross, A.H.; Benzinger, T.L. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage 2011, 55, 1454–1460. [Google Scholar] [CrossRef]
- Song, S.K.; Yoshino, J.; Le, T.Q.; Lin, S.J.; Sun, S.W.; Cross, A.H.; Armstrong, R.C. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 2005, 26, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Villoz, F.; Filippini, T.; Ortega, N.; Kopp-Heim, D.; Voortman, T.; Blum, M.R.; Del Giovane, C.; Vinceti, M.; Rodondi, N.; Chocano-Bedoya, P.O. Dairy Intake and Risk of Cognitive Decline and Dementia: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2024, 15, 100160. [Google Scholar] [CrossRef]
- Kim, B.; Hong, V.M.; Yang, J.; Hyun, H.; Im, J.J.; Hwang, J.; Yoon, S.; Kim, J.E. A Review of Fermented Foods with Beneficial Effects on Brain and Cognitive Function. Prev. Nutr. Food Sci. 2016, 21, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Camfield, D.A.; Owen, L.; Scholey, A.B.; Pipingas, A.; Stough, C. Dairy constituents and neurocognitive health in ageing. Br. J. Nutr. 2011, 106, 159–174. [Google Scholar] [CrossRef]
- Elwood, P.C.; Pickering, J.E.; Givens, D.I.; Gallacher, J.E. The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: An overview of the evidence. Lipids 2010, 45, 925–939. [Google Scholar] [CrossRef]
- Mahdavi, A.; Trottier, J.; Barbier, O.; Lebel, M.; Rudkowska, I. Dairy Intake Modifies the Level of the Bile Acid Precursor and Its Correlation with Serum Proteins Associated with Cholesterol Clearance in Subjects with Hyperinsulinemia. Nutrients 2023, 15, 4707. [Google Scholar] [CrossRef]
- Pushpass, R.G.; Alzoufairi, S.; Jackson, K.G.; Lovegrove, J.A. Circulating bile acids as a link between the gut microbiota and cardiovascular health: Impact of prebiotics, probiotics and polyphenol-rich foods. Nutr. Res. Rev. 2022, 35, 161–180. [Google Scholar] [CrossRef]
- Baloni, P.; Funk, C.C.; Yan, J.; Yurkovich, J.T.; Kueider-Paisley, A.; Nho, K.; Heinken, A.; Jia, W.; Mahmoudiandehkordi, S.; Louie, G.; et al. Metabolic Network Analysis Reveals Altered Bile Acid Synthesis and Metabolism in Alzheimer’s Disease. Cell Rep. Med. 2020, 1, 100138. [Google Scholar] [CrossRef]
- Kaur, H.; Seeger, D.; Golovko, S.; Golovko, M.; Combs, C.K. Liver Bile Acid Changes in Mouse Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 7451. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, K.; Mulak, A. Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J. Neurogastroenterol. Motil. 2019, 25, 48–60. [Google Scholar] [CrossRef] [PubMed]
- van de Rest, O.; van der Zwaluw, N.L.; de Groot, L.C. Literature review on the role of dietary protein and amino acids in cognitive functioning and cognitive decline. Amino Acids 2013, 45, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Norde, M.M.; Collese, T.S.; Giovannucci, E.; Rogero, M.M. A posteriori dietary patterns and their association with systemic low-grade inflammation in adults: A systematic review and meta-analysis. Nutr. Rev. 2021, 79, 331–350. [Google Scholar] [CrossRef] [PubMed]
- Macho-Gonzalez, A.; Bastida, S.; Garcimartin, A.; Lopez-Oliva, M.E.; Gonzalez, P.; Benedi, J.; Gonzalez-Munoz, M.J.; Sanchez-Muniz, F.J. Functional Meat Products as Oxidative Stress Modulators: A Review. Adv. Nutr. 2021, 12, 1514–1539. [Google Scholar] [CrossRef]
- Wiegman, C.H.; Michaeloudes, C.; Haji, G.; Narang, P.; Clarke, C.J.; Russell, K.E.; Bao, W.; Pavlidis, S.; Barnes, P.J.; Kanerva, J.; et al. Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2015, 136, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Barnard, N.D.; Bunner, A.E.; Agarwal, U. Saturated and trans fats and dementia: A systematic review. Neurobiol. Aging 2014, 35 (Suppl. 2), S65–S73. [Google Scholar] [CrossRef]
- Puglielli, L.; Konopka, G.; Pack-Chung, E.; Ingano, L.A.; Berezovska, O.; Hyman, B.T.; Chang, T.Y.; Tanzi, R.E.; Kovacs, D.M. Acyl-coenzyme A: Cholesterol acyltransferase modulates the generation of the amyloid beta-peptide. Nat. Cell Biol. 2001, 3, 905–912. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Neusner, A.; Chu, J.; Lawton, M. Epidemilogical trends strongly suggest exposures as etiologic agents in the pathogenesis of sporadic Alzheimer’s disease, diabetes mellitus, and non-alcoholic steatohepatitis. J. Alzheimers Dis. 2009, 17, 519–529. [Google Scholar] [CrossRef]
- Ledesma, E.; Rendueles, M.; Diaz, M. Contamination of meat products during smoking by polycyclic aromatic hydrocarbons: Processes and prevention. Food Control 2016, 60, 64–87. [Google Scholar] [CrossRef]
- Edelmann, E.; Cepeda-Prado, E.; Franck, M.; Lichtenecker, P.; Brigadski, T.; Lessmann, V. Theta Burst Firing Recruits BDNF Release and Signaling in Postsynaptic CA1 Neurons in Spike-Timing-Dependent LTP. Neuron 2015, 86, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Xiang, J.; Liu, X.; Yu, S.P.; Manfredsson, F.P.; Sandoval, I.M.; Wu, S.; Wang, J.Z.; Ye, K. Deficiency in BDNF/TrkB Neurotrophic Activity Stimulates delta-Secretase by Upregulating C/EBPbeta in Alzheimer’s Disease. Cell Rep. 2019, 28, 655–669.e5. [Google Scholar] [CrossRef] [PubMed]
- Buljeta, I.; Pichler, A.; Simunovic, J.; Kopjar, M. Beneficial Effects of Red Wine Polyphenols on Human Health: Comprehensive Review. Curr. Issues Mol. Biol. 2023, 45, 782–798. [Google Scholar] [CrossRef]
- Caruana, M.; Cauchi, R.; Vassallo, N. Putative Role of Red Wine Polyphenols against Brain Pathology in Alzheimer’s and Parkinson’s Disease. Front. Nutr. 2016, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Bastianetto, S.; Menard, C.; Quirion, R. Neuroprotective action of resveratrol. Biochim. Biophys. Acta 2015, 1852, 1195–1201. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S.; et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Sawda, C.; Moussa, C.; Turner, R.S. Resveratrol for Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2017, 1403, 142–149. [Google Scholar] [CrossRef]
- Ayabe, T.; Fukuda, T.; Ano, Y. Improving Effects of Hop-Derived Bitter Acids in Beer on Cognitive Functions: A New Strategy for Vagus Nerve Stimulation. Biomolecules 2020, 10, 131. [Google Scholar] [CrossRef]
- Gronbaek, M. Alcohol, type of alcohol, and all-cause and coronary heart disease mortality. Ann. N. Y. Acad. Sci. 2002, 957, 16–20. [Google Scholar] [CrossRef]
- Bell, S.; Daskalopoulou, M.; Rapsomaniki, E.; George, J.; Britton, A.; Bobak, M.; Casas, J.P.; Dale, C.E.; Denaxas, S.; Shah, A.D.; et al. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: Population based cohort study using linked health records. BMJ 2017, 356, j909. [Google Scholar] [CrossRef]
- Yu, A.; Cooke, A.B.; Scheffler, P.; Doonan, R.J.; Daskalopoulou, S.S. Alcohol Exerts a Shifted U-Shaped Effect on Central Blood Pressure in Young Adults. J. Gen. Intern. Med. 2021, 36, 2975–2981. [Google Scholar] [CrossRef] [PubMed]
- Rimm, E.B.; Williams, P.; Fosher, K.; Criqui, M.; Stampfer, M.J. Moderate alcohol intake and lower risk of coronary heart disease: Meta-analysis of effects on lipids and haemostatic factors. BMJ 1999, 319, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, B.C.; Eichenbaum, H. The episodic memory system: Neurocircuitry and disorders. Neuropsychopharmacology 2010, 35, 86–104. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Cummings, J.L.; Dekosky, S.T.; Barberger-Gateau, P.; Delacourte, A.; Frisoni, G.; Fox, N.C.; Galasko, D.; et al. Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol. 2010, 9, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, C.A.; Yannakoulia, M.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; Arampatzi, X.; Bougea, A.; Labropoulos, I.; Scarmeas, N. Mediterranean diet and cognitive health: Initial results from the Hellenic Longitudinal Investigation of Ageing and Diet. PLoS ONE 2017, 12, e0182048. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Manly, J.J.; Schupf, N.; Luchsinger, J.A. Mediterranean diet and mild cognitive impairment. Arch. Neurol. 2009, 66, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef]
- Loughrey, D.G.; Lavecchia, S.; Brennan, S.; Lawlor, B.A.; Kelly, M.E. The Impact of the Mediterranean Diet on the Cognitive Functioning of Healthy Older Adults: A Systematic Review and Meta-Analysis. Adv. Nutr. 2017, 8, 571–586. [Google Scholar] [CrossRef]
- Dalile, B.; Kim, C.; Challinor, A.; Geurts, L.; Gibney, E.R.; Galdos, M.V.; La Fata, G.; Laye, S.; Mathers, J.C.; Vauzour, D.; et al. The EAT-Lancet reference diet and cognitive function across the life course. Lancet Planet. Health 2022, 6, e749–e759. [Google Scholar] [CrossRef]
- Lee, L.; Kang, S.A.; Lee, H.O.; Lee, B.H.; Park, J.S.; Kim, J.H.; Jung, I.K.; Park, Y.J.; Lee, J.E. Relationships between dietary intake and cognitive function level in Korean elderly people. Public Health 2001, 115, 133–138. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Cao, L.; Shi, M.; Liu, H.; Zhao, Y.; Xia, Y. Fruit and Vegetable Consumption and Cognitive Disorders in Older Adults: A Meta-Analysis of Observational Studies. Front. Nutr. 2022, 9, 871061. [Google Scholar] [CrossRef] [PubMed]
- Hogervorst, E.; Sadjimim, T.; Yesufu, A.; Kreager, P.; Rahardjo, T.B. High tofu intake is associated with worse memory in elderly Indonesian men and women. Dement. Geriatr. Cogn. Disord. 2008, 26, 50–57. [Google Scholar] [CrossRef]
- Lin, H.C.; Peng, C.H.; Huang, C.N.; Chiou, J.Y. Soy-Based Foods Are Negatively Associated with Cognitive Decline in Taiwan’s Elderly. J. Nutr. Sci. Vitaminol. 2018, 64, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Custodero, C.; Lozupone, M.; Imbimbo, B.P.; Valiani, V.; Agosti, P.; Schilardi, A.; D’Introno, A.; La Montagna, M.; Calvani, M.; et al. Relationships of Dietary Patterns, Foods, and Micro- and Macronutrients with Alzheimer’s Disease and Late-Life Cognitive Disorders: A Systematic Review. J. Alzheimers Dis. 2017, 59, 815–849. [Google Scholar] [CrossRef] [PubMed]
- Petruski-Ivleva, N.; Kucharska-Newton, A.; Palta, P.; Couper, D.; Meyer, K.; Graff, M.; Haring, B.; Sharrett, R.; Heiss, G. Milk Intake at Midlife and Cognitive Decline over 20 Years. The Atherosclerosis Risk in Communities (ARIC) Study. Nutrients 2017, 9, 1134. [Google Scholar] [CrossRef]
- Almeida, O.P.; Norman, P.; Hankey, G.; Jamrozik, K.; Flicker, L. Successful mental health aging: Results from a longitudinal study of older Australian men. Am. J. Geriatr. Psychiatry 2006, 14, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Paul, T.L.; Fleming, S.A. Dietary consumption of beef and red meat: A scoping review and evidence map on cognitive outcomes across the lifespan. Public. Health Nutr. 2023, 26, 2912–2926. [Google Scholar] [CrossRef]
- Zhang, H.; Hardie, L.; Bawajeeh, A.O.; Cade, J. Meat Consumption, Cognitive Function and Disorders: A Systematic Review with Narrative Synthesis and Meta-Analysis. Nutrients 2020, 12, 1528. [Google Scholar] [CrossRef]
- Granic, A.; Davies, K.; Adamson, A.; Kirkwood, T.; Hill, T.R.; Siervo, M.; Mathers, J.C.; Jagger, C. Dietary Patterns High in Red Meat, Potato, Gravy, and Butter Are Associated with Poor Cognitive Functioning but Not with Rate of Cognitive Decline in Very Old Adults. J. Nutr. 2016, 146, 265–274. [Google Scholar] [CrossRef]
- Crichton, G.E.; Elias, M.F.; Davey, A.; Alkerwi, A.; Dore, G.A. Higher Cognitive Performance Is Prospectively Associated with Healthy Dietary Choices: The Maine Syracuse Longitudinal Study. J. Prev. Alzheimers Dis. 2015, 2, 24–32. [Google Scholar] [CrossRef]
- Harper, C. The neuropathology of alcohol-related brain damage. Alcohol Alcohol. 2009, 44, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Stavro, K.; Pelletier, J.; Potvin, S. Widespread and sustained cognitive deficits in alcoholism: A meta-analysis. Addict. Biol. 2013, 18, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A.; Chandra, M.; Choudhary, M.; Dayal, P.; Anand, K.S. Alcohol-Related Dementia and Neurocognitive Impairment: A Review Study. Int. J. High. Risk Behav. Addict. 2016, 5, e27976. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Wan, Y.; Tan, C.; Li, J.; Tan, L.; Yu, J.T. Alcohol consumption and dementia risk: A dose-response meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Kalev-Zylinska, M.L.; During, M.J. Paradoxical facilitatory effect of low-dose alcohol consumption on memory mediated by NMDA receptors. J. Neurosci. 2007, 27, 10456–10467. [Google Scholar] [CrossRef]
- Chosy, E.J.; Edland, S.; Launer, L.; White, L.R. Midlife alcohol consumption and later life cognitive impairment: Light drinking is not protective and APOE genotype does not change this relationship. PLoS ONE 2022, 17, e0264575. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.; Jones, D.K.; Summers, P.E.; Morris, R.G.; Williams, S.C.; Markus, H.S. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology 2001, 57, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, W.; Wan, Y.; Shen, H.; Wang, T.; Ping, L.; Liu, C.; Chen, M.; Yu, H.; Jin, S.; et al. White matter alterations in mild cognitive impairment revealed by meta-analysis of diffusion tensor imaging using tract-based spatial statistics. Brain Imaging Behav. 2023, 17, 639–651. [Google Scholar] [CrossRef]
- Kantarci, K.; Petersen, R.C.; Boeve, B.F.; Knopman, D.S.; Weigand, S.D.; O’Brien, P.C.; Shiung, M.M.; Smith, G.E.; Ivnik, R.J.; Tangalos, E.G.; et al. DWI predicts future progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology 2005, 64, 902–904. [Google Scholar] [CrossRef]
- Fellgiebel, A.; Wille, P.; Muller, M.J.; Winterer, G.; Scheurich, A.; Vucurevic, G.; Schmidt, L.G.; Stoeter, P. Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: A diffusion tensor imaging study. Dement. Geriatr. Cogn. Disord. 2004, 18, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.J.; Greverus, D.; Dellani, P.R.; Weibrich, C.; Wille, P.R.; Scheurich, A.; Stoeter, P.; Fellgiebel, A. Functional implications of hippocampal volume and diffusivity in mild cognitive impairment. Neuroimage 2005, 28, 1033–1042. [Google Scholar] [CrossRef]
- Liu, J.; Yin, C.; Xia, S.; Jia, L.; Guo, Y.; Zhao, Z.; Li, X.; Han, Y.; Jia, J. White matter changes in patients with amnestic mild cognitive impairment detected by diffusion tensor imaging. PLoS ONE 2013, 8, e59440. [Google Scholar] [CrossRef] [PubMed]
- Sheelakumari, R.; Sarma, S.P.; Kesavadas, C.; Thomas, B.; Sasi, D.; Sarath, L.V.; Justus, S.; Mathew, M.; Menon, R.N. Multimodality Neuroimaging in Mild Cognitive Impairment: A Cross-sectional Comparison Study. Ann. Indian. Acad. Neurol. 2018, 21, 133–139. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013, 12, 207–216. [Google Scholar] [CrossRef]
- Karavasilis, E.; Christidi, F.; Velonakis, G.; Tzanetakos, D.; Zalonis, I.; Potagas, C.; Andreadou, E.; Efstathopoulos, E.; Kilidireas, C.; Kelekis, N.; et al. Hippocampal structural and functional integrity in multiple sclerosis patients with or without memory impairment: A multimodal neuroimaging study. Brain Imaging Behav. 2019, 13, 1049–1059. [Google Scholar] [CrossRef]
- Huang, X.; He, Q.; Ruan, X.; Li, Y.; Kuang, Z.; Wang, M.; Guo, R.; Bu, S.; Wang, Z.; Yu, S.; et al. Structural connectivity from DTI to predict mild cognitive impairment in de novo Parkinson’s disease. Neuroimage Clin. 2024, 41, 103548. [Google Scholar] [CrossRef]
- Hugenschmidt, C.E.; Peiffer, A.M.; Kraft, R.A.; Casanova, R.; Deibler, A.R.; Burdette, J.H.; Maldjian, J.A.; Laurienti, P.J. Relating imaging indices of white matter integrity and volume in healthy older adults. Cereb. Cortex 2008, 18, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Braskie, M.N.; Jahanshad, N.; Stein, J.L.; Barysheva, M.; McMahon, K.L.; de Zubicaray, G.I.; Martin, N.G.; Wright, M.J.; Ringman, J.M.; Toga, A.W.; et al. Common Alzheimer’s disease risk variant within the CLU gene affects white matter microstructure in young adults. J. Neurosci. 2011, 31, 6764–6770. [Google Scholar] [CrossRef] [PubMed]
- Imaeda, N.; Goto, C.; Tokudome, Y.; Hirose, K.; Tajima, K.; Tokudome, S. Reproducibility of a short food frequency questionnaire for Japanese general population. J. Epidemiol. 2007, 17, 100–107. [Google Scholar] [CrossRef]
- de Vries, J.H.; de Groot, L.C.; van Staveren, W.A. Dietary assessment in elderly people: Experiences gained from studies in the Netherlands. Eur. J. Clin. Nutr. 2009, 63 (Suppl. 1), S69–S74. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Luchsinger, J.A.; Schupf, N.; Brickman, A.M.; Cosentino, S.; Tang, M.X.; Stern, Y. Physical activity, diet, and risk of Alzheimer disease. JAMA 2009, 302, 627–637. [Google Scholar] [CrossRef]
- Freedman, L.S.; Midthune, D.; Carroll, R.J.; Krebs-Smith, S.; Subar, A.F.; Troiano, R.P.; Dodd, K.; Schatzkin, A.; Bingham, S.A.; Ferrari, P.; et al. Adjustments to improve the estimation of usual dietary intake distributions in the population. J. Nutr. 2004, 134, 1836–1843. [Google Scholar] [CrossRef]
- Nagel, G.; Zoller, D.; Ruf, T.; Rohrmann, S.; Linseisen, J. Long-term reproducibility of a food-frequency questionnaire and dietary changes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Heidelberg cohort. Br. J. Nutr. 2007, 98, 194–200. [Google Scholar] [CrossRef]
- Gu, Y.; Vorburger, R.; Scarmeas, N.; Luchsinger, J.A.; Manly, J.J.; Schupf, N.; Mayeux, R.; Brickman, A.M. Circulating inflammatory biomarkers in relation to brain structural measurements in a non-demented elderly population. Brain Behav. Immun. 2017, 65, 150–160. [Google Scholar] [CrossRef]
- Tsamou, M.; Kalligerou, F.; Ntanasi, E.; Scarmeas, N.; Skalicky, S.; Hackl, M.; Roggen, E.L. A Candidate microRNA Profile for Early Diagnosis of Sporadic Alzheimer’s Disease. J. Alzheimers Dis. Rep. 2023, 7, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Sampatakakis, S.N.; Mamalaki, E.; Ntanasi, E.; Kalligerou, F.; Liampas, I.; Yannakoulia, M.; Gargalionis, A.N.; Scarmeas, N. Objective Physical Function in the Alzheimer’s Disease Continuum: Association with Cerebrospinal Fluid Biomarkers in the ALBION Study. Int. J. Mol. Sci. 2023, 24, 14079. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Schupf, N.; Cosentino, S.A.; Luchsinger, J.A.; Scarmeas, N. Nutrient intake and plasma beta-amyloid. Neurology 2012, 78, 1832–1840. [Google Scholar] [CrossRef]
- Brikou, D.; Dimopoulou, M.A.; Drouka, A.; Ntanasi, E.; Mamalaki, E.; Gu, Y.; Scarmeas, N.; Yannakoulia, M. Eating Frequency, Timing, and Duration in Relation to Cognitive Performance and Alzheimer Disease Biomarkers in Adults. J. Nutr. 2024, 154, 2167–2175. [Google Scholar] [CrossRef]
- Ritchie, K.; Ritchie, C.W.; Yaffe, K.; Skoog, I.; Scarmeas, N. Is late-onset Alzheimer’s disease really a disease of midlife? Alzheimers Dement. 2015, 1, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Shaheen, M.; Grover, B. Nutrition and cognitive health: A life course approach. Front. Public. Health 2023, 11, 1023907. [Google Scholar] [CrossRef] [PubMed]
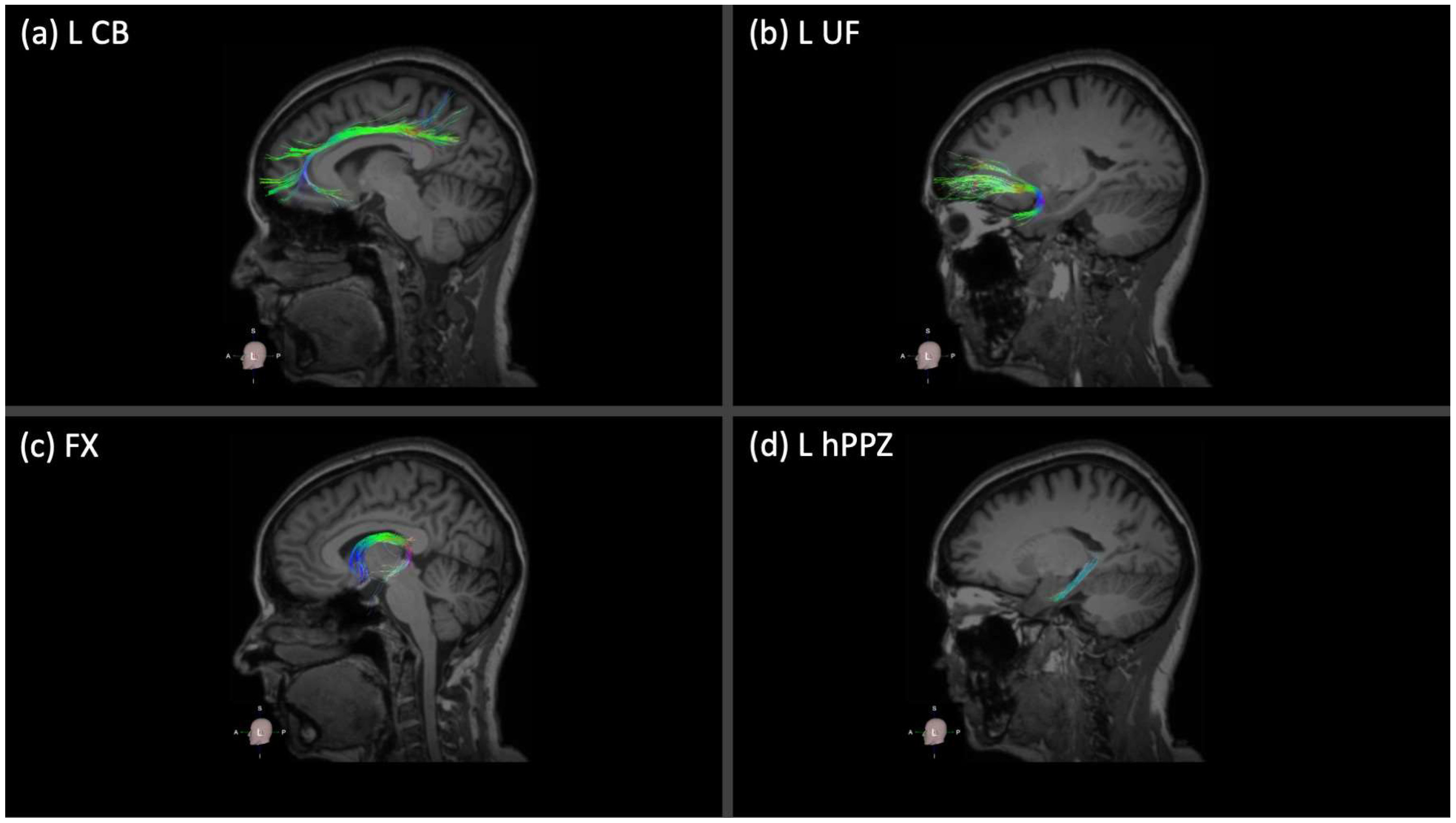
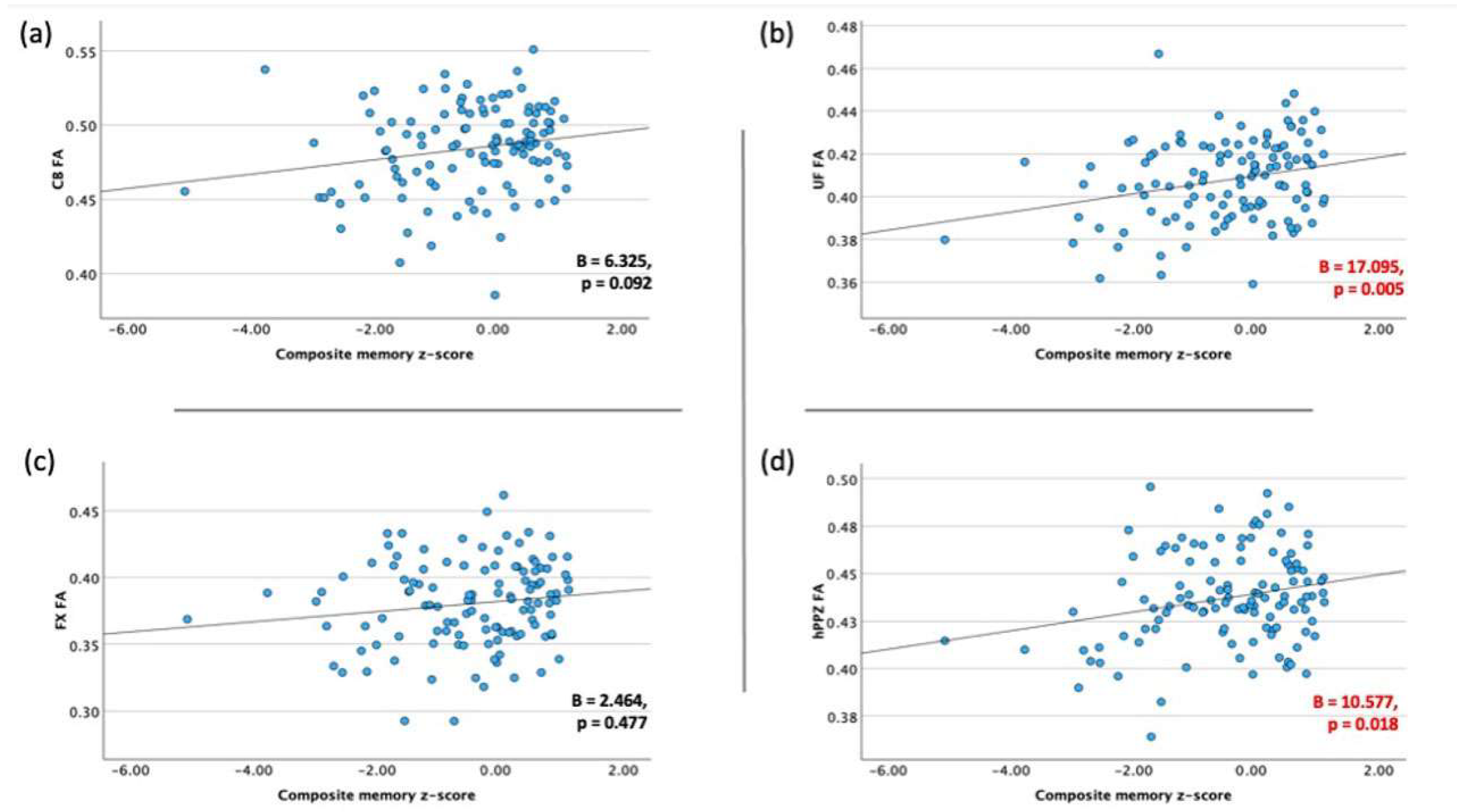
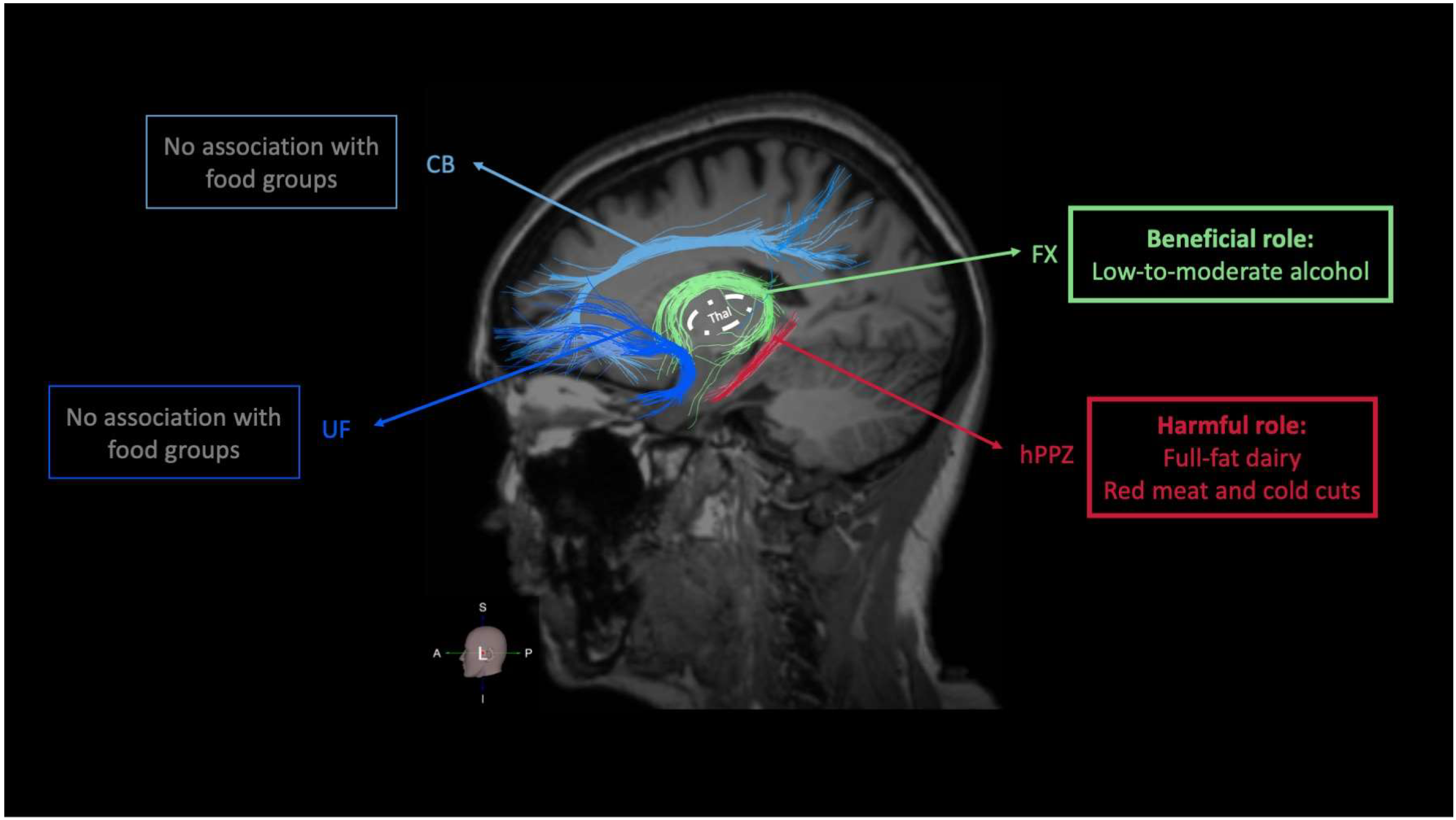
| Study Variables | Descriptive Measures |
|---|---|
| Demographic data | |
| Age (years) | 63.58 ± 9.19 |
| Sex (M/F) | 39/78 |
| Education (years) | 13.39 ± 3.78 |
| Cognitive data | |
| MMSE | 28.22 ± 1.98 |
| ACE-R total | 90.90 ± 7.15 |
| Memory composite z-score | −0.47 ± 1.17 |
| Neuroimaging data (DTI) | |
| CB FA | 0.48 ± 0.03 |
| CB Dax (×10−3) | 1.27 ± 0.04 |
| CB Drad (×10−3) | 0.56 ± 0.04 |
| UF FA | 0.41 ± 0.02 |
| UF Dax (×10−3) | 1.26 ± 0.06 |
| UF Drad (×10−3) | 0.65 ± 0.05 |
| FX FA | 0.38 ± 0.03 |
| FX Dax (×10−3) | 1.97 ± 0.26 |
| FX Drad (×10−3) | 1.13 ± 0.19 |
| hPPZ FA | 0.44 ± 0.02 |
| hPPZ Dax (×10−3) | 1.26 ± 0.05 |
| hPPZ Drad (×10−3) | 0.62 ± 0.04 |
| Dietary data | |
| Total energy intake (kcal/day) | 1723.64 ± 492.20 |
| Non-refined cereals (servings/day) | 1.70 ± 1.66 |
| Fruits, vegetables, legumes (servings/day) | 4.22 ± 2.66 |
| Fish (servings/day) | 0.43 ± 0.62 |
| Full-fat dairy (servings/day) | 0.73 ± 0.87 |
| Red meat and cold cuts (servings/day) | 0.99 ± 1.09 |
| Alcohol (servings/day) | 0.26 ± 0.55 |
| Independent Variable: Food Groups | Dependent Variable: FA Value of WM Tract | |||
|---|---|---|---|---|
| CB FA | UF FA | FX FA | hPPZ FA | |
| Non-refined cereals | B = 3.078E-5 [CI: −0.003 to 0.003], p = 0.985 | B = 0.002 [CI: 0.000 to 0.004], p = 0.114 | B = 0.001 [CI: −0.002 to 0.005], p = 0.551 | B = 0.002 [CI: −0.001 to 0.005], p = 0.164 |
| Fruits, vegetables, legumes | B = 0.000 [CI: −0.002 to 0.002], p = 0.864 | B = −0.001 [CI: −0.002 to 0.001], p = 0.351 | B = −0.001 [CI: −0.003 to 0.002], p = 0.597 | B = 0.000 [CI: −0.002 to 0.002], p = 0.797 |
| Fish | B = 0.004 [CI: −0.004 to 0.012], p = 0.359 | B = 0.002 [CI: −0.003 to 0.007], p = 0.421 | B = 0.000 [CI: −0.009 to 0.009], p = 0.965 | B = 0.004 [CI: −0.003 to 0.011], p = 0.315 |
| Full-fat dairy | B = −0.002 [CI: −0.009 to 0.004], p = 0.515 | B = −0.003 [CI: −0.007 to 0.001], p = 0.125 | B = −0.001 [CI: −0.008 to 0.006], p = 0.732 | B = −0.006 [CI: −0.011 to −0.001], p = 0.029 |
| Red meat and cold cuts | B = −0.003 [CI: −0.008 to 0.002], p = 0.276 | B = −0.002 [CI: −0.005 to 0.001], p = 0.229 | B = −0.002 [CI: −0.008 to 0.004], p = 0.473 | B = −0.007 [CI: −0.011 to −0.003], p = 0.002 |
| Alcohol | B = 0.000 [CI: −0.010 to 0.010], p = 0.968 | B = 0.003 [CI: −0.003 to 0.009], p = 0.337 | B = 0.014 [CI: 0.004 to 0.025], p = 0.009 | B = 0.006 [CI: −0.002 to 0.014], p = 0.141 |
| Independent Variable: Food Groups | Dependent Variable: Composite Memory z-Score |
|---|---|
| Non-refined cereals | B = 0.002 [CI: −0.127 to 0.131], p = 0.974 |
| Fruits, vegetables, legumes | B = −0.010 [CI: −0.092 to 0.073], p = 0.821 |
| Fish | B = 0.192 [CI: −0.140 to 0.524], p = 0.253 |
| Full-fat dairy | B = −0.129 [CI: −0.384 to 0.125], p = 0.317 |
| Red meat and cold cuts | B = 0.129 [CI: −0.083 to 0.342], p = 0.231 |
| Alcohol | B = 0.047 [CI: −0.350 to 0.445], p = 0.814 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christidi, F.; Drouka, A.; Brikou, D.; Mamalaki, E.; Ntanasi, E.; Karavasilis, E.; Velonakis, G.; Angelopoulou, G.; Tsapanou, A.; Gu, Y.; et al. The Association between Individual Food Groups, Limbic System White Matter Tracts, and Episodic Memory: Initial Data from the Aiginition Longitudinal Biomarker Investigation of Neurodegeneration (ALBION) Study. Nutrients 2024, 16, 2766. https://doi.org/10.3390/nu16162766
Christidi F, Drouka A, Brikou D, Mamalaki E, Ntanasi E, Karavasilis E, Velonakis G, Angelopoulou G, Tsapanou A, Gu Y, et al. The Association between Individual Food Groups, Limbic System White Matter Tracts, and Episodic Memory: Initial Data from the Aiginition Longitudinal Biomarker Investigation of Neurodegeneration (ALBION) Study. Nutrients. 2024; 16(16):2766. https://doi.org/10.3390/nu16162766
Chicago/Turabian StyleChristidi, Foteini, Archontoula Drouka, Dora Brikou, Eirini Mamalaki, Eva Ntanasi, Efstratios Karavasilis, Georgios Velonakis, Georgia Angelopoulou, Angeliki Tsapanou, Yian Gu, and et al. 2024. "The Association between Individual Food Groups, Limbic System White Matter Tracts, and Episodic Memory: Initial Data from the Aiginition Longitudinal Biomarker Investigation of Neurodegeneration (ALBION) Study" Nutrients 16, no. 16: 2766. https://doi.org/10.3390/nu16162766
APA StyleChristidi, F., Drouka, A., Brikou, D., Mamalaki, E., Ntanasi, E., Karavasilis, E., Velonakis, G., Angelopoulou, G., Tsapanou, A., Gu, Y., Yannakoulia, M., & Scarmeas, N. (2024). The Association between Individual Food Groups, Limbic System White Matter Tracts, and Episodic Memory: Initial Data from the Aiginition Longitudinal Biomarker Investigation of Neurodegeneration (ALBION) Study. Nutrients, 16(16), 2766. https://doi.org/10.3390/nu16162766






