Serum Homocysteine Levels and All-Cause and Cause-Specific Mortality in Korean Adult Men: A Cohort Study
Abstract
1. Introduction
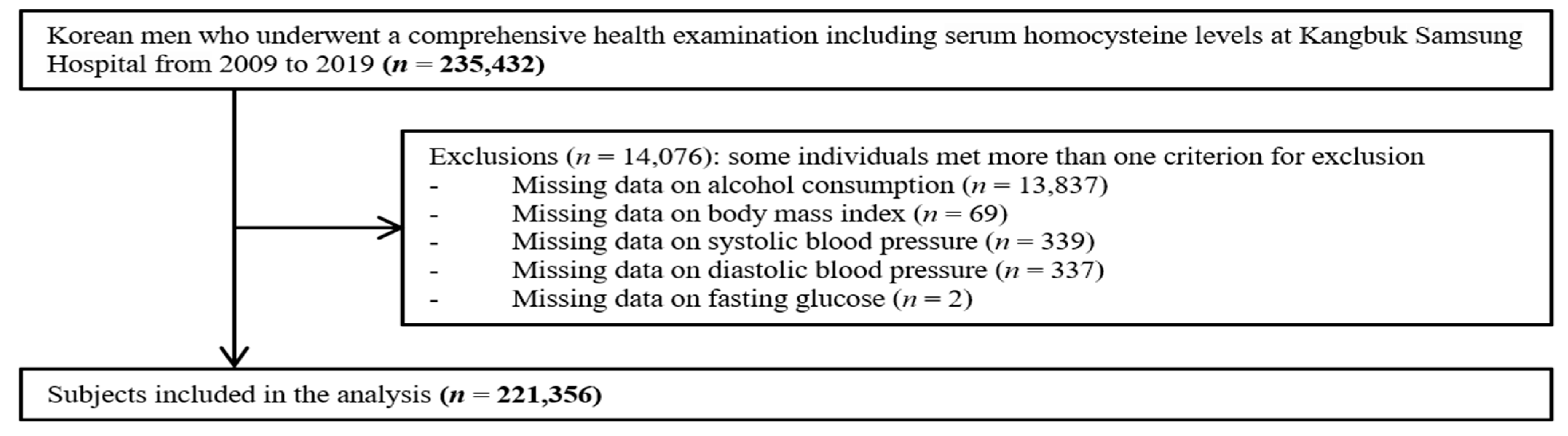
2. Materials and Methods
2.1. Study Population
2.2. Measurements
2.3. Mortality Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Study Subjects
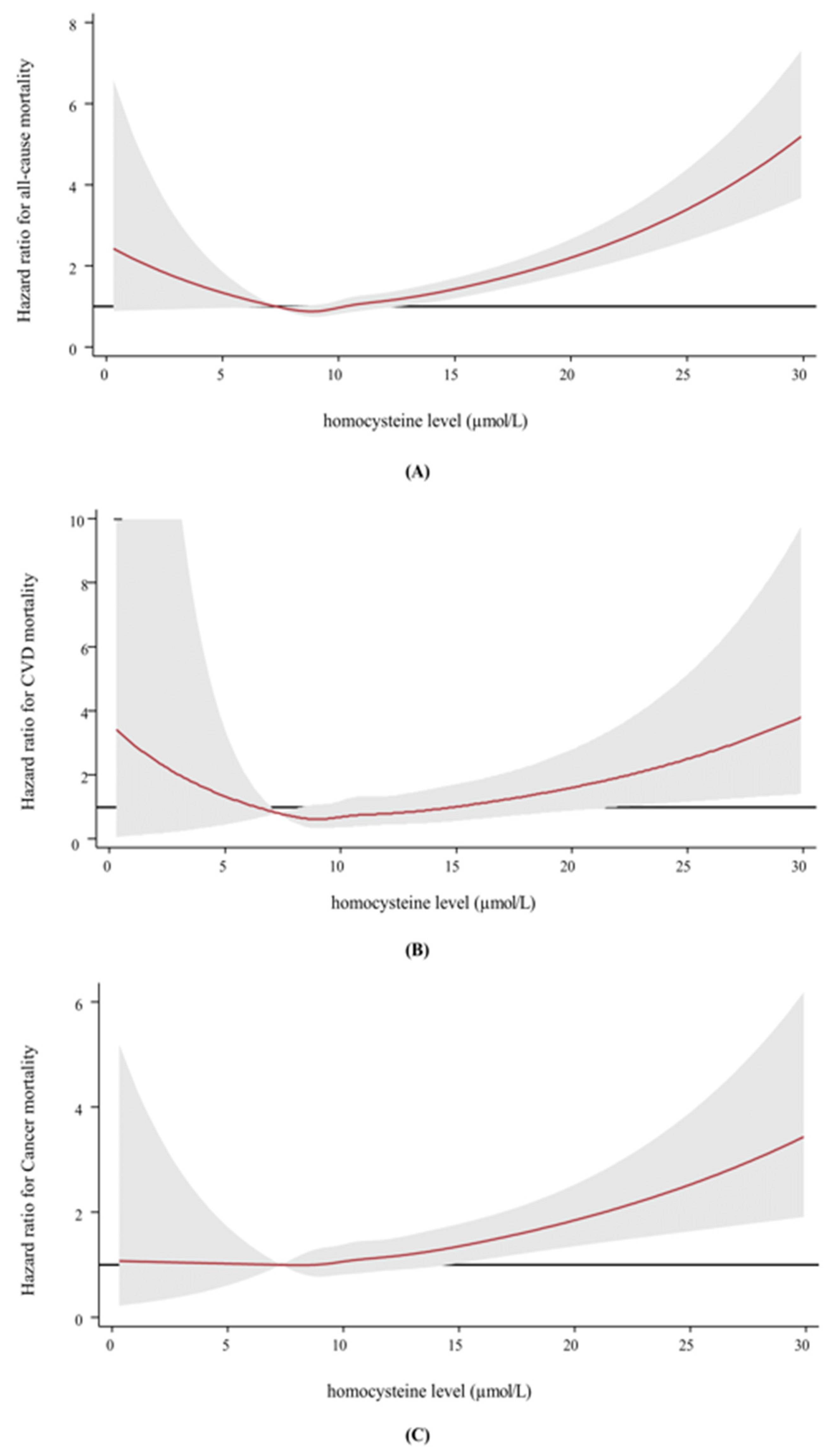
3.2. Association between Homocysteine and Mortality
3.3. Subgroup Analysis: Homocysteine and Mortality Based on Vitamin Uses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pizzorno, J. Homocysteine: Friend or foe? Integr. Med. A Clin. J. 2014, 13, 8–14. Available online: https://pubmed.ncbi.nlm.nih.gov/26770102/ (accessed on 15 August 2024).
- Pizzorno, J. Glutathione! Integr. Med. A Clin. J. 2014, 13, 8–13. Available online: https://pubmed.ncbi.nlm.nih.gov/26770075/ (accessed on 15 August 2024).
- Son, P.; Lewis, L. Hyperhomocysteinemia. In StatPearls; StatPearls Publishing: Petersburg, FL, USA, 2022. [Google Scholar]
- Chen, S.; Honda, T.; Ohara, T.; Hata, J.; Hirakawa, Y.; Yoshida, D.; Shibata, M.; Sakata, S.; Oishi, E.; Furuta, Y. Serum homocysteine and risk of dementia in Japan. J. Neurol. Neurosurg. Psychiatry 2020, 91, 540–546. [Google Scholar] [CrossRef]
- Özkan, Y.; Yardim-Akaydin, S.; Firat, H.; Calişkan-Can, E.; Ardic, S.; Şimşek, B. Usefulness of homocysteine as a cancer marker: Total thiol compounds and folate levels in untreated lung cancer patients. Anticancer Res. 2007, 27, 1185–1189. [Google Scholar] [PubMed]
- Wald, D.S.; Law, M.; Morris, J.K. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ 2002, 325, 1202. [Google Scholar] [CrossRef] [PubMed]
- Futschek, I.E.; Schernhammer, E.; Haslacher, H.; Stögmann, E.; Lehrner, J. Homocysteine—A predictor for five year-mortality in patients with subjective cognitive decline, mild cognitive impairment and Alzheimer’s dementia. Exp. Gerontol. 2023, 172, 112045. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Zhang, A.; Zhong, F. Association between homocysteine levels and all-cause mortality: A dose-response meta-analysis of prospective studies. Sci. Rep. 2017, 7, 4769. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Song, B.M.; Park, H.-Y. Associations of Serum Folate and Homocysteine Concentrations with All-Cause, Cardiovascular Disease, and Cancer Mortality in Men and Women in Korea: The Cardiovascular Disease Association Study. J. Nutr. 2023, 153, 760–770. [Google Scholar] [CrossRef]
- Tseng, F.-C.; Huang, T.-C. Using data mining technology to explore homocysteine at low levels. Medicine 2021, 100, e26893. [Google Scholar] [CrossRef] [PubMed]
- Cullen, C.E.; Carter, G.T.; Weiss, M.D.; Grant, P.A.; Saperstein, D.S. Hypohomocysteinemia: A potentially treatable cause of peripheral neuropathology? Phys. Med. Rehabil. Clin. 2012, 23, 59–65. [Google Scholar] [CrossRef]
- Bae, J.B.; Han, J.W.; Song, J.; Lee, K.; Kim, T.H.; Kwak, K.P.; Kim, B.J.; Kim, S.G.; Kim, J.L.; Moon, S.W. Hypohomocysteinemia may increases the risk of dementia and Alzheimer’s disease: A nationwide population-based prospective cohort study. Clin. Nutr. 2021, 40, 4579–4584. [Google Scholar] [CrossRef] [PubMed]
- Xiu, L.-L.; Lee, M.-S.; Wahlqvist, M.L.; Chen, R.C.-Y.; Huang, Y.-C.; Chen, K.-J.; Li, D. Low and high homocysteine are associated with mortality independent of B group vitamins but interactive with cognitive status in a free-living elderly cohort. Nutr. Res. 2012, 32, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Kalra, D.K. Homocysteine and cardiovascular disease. Curr. Atheroscler. Rep. 2004, 6, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Hak, A.E.; Polderman, K.H.; Westendorp, I.C.; Jakobs, C.; Hofman, A.; Witteman, J.C.; Stehouwer, C.D. Increased plasma homocysteine after menopause. Atherosclerosis 2000, 149, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Tallova, J.; Tomandl, J.; Bicikova, M.; Hill, M. Changes of plasma total homocysteine levels during the menstrual cycle. Eur. J. Clin. Investig. 1999, 29, 1041–1044. [Google Scholar] [CrossRef]
- Ryu, S.; Chang, Y.; Yun, K.E.; Jung, H.-S.; Shin, J.H.; Shin, H. Gallstones and the risk of gallbladder cancer mortality: A cohort study. Off. J. Am. Coll. Gastroenterol. ACG 2016, 111, 1476–1487. [Google Scholar] [CrossRef]
- Chang, Y.; Ryu, S.; Choi, Y.; Zhang, Y.; Cho, J.; Kwon, M.-J.; Hyun, Y.Y.; Lee, K.-B.; Kim, H.; Jung, H.-S. Metabolically healthy obesity and development of chronic kidney disease: A cohort study. Ann. Intern. Med. 2016, 164, 305–312. [Google Scholar] [CrossRef]
- Kim, H.C.; Ihm, S.-H.; Kim, G.-H.; Kim, J.H.; Kim, K.-I.; Lee, H.-Y.; Lee, J.H.; Park, J.-M.; Park, S.; Pyun, W.B. 2018 Korean Society of Hypertension guidelines for the management of hypertension: Part I-epidemiology of hypertension. Clin. Hypertens. 2019, 25, 16. [Google Scholar] [CrossRef]
- Hur, K.Y.; Moon, M.K.; Park, J.S.; Kim, S.-K.; Lee, S.-H.; Yun, J.-S.; Baek, J.H.; Noh, J.; Lee, B.-W.; Oh, T.J. 2021 clinical practice guidelines for diabetes mellitus in Korea. Diabetes Metab. J. 2021, 45, 461–481. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M. New creatinine-and cystatin C–based equations to estimate GFR without race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, B.S.; Kang, J.H. Plasma homocysteine and coronary artery calcification in Korean men. Eur. J. Prev. Cardiol. 2015, 22, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.K.; Rhim, H.C.; Lee, Y.-T.; Yoon, K.J.; Park, C.-H. Relationship between hyperhomocysteinemia and coexisting obesity with low skeletal muscle mass in asymptomatic adult population. Sci. Rep. 2022, 12, 12439. [Google Scholar] [CrossRef] [PubMed]
- Pavillon, G.; Maguin, P. The 10th revision of the International Classification of Diseases. Rev. D’epidemiol. Sante Publique 1993, 41, 253–255. [Google Scholar]
- Sreckovic, B.; Sreckovic, V.D.; Soldatovic, I.; Colak, E.; Sumarac-Dumanovic, M.; Janeski, H.; Janeski, N.; Gacic, J.; Mrdovic, I. Homocysteine is a marker for metabolic syndrome and atherosclerosis. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Dierkes, J.; Luley, C.; Westphal, S. Effect of lipid-lowering and anti-hypertensive drugs on plasma homocysteine levels. Vasc. Health Risk Manag. 2007, 3, 99–108. [Google Scholar] [PubMed]
- Stehouwer, C.D.; van Guldener, C. Does homocysteine cause hypertension? Clin. Chem. Lab. Med. (CCLM) 2003, 41, 1408–1411. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Wu, J.T. Hyperhomocysteinemia is a risk factor for cancer and a new potential tumor marker. Clin. Chim. Acta 2002, 322, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Welch, G.N.; Loscalzo, J. Homocysteine and atherothrombosis. N. Engl. J. Med. 1998, 338, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Charpio, P.; Bescond, A.; Augier, T.; Chareyre, C.; Fraterno, M.; Rolland, P.-H.; Garçon, D. Hyperhomocysteinemia induces elastolysis in minipig arteries: Structural consequences, arterial site specificity and effect of captopril-hydrochlorothiazide. Matrix Biol. 1998, 17, 559–574. [Google Scholar] [CrossRef]
- Tawakol, A.; Omland, T.; Gerhard, M.; Wu, J.T.; Creager, M.A. Hyperhomocyst (e) inemia is associated with impaired endothelium-dependent vasodilation in humans. Circulation 1997, 95, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.K.; Kweon, S.-S.; Lee, Y.-H.; Nam, H.-S.; Choi, S.-W.; Kim, H.-Y.; Shin, M.-H. Association between plasma homocysteine level and mortality: A Mendelian randomization study. Korean Circ. J. 2023, 53, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chen, K.; Chen, W.; Liu, C.; Jiang, X.; Ma, Z.; Li, D.; Shen, Y.; Tian, H. Association of Serum Homocysteine with Cardiovascular and All-Cause Mortality in Adults with Diabetes: A Prospective Cohort Study. Oxidative Med. Cell. Longev. 2022, 2022, 2156483. [Google Scholar] [CrossRef] [PubMed]
- Liperoti, R.; Vetrano, D.L.; Palmer, K.; Targowski, T.; Cipriani, M.C.; Lo Monaco, M.R.; Giovannini, S.; Acampora, N.; Villani, E.R.; Bernabei, R. Association between frailty and ischemic heart disease: A systematic review and meta-analysis. BMC Geriatr. 2021, 21, 357. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.Y.; Almeida, O.P.; McCaul, K.A.; Yeap, B.B.; Hankey, G.J.; Flicker, L. Homocysteine, frailty, and all-cause mortality in older men: The health in men study. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 590–598. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Yang, Z.; Sun, Y.; Qu, Z.; Jia, X.; Li, J.; Lin, Y.; Luo, Y. The impact of homocysteine on the risk of hormone-related cancers: A Mendelian randomization study. Front. Nutr. 2021, 8, 645371. [Google Scholar] [CrossRef] [PubMed]
- Fuso, A.; Seminara, L.; Cavallaro, R.A.; D’Anselmi, F.; Scarpa, S. S-adenosylmethionine/homocysteine cycle alterations modify DNA methylation status with consequent deregulation of PS1 and BACE and beta-amyloid production. Mol. Cell. Neurosci. 2005, 28, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Halsey, J.; Lewington, S.; Lonn, E.; Armitage, J.; Manson, J.E.; Bønaa, K.H.; Spence, J.D.; Nygård, O.; Jamison, R. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37 485 individuals. Arch. Intern. Med. 2010, 170, 1622–1631. [Google Scholar]
- Huang, T.; Chen, Y.; Yang, B.; Yang, J.; Wahlqvist, M.L.; Li, D. Meta-analysis of B vitamin supplementation on plasma homocysteine, cardiovascular and all-cause mortality. Clin. Nutr. 2012, 31, 448–454. [Google Scholar] [CrossRef]
- Ji, Y.; Tan, S.; Xu, Y.; Chandra, A.; Shi, C.; Song, B.; Qin, J.; Gao, Y. Vitamin B supplementation, homocysteine levels, and the risk of cerebrovascular disease: A meta-analysis. Neurology 2013, 81, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Homocysteine Level (µmol/L) | p for Trend | ||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| (1.1–8.7) | (8.8–9.9) | (10.0–11.1) | (11.2–12.9) | (13.0–179.9) | ||
| Number | 41,608 | 43,874 | 44,676 | 46,626 | 44,572 | |
| Age (years) 1 | 38.2 (9.7) | 39.0 (10.0) | 39.2 (10.1) | 39.6 (10.5) | 40.0 (11.3) | 0.002 |
| BMI (kg/m2) 1 | 24.5 (3.0) | 24.6 (3.0) | 24.7 (3.0) | 24.7 (3.1) | 24.6 (3.2) | <0.001 |
| Current smoker (%) | 29.8 | 30.5 | 31.4 | 33.3 | 37.5 | <0.001 |
| Alcohol intake (%) 3 | 33.1 | 33.8 | 33.8 | 33.2 | 33.8 | 0.450 |
| Exercise (%) 4 | 18.5 | 18.8 | 18.3 | 18.5 | 17.5 | 0.005 |
| Higher education (%) 5 | 71.2 | 67.0 | 64.2 | 61.4 | 58.0 | 0.009 |
| History of diabetes (%) | 5.7 | 5.6 | 5.8 | 6.0 | 6.8 | <0.001 |
| History of hypertension (%) | 13.8 | 16.0 | 17.6 | 19.1 | 21.7 | 0.653 |
| Medication for dyslipidemia (%) | 3.2 | 2.8 | 2.8 | 2.8 | 3.5 | <0.001 |
| Use of vitamins (%) | 28.6 | 24.3 | 21.7 | 18.7 | 15.1 | 0.033 |
| Systolic BP (mmHg) 1 | 114.8 (11.1) | 115.6 (11.4) | 116.0 (11.5) | 116.3 (11.8) | 116.8 (12.1) | 0.002 |
| Diastolic BP (mmHg) 1 | 73.3 (8.9) | 74.0 (9.0) | 74.3 (9.1) | 74.6 (9.2) | 74.9 (9.4) | <0.001 |
| Estimated GFR (mL/min/1.73 m2) 1 | 101.1 (13.2) | 98.1 (13.8) | 96.1 (14.1) | 93.9 (14.6) | 91.3 (16.2) | 0.044 |
| Glucose (mg/dL) 1 | 98.3 (17.6) | 97.9 (16.8) | 97.5 (16.6) | 96.9 (16.2) | 96.8 (16.6) | 0.066 |
| HbA1c (%) 1 | 5.6 (0.6) | 5.6 (0.6) | 5.6 (0.6) | 5.6 (0.6) | 5.6 (0.6) | 0.041 |
| HOMA-IR value 2 | 1.34 (0.88–2.00) | 1.33 (0.88–1.99) | 1.32 (0.86–2.00) | 1.31 (0.85–2.00) | 1.30 (0.84–2.00) | 0.509 |
| Total cholesterol (mg/dL) 1 | 194.9 (34.5) | 196.3 (34.1) | 196.9 (34.5) | 197.9 (35.1) | 196.9 (35.5) | <0.001 |
| HDL-C (mg/dL) 1 | 53.3 (12.9) | 53.3 (12.8) | 53.1 (12.8) | 53.0 (12.7) | 52.9 (13.0) | 0.521 |
| LDL-C (mg/dL) 1 | 124.5 (31.6) | 125.7 (31.5) | 125.6 (31.9) | 126.3 (32.4) | 125.2 (32.9) | <0.001 |
| Triglycerides (mg/dL) 2 | 112 (79–163) | 113 (79–164) | 113 (79–164) | 112 (80–164) | 113 (80–165) | 0.006 |
| hsCRP (mg/dL) 2 | 0.05 (0.03–0.10) | 0.05 (0.03–0.10) | 0.05 (0.03–0.11) | 0.05 (0.03–0.11) | 0.06 (0.03–0.11) | 0.043 |
| Homocysteine Level (µmol/L) | Person-Years (PY) | Number of Events | Mortality Rate (per 105 PY) | Age-Adjusted HR (95% CI) | Multivariable-Adjusted HR (95% CI) | |
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| All-cause mortality | ||||||
| Q1 (1.1–8.7) | 1,891,440.2 | 216 | 11.4 | 1.11 (0.92–1.33) | 1.12 (0.93–1.35) | 1.06 (0.89–1.28) |
| Q2 (8.8–9.9) | 2,034,626 | 243 | 11.9 | 1.00 | 1.00 | 1.00 |
| Q3 (10.0–11.1) | 2,093,202.6 | 311 | 14.9 | 1.13 (0.95–1.34) | 1.10 (0.93–1.30) | 1.15 (0.98–1.37) |
| Q4 (11.2–12.9) | 2,211,819.1 | 389 | 17.6 | 1.18 (1.00–1.38) | 1.14 (0.97–1.34) | 1.27 (1.08–1.49) |
| Q5 (13.0–179.9) | 2,129,261.1 | 590 | 27.7 | 1.49 (1.28–1.74) | 1.38 (1.19–1.61) | 1.67 (1.43–1.95) |
| p for trend | 0.004 | 0.010 | 0.002 | |||
| CVD mortality | ||||||
| Q1 (1.1–8.7) | 1,891,440.2 | 30 | 1.6 | 1.08 (0.66–1.76) | 1.10 (0.67–1.79) | 1.08 (0.66–1.76) |
| Q2 (8.8–9.9) | 2,034,626 | 35 | 1.7 | 1.00 | 1.00 | 1.00 |
| Q3 (10.0–11.1) | 2,093,202.6 | 42 | 2.0 | 1.05 (0.67–1.65) | 1.03 (0.65–1.61) | 1.05 (0.67–1.64) |
| Q4 (11.2–12.9) | 2,211,819.1 | 60 | 2.7 | 1.25 (0.82–1.89) | 1.19 (0.78–1.81) | 1.25 (0.82–1.90) |
| Q5 (13.0–179.9) | 2,129,261.1 | 86 | 4.0 | 1.47 (0.99–2.19) | 1.34 (0.90–2.00) | 1.46 (0.97–2.20) |
| p for trend | 0.303 | 0.365 | 0.325 | |||
| Cancer mortality | ||||||
| Q1 (1.1–8.7) | 1,891,440.2 | 89 | 4.7 | 0.95 (0.72–1.25) | 0.97 (0.74–1.28) | 0.92 (0.70–1.21) |
| Q2 (8.8–9.9) | 2,034,626 | 119 | 5.9 | 1.00 | 1.00 | 1.00 |
| Q3 (10.0–11.1) | 2,093,202.6 | 156 | 7.5 | 1.15 (0.90–1.46) | 1.11 (0.87–1.41) | 1.17 (0.92–1.49) |
| Q4 (11.2–12.9) | 2,211,819.1 | 162 | 7.3 | 0.99 (0.78–1.25) | 0.95 (0.75–1.21) | 1.07 (0.84–1.36) |
| Q5 (13.0–179.9) | 2,129,261.1 | 249 | 11.7 | 1.25 (1.00–1.55) | 1.15 (0.92–1.43) | 1.44 (1.15–1.80) |
| p for trend | 0.621 | 0.691 | 0.422 | |||
| Homocysteine Level (µmol/L) | Person-Years (PY) | Number of Events | Mortality Rate (per 105 PY) | Age-Adjusted HR (95% CI) | Multivariable-Adjusted HR (95% CI) | |
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| Users of vitamin supplements (n = 47,727) | ||||||
| Q1 (1.1–8.7) | 587,149.9 | 83 | 14.1 | 1.02 (0.75–1.37) | 1.04 (0.77–1.41) | 0.98 (0.72–1.32) |
| Q2 (8.8–9.9) | 538,744.5 | 87 | 16.2 | 1.00 | 1.00 | 1.00 |
| Q3 (10.0–11.1) | 498,566.2 | 102 | 20.5 | 1.09 (0.82–1.45) | 1.06 (0.79–1.41) | 1.12 (0.84–1.49) |
| Q4 (11.2–12.9) | 454,006.1 | 96 | 21.2 | 1.01 (0.75–1.35) | 0.98 (0.73–1.31) | 1.09 (0.81–1.46) |
| Q5 (13.0–179.9) | 352,808.9 | 125 | 35.4 | 1.35 (1.02–1.79) | 1.24 (0.94–1.65) | 1.57 (1.18–2.09) |
| p for trend | 0.207 | 0.248 | 0.134 | |||
| Non-users of vitamin supplements (n = 173,629) | ||||||
| Q1 (1.1–8.7) | 1,304,290.3 | 133 | 10.2 | 1.19 (0.95–1.50) | 1.21 (0.96–1.52) | 1.15 (0.91–1.44) |
| Q2 (8.8–9.9) | 1,495,881.6 | 156 | 10.4 | 1.00 | 1.00 | 1.00 |
| Q3 (10.0–11.1) | 1,594,636.4 | 209 | 13.1 | 1.15 (0.93–1.41) | 1.11 (0.91–1.37) | 1.17 (0.95–1.44) |
| Q4 (11.2–12.9) | 1,757,813.1 | 293 | 16.7 | 1.24 (1.02–1.50) | 1.19 (0.98–1.45) | 1.34 (1.10–1.62) |
| Q5 (13.0–179.9) | 1,776,452.2 | 465 | 26.2 | 1.49 (1.24–1.79) | 1.38 (1.15–1.66) | 1.67 (1.38–2.01) |
| p for trend | 0.014 | 0.025 | 0.012 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Shin, S.; Yoo, E.; Kang, J.-H.; Sung, E.; Kim, C.-H.; Shin, H.; Lee, M.Y. Serum Homocysteine Levels and All-Cause and Cause-Specific Mortality in Korean Adult Men: A Cohort Study. Nutrients 2024, 16, 2759. https://doi.org/10.3390/nu16162759
Kim M, Shin S, Yoo E, Kang J-H, Sung E, Kim C-H, Shin H, Lee MY. Serum Homocysteine Levels and All-Cause and Cause-Specific Mortality in Korean Adult Men: A Cohort Study. Nutrients. 2024; 16(16):2759. https://doi.org/10.3390/nu16162759
Chicago/Turabian StyleKim, Minyoung, Sujeong Shin, Eunsol Yoo, Jae-Heon Kang, Eunju Sung, Cheol-Hwan Kim, Hocheol Shin, and Mi Yeon Lee. 2024. "Serum Homocysteine Levels and All-Cause and Cause-Specific Mortality in Korean Adult Men: A Cohort Study" Nutrients 16, no. 16: 2759. https://doi.org/10.3390/nu16162759
APA StyleKim, M., Shin, S., Yoo, E., Kang, J.-H., Sung, E., Kim, C.-H., Shin, H., & Lee, M. Y. (2024). Serum Homocysteine Levels and All-Cause and Cause-Specific Mortality in Korean Adult Men: A Cohort Study. Nutrients, 16(16), 2759. https://doi.org/10.3390/nu16162759






