The Role of S-Glutathionylation in Health and Disease: A Bird’s Eye View
Abstract
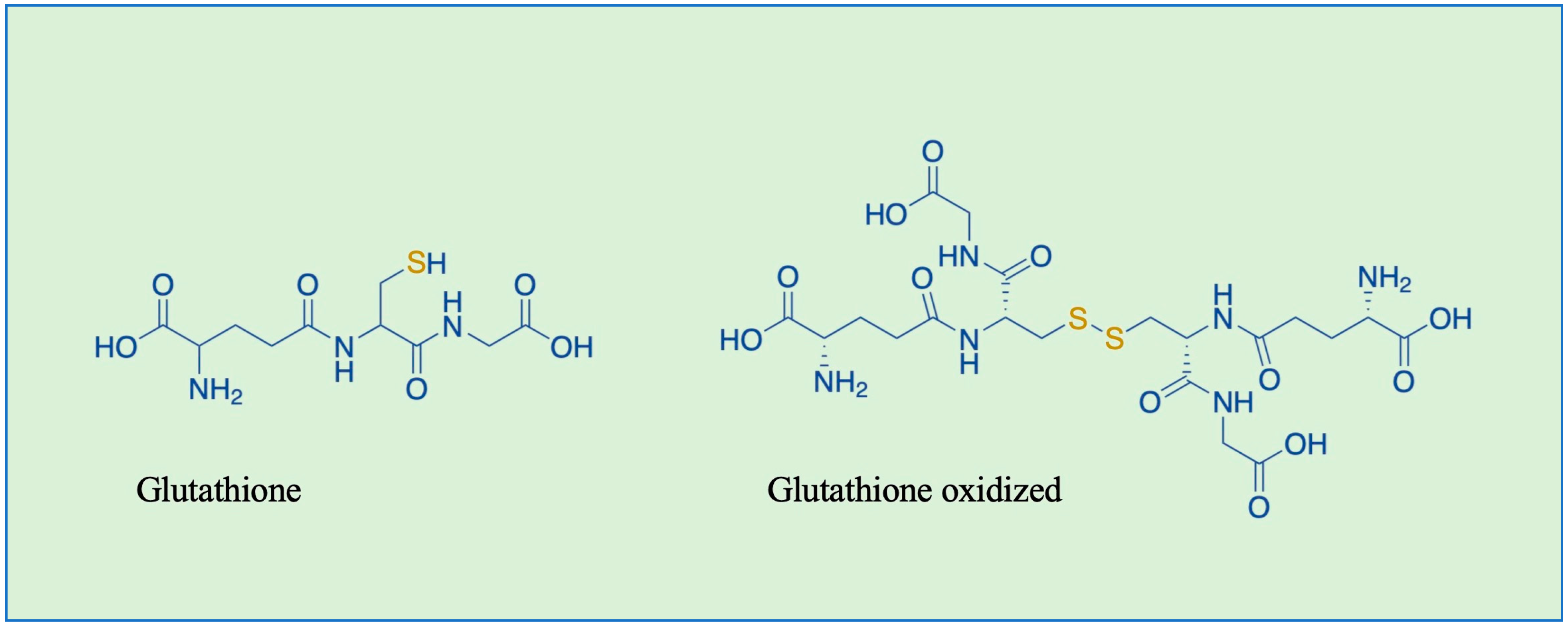
1. Introduction
2. Literature Search
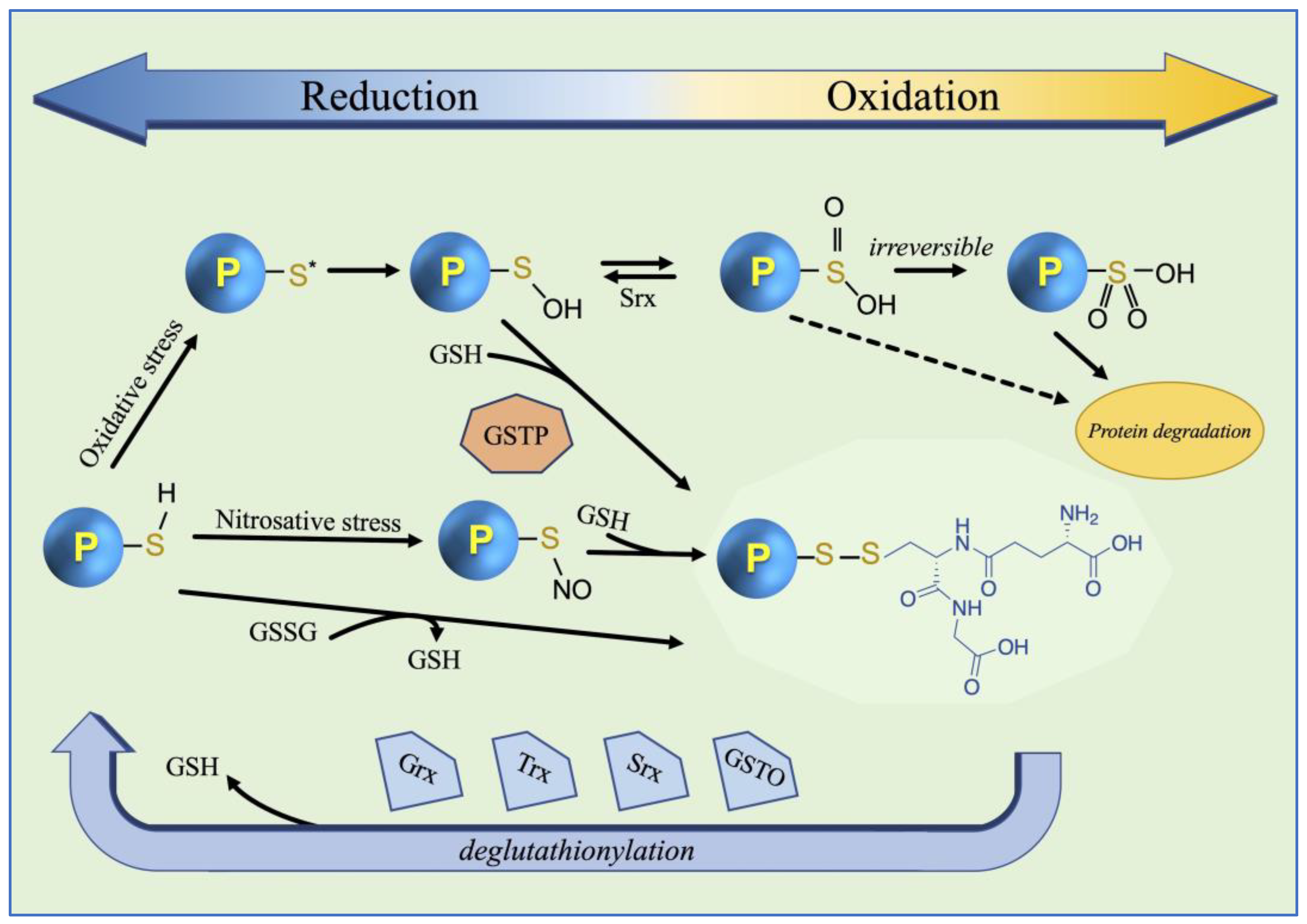
3. S-Glutathionylation Physiological Role
4. S-Glutathionylation in Disease
4.1. Cardiovascular Diseases
4.1.1. GS-Ylated Na+-K+ Pump
4.1.2. GS-Ylation of cMyBP-C
4.1.3. Hypertrophic Cardiomyopathy
4.1.4. GS-Ylation of Rac1
4.1.5. Cerebrovascular Diseases
4.2. Vascular Diseases
GS-Ylation of SirT1
4.3. Neurodegenerative Diseases
4.3.1. Alzheimer’s Disease
4.3.2. Parkinson’s Disease
4.3.3. Huntington’s Disease
4.3.4. Amyotrophic Lateral Sclerosis
4.3.5. Multiple Sclerosis
4.3.6. Friedreich’s Ataxia
4.3.7. GS-Ylation in Microglia Cells
4.3.8. Leber Hereditary Optic Neuropathy
4.4. Kidney Diseases
GS-Ylation of Cystathionine β-Synthase
4.5. Lung Disease
Idiopathic Pulmonary Fibrosis
4.6. Liver Disease
GS-Ylation of Notch1
4.7. Cancer
4.7.1. GS-Ylated NF-κB
4.7.2. GS-Ylated PKC
4.7.3. GS-Ylated p53
| Pathological Conditions | Target Protein | Function | Functional Impact/ Effect of GS-Ylation | Ref. |
|---|---|---|---|---|
| Cardiovascular disease | ||||
| Diastolic dysfunction | cMyBP-C | Cardiac contraction and relaxation | Heart failure | [42] |
| Vascular barrier dysfunction | Rac1 | Correct function of the cell barrier | Cell hyperpermeability | [48] |
| Cerebrovascular disease | ||||
| Different and multifactorial disorders of the blood vessels | eNOS | Prominent enzymatic source of NO in the vascular wall | Functional uncoupling, reduction of NO synthesis, increased | [49] |
| Vascular disease | ||||
| Maintaining the endothelial barrier function | Src tyrosine kinase | Phosphorylation of VE-cadherin | Inhibition of phosphorylation | [53] |
| Marfan syndrome | SirT1 | Prevention of aortic dissections | Contributing to thoracic aortic aneurysm in Marfan syndrome | [55] |
| Neurodegenerative disease | ||||
| Alzheimer’s | ||||
| Slowdown of glycolysis | GAPDH | Glycolytic enzyme | Inhibition of enzymatic activity | [64] |
| Inhibit functionality of p53 | p53 | Control the expression of a wide array of genes | Prevents the formation of the tetramer form of p53 | [70] |
| Parkinson’s | ||||
| Positive feedback regulatory mechanism | KEAP1 | Regulator of Nrf2 activity | Nrf2 activation and subsequently expression of its augments | [75] |
| Huntington’s | ||||
| Striatal neuron loss | TRPC5 | Calcium channels | Enhancement of calcium ions in cytosol | [81] |
| Amyotrophic lateral sclerosis | ||||
| Excessive release of ER calcium into cytoplasm | STIM1 | Maintain cellular Ca2+ balance | Dysregulation of Ca2+ entry | [82] |
| Friedreich’s ataxia | ||||
| Impairment of cytoskeletal functions | Actin | Cytoskeletal proteins | Compromission of microfilament organization in FDRA fibroblasts | [91] |
| Neuroinflammation | STAT1 | Regulation of inflammatory response | Hyper-activation of its signaling in microglia cells | [95] |
| Other organ diseases | ||||
| Kidney | ||||
| Influencing renal salt and water reabsorption | Na+-K+ ATPase | Maintains the balance of sodium and potassium ions in cells | Affect pump activity | [100] |
| Lung | ||||
| Implicated in the pathogenesis of IPF | Fas receptor | Trigger epithelial cell apoptosis | Amplification of epithelial cells apoptosis | [108] |
| Liver | ||||
| Increased inflammation | Notch1 | Involved in various biological processes | Enhanced of Notch1 signaling pathway | [112] |
| Cancer | ||||
| NF-κB highly expressed in NSCLC | NF-κB | Involved in various biological processes | Enhanced lung inflammation | [118] |
| Dysregulation of PKC signaling | PKC | Involved in signaling pathways | Complete inactivation of the enzyme | [121] |
| Inhibit functionality of p53 | p53 | Control the expression of a wide array of genes | Complete inactivation of transcription factor | [124] |
4.7.4. GS-Ylated BiP
5. GS-Ylated Proteins as Biomarkers
6. Therapeutical Applications
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Ramazi, S.; Zahiri, J. Post-Translational Modifications in Proteins: Resources, Tools and Prediction Methods. Database 2021, 2021, baab012. [Google Scholar] [CrossRef]
- Ghezzi, P. Protein Glutathionylation in Health and Disease. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2013, 1830, 3165–3172. [Google Scholar] [CrossRef]
- Bartolini, D.; Torquato, P.; Piroddi, M.; Galli, F. Targeting Glutathione S-Transferase P and Its Interactome with Selenium Compounds in Cancer Therapy. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2019, 1863, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Gill, R.; Mailloux, R.J. Protein S-Glutathionylation: The Linchpin for the Transmission of Regulatory Information on Redox Buffering Capacity in Mitochondria. Chem. -Biol. Interact. 2019, 299, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Musaogullari, A.; Chai, Y.-C. Redox Regulation by Protein S-Glutathionylation: From Molecular Mechanisms to Implications in Health and Disease. Int. J. Mol. Sci. 2020, 21, 8113. [Google Scholar] [CrossRef] [PubMed]
- Kukulage, D.S.K.; Matarage Don, N.N.J.; Ahn, Y.-H. Emerging Chemistry and Biology in Protein Glutathionylation. Curr. Opin. Chem. Biol. 2022, 71, 102221. [Google Scholar] [CrossRef]
- Bak, D.W.; Bechtel, T.J.; Falco, J.A.; Weerapana, E. Cysteine Reactivity across the Subcellular Universe. Curr. Opin. Chem. Biol. 2019, 48, 96–105. [Google Scholar] [CrossRef]
- Miseta, A.; Csutora, P. Relationship Between the Occurrence of Cysteine in Proteins and the Complexity of Organisms. Mol. Biol. Evol. 2000, 17, 1232–1239. [Google Scholar] [CrossRef]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New Roles in Redox Signaling for an Old Antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.W.K.; Gan, Y.-H. New Roles for Glutathione: Modulators of Bacterial Virulence and Pathogenesis. Redox Biol. 2021, 44, 102012. [Google Scholar] [CrossRef]
- Scirè, A.; Cianfruglia, L.; Minnelli, C.; Bartolini, D.; Torquato, P.; Principato, G.; Galli, F.; Armeni, T. Glutathione Compartmentalization and Its Role in Glutathionylation and Other Regulatory Processes of Cellular Pathways. BioFactors 2019, 45, 152–168. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Thiel, A.; Weishaupt, A.-K.; Nicolai, M.M.; Lossow, K.; Kipp, A.P.; Schwerdtle, T.; Bornhorst, J. Simultaneous Quantitation of Oxidized and Reduced Glutathione via LC-MS/MS to Study the Redox State and Drug-Mediated Modulation in Cells, Worms and Animal Tissue. J. Chromatogr. B 2023, 1225, 123742. [Google Scholar] [CrossRef] [PubMed]
- Allocati, N.; Masulli, M.; Di Ilio, C.; Federici, L. Glutathione Transferases: Substrates, Inihibitors and pro-Drugs in Cancer and Neurodegenerative Diseases. Oncogenesis 2018, 7, 8. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive Oxygen Species (ROS) Homeostasis and Redox Regulation in Cellular Signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Vrettou, S.; Wirth, B. S-Glutathionylation and S-Nitrosylation in Mitochondria: Focus on Homeostasis and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 15849. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Martínez, M.C.; Andriantsitohaina, R. Reactive Nitrogen Species: Molecular Mechanisms and Potential Significance in Health and Disease. Antioxid. Redox Signal. 2009, 11, 669–702. [Google Scholar] [CrossRef]
- Dominko, K.; Đikić, D. Glutathionylation: A Regulatory Role of Glutathione in Physiological Processes. Arch. Ind. Hyg. Toxicol. 2018, 69, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.B.; Mailloux, R.J. Mitochondria Need Their Sleep: Redox, Bioenergetics, and Temperature Regulation of Circadian Rhythms and the Role of Cysteine-Mediated Redox Signaling, Uncoupling Proteins, and Substrate Cycles. Antioxidants 2023, 12, 674. [Google Scholar] [CrossRef]
- Ghezzi, P.; Di Simplicio, P. Glutathionylation Pathways in Drug Response. Curr. Opin. Pharmacol. 2007, 7, 398–403. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, Z.; Singh, S.; Townsend, D.M.; Tew, K.D. An Evolving Understanding of the S-Glutathionylation Cycle in Pathways of Redox Regulation. Free Radic. Biol. Med. 2018, 120, 204–216. [Google Scholar] [CrossRef]
- Tew, K.D. Redox in Redux: Emergent Roles for Glutathione S-Transferase P (GSTP) in Regulation of Cell Signaling and S-Glutathionylation. Biochem. Pharmacol. 2007, 73, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.M.; Manevich, Y.; He, L.; Hutchens, S.; Pazoles, C.J.; Tew, K.D. Novel Role for Glutathione S-Transferase π. J. Biol. Chem. 2009, 284, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, E.; Novichkova, M. Glutathione in Protein Redox Modulation through S-Glutathionylation and S-Nitrosylation. Molecules 2021, 26, 435. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Uys, J.D.; Tew, K.D.; Townsend, D.M. S-Glutathionylation: From Molecular Mechanisms to Health Outcomes. Antioxid. Redox Signal. 2011, 15, 233–270. [Google Scholar] [CrossRef]
- Galeazzi, R.; Laudadio, E.; Falconi, E.; Massaccesi, L.; Ercolani, L.; Mobbili, G.; Minnelli, C.; Scirè, A.; Cianfruglia, L.; Armeni, T. Protein–Protein Interactions of Human Glyoxalase II: Findings of a Reliable Docking Protocol. Org. Biomol. Chem. 2018, 16, 5167–5177. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.G.; Bhatnagar, A. Protein S-Glutathiolation: Redox-Sensitive Regulation of Protein Function. J. Mol. Cell. Cardiol. 2012, 52, 559–567. [Google Scholar] [CrossRef]
- Greetham, D.; Vickerstaff, J.; Shenton, D.; Perrone, G.G.; Dawes, I.W.; Grant, C.M. Thioredoxins Function as Deglutathionylase Enzymes in the Yeast Saccharomyces Cerevisiae. BMC Biochem. 2010, 11, 3. [Google Scholar] [CrossRef]
- Findlay, V.J.; Townsend, D.M.; Morris, T.E.; Fraser, J.P.; He, L.; Tew, K.D. A Novel Role for Human Sulfiredoxin in the Reversal of Glutathionylation. Cancer Res. 2006, 66, 6800–6806. [Google Scholar] [CrossRef]
- Ramesh, A.; Varghese, S.S.; Doraiswamy, J.; Malaiappan, S. Role of Sulfiredoxin in Systemic Diseases Influenced by Oxidative Stress. Redox Biol. 2014, 2, 1023–1028. [Google Scholar] [CrossRef]
- Menon, D.; Board, P.G. A Role for Glutathione Transferase Omega 1 (GSTO1-1) in the Glutathionylation Cycle. J. Biol. Chem. 2013, 288, 25769–25779. [Google Scholar] [CrossRef]
- Starke, D.W.; Chock, P.B.; Mieyal, J.J. Glutathione-Thiyl Radical Scavenging and Transferase Properties of Human Glutaredoxin (Thioltransferase). J. Biol. Chem. 2003, 278, 14607–14613. [Google Scholar] [CrossRef] [PubMed]
- Gallogly, M.M.; Starke, D.W.; Leonberg, A.K.; Ospina, S.M.E.; Mieyal, J.J. Kinetic and Mechanistic Characterization and Versatile Catalytic Properties of Mammalian Glutaredoxin 2: Implications for Intracellular Roles. Biochemistry 2008, 47, 11144–11157. [Google Scholar] [CrossRef] [PubMed]
- Hanna, P.E.; Anders, M.W. The Mercapturic Acid Pathway. Crit. Rev. Toxicol. 2019, 49, 819–929. [Google Scholar] [CrossRef]
- Pastore, A.; Piemonte, F. Protein Glutathionylation in Cardiovascular Diseases. Int. J. Mol. Sci. 2013, 14, 20845–20876. [Google Scholar] [CrossRef]
- Bubb, K.J.; Birgisdottir, A.B.; Tang, O.; Hansen, T.; Figtree, G.A. Redox Modification of Caveolar Proteins in the Cardiovascular System- Role in Cellular Signalling and Disease. Free Radic. Biol. Med. 2017, 109, 61–74. [Google Scholar] [CrossRef]
- Lian, X.; Matthaeus, C.; Kaßmann, M.; Daumke, O.; Gollasch, M. Pathophysiological Role of Caveolae in Hypertension. Front. Med. 2019, 6, 153. [Google Scholar] [CrossRef]
- Figtree, G.A.; Keyvan Karimi, G.; Liu, C.-C.; Rasmussen, H.H. Oxidative Regulation of the Na+–K+ Pump in the Cardiovascular System. Free Radic. Biol. Med. 2012, 53, 2263–2268. [Google Scholar] [CrossRef] [PubMed]
- Petrushanko, I.Y.; Yakushev, S.; Mitkevich, V.A.; Kamanina, Y.V.; Ziganshin, R.H.; Meng, X.; Anashkina, A.A.; Makhro, A.; Lopina, O.D.; Gassmann, M.; et al. S-Glutathionylation of the Na,K-ATPase Catalytic α Subunit Is a Determinant of the Enzyme Redox Sensitivity. J. Biol. Chem. 2012, 287, 32195–32205. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulou, K.; Wittig, I.; Heidler, J.; Piasecki, A.; Richter, F.; Diering, S.; Velden, J.; Buck, F.; Donzelli, S.; Schröder, E.; et al. S-glutathiolation Impairs Phosphoregulation and Function of Cardiac Myosin-binding Protein C in Human Heart Failure. FASEB J. 2016, 30, 1849–1864. [Google Scholar] [CrossRef]
- Zhou, X.; Jeong, E.; Liu, H.; Kaseer, B.; Liu, M.; Shrestha, S.; Imran, H.; Kavanagh, K.; Jiang, N.; Desimone, L.; et al. Circulating S-Glutathionylated cMyBP-C as a Biomarker for Cardiac Diastolic Dysfunction. J. Am. Hear. Assoc. 2022, 11, e025295. [Google Scholar] [CrossRef]
- Patel, B.G.; Wilder, T.; Solaro, R.J. Novel Control of Cardiac Myofilament Response to Calcium by S-Glutathionylation at Specific Sites of Myosin Binding Protein C. Front. Physiol. 2013, 4, 68150. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.M. Hypertrophic Cardiomyopathy: Genetics and Clinical Perspectives. Cardiovasc. Diagn. Ther. 2019, 9, S388–S415. [Google Scholar] [CrossRef]
- Ryba, D.M.; Warren, C.M.; Karam, C.N.; Davis, R.T.; Chowdhury, S.A.K.; Alvarez, M.G.; McCann, M.; Liew, C.W.; Wieczorek, D.F.; Varga, P.; et al. Sphingosine-1-Phosphate Receptor Modulator, FTY720, Improves Diastolic Dysfunction and Partially Reverses Atrial Remodeling in a Tm-E180G Mouse Model Linked to Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2019, 12, e005835. [Google Scholar] [CrossRef]
- Etienne-Manneville, S.; Hall, A. Rho GTPases in Cell Biology. Nature 2002, 420, 629–635. [Google Scholar] [CrossRef]
- Strassheim, D.; Gerasimovskaya, E.; Irwin, D.; Dempsey, E.C.; Stenmark, K.; Karoor, V. RhoGTPase in Vascular Disease. Cells 2019, 8, 551. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Weisbrod, R.M.; Shao, D.; Watanabe, Y.; Yin, X.; Bachschmid, M.M.; Seta, F.; Janssen-Heininger, Y.M.W.; Matsui, R.; Zang, M.; et al. The Redox Mechanism for Vascular Barrier Dysfunction Associated with Metabolic Disorders: Glutathionylation of Rac1 in Endothelial Cells. Redox Biol. 2016, 9, 306–319. [Google Scholar] [CrossRef]
- Chen, C.-A.; Wang, T.-Y.; Varadharaj, S.; Reyes, L.A.; Hemann, C.; Talukder, M.A.H.; Chen, Y.-R.; Druhan, L.J.; Zweier, J.L. S-Glutathionylation Uncouples eNOS and Regulates Its Cellular and Vascular Function. Nature 2010, 468, 1115–1118. [Google Scholar] [CrossRef]
- Matsuo, K.; Yabuki, Y.; Fukunaga, K. Combined L-Citrulline and Glutathione Administration Prevents Neuronal Cell Death Following Transient Brain Ischemia. Brain Res. 2017, 1663, 123–131. [Google Scholar] [CrossRef]
- Dejana, E.; Giampietro, C. Vascular Endothelial-Cadherin and Vascular Stability. Curr. Opin. Hematol. 2012, 19, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Goswami, S.; Dash, S.; Samanta, D. Structural Basis of Molecular Recognition among Classical Cadherins Mediating Cell Adhesion. Biochem. Soc. Trans. 2023, 51, 2103–2115. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dong, X.; Zheng, S.; Sun, J.; Ye, J.; Chen, J.; Fang, Y.; Zhao, B.; Yin, Z.; Cao, P.; et al. GSTpi Regulates VE-Cadherin Stabilization through Promoting S-Glutathionylation of Src. Redox Biol. 2020, 30, 101416. [Google Scholar] [CrossRef]
- Wallez, Y.; Cand, F.; Cruzalegui, F.; Wernstedt, C.; Souchelnytskyi, S.; Vilgrain, I.; Huber, P. Src Kinase Phosphorylates Vascular Endothelial-Cadherin in Response to Vascular Endothelial Growth Factor: Identification of Tyrosine 685 as the Unique Target Site. Oncogene 2007, 26, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Budbazar, E.; Sulser Ponce De Leon, S.; Tsukahara, Y.; Liu, H.; Huangfu, Y.; Wang, Y.; Seabra, P.M.; Yang, X.; Goodman, J.B.; Wan, X.; et al. Redox Dysregulation of Vascular Smooth Muscle Sirtuin-1 in Thoracic Aortic Aneurysm in Marfan Syndrome. Arter. Thromb. Vasc. Biol. 2023, 43, E339–E357. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yao, L.; Ma, X.; Xu, X. Small Molecules as SIRT Modulators. Mini-Reviews Med. Chem. 2018, 18, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Fry, J.L.; Shiraishi, Y.; Turcotte, R.; Yu, X.; Gao, Y.Z.; Akiki, R.; Bachschmid, M.; Zhang, Y.; Morgan, K.G.; Cohen, R.A.; et al. Vascular Smooth Muscle Sirtuin-1 Protects Against Aortic Dissection During Angiotensin II–Induced Hypertension. J. Am. Hear. Assoc. 2015, 4, e002384. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 Reasons Why the Brain Is Susceptible to Oxidative Stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer Disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef]
- Rani, P.; Krishnan, S.; Rani Cathrine, C. Study on Analysis of Peripheral Biomarkers for Alzheimer’s Disease Diagnosis. Front. Neurol. 2017, 8, 328. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kim, S.-H.; Bishayee, K. Dysfunctional Glucose Metabolism in Alzheimer’s Disease Onset and Potential Pharmacological Interventions. Int. J. Mol. Sci. 2022, 23, 9540. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guglielmetti, C.; Sei, Y.J.; Zilberter, M.; Le Page, L.M.; Shields, L.; Yang, J.; Nguyen, K.; Tiret, B.; Gao, X.; et al. Neurons Require Glucose Uptake and Glycolysis In Vivo. Cell Rep. 2023, 42, 112335. [Google Scholar] [CrossRef]
- Hyslop, P.A.; Boggs, L.N.; Chaney, M.O. Origin of Elevated S-Glutathionylated GAPDH in Chronic Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 5529. [Google Scholar] [CrossRef]
- Newman, S.F.; Sultana, R.; Perluigi, M.; Coccia, R.; Cai, J.; Pierce, W.M.; Klein, J.B.; Turner, D.M.; Butterfield, D.A. An Increase in S-Glutathionylated Proteins in the Alzheimer’s Disease Inferior Parietal Lobule, a Proteomics Approach. J. Neurosci. Res. 2007, 85, 1506–1514. [Google Scholar] [CrossRef]
- Cortez, L.M.; Ávila, C.L.; Torres Bugeau, C.M.; Farías, R.N.; Morero, R.D.; Chehín, R.N. Glyceraldehyde-3-Phosphate Dehydrogenase Tetramer Dissociation and Amyloid Fibril Formation Induced by Negatively Charged Membranes. FEBS Lett. 2010, 584, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Arutyunova, E.I.; Danshina, P.V.; Domnina, L.V.; Pleten, A.P.; Muronetz, V.I. Oxidation of Glyceraldehyde-3-Phosphate Dehydrogenase Enhances Its Binding to Nucleic Acids. Biochem. Biophys. Res. Commun. 2003, 307, 547–552. [Google Scholar] [CrossRef]
- Tsai, C.W.; Tsai, C.F.; Lin, K.H.; Chen, W.J.; Lin, M.S.; Hsieh, C.C.; Lin, C.C. An Investigation of the Correlation between the S-Glutathionylated GAPDH Levels in Blood and Alzheimer’s Disease Progression. PLoS ONE 2020, 15, e0233289. [Google Scholar] [CrossRef]
- Kruiswijk, F.; Labuschagne, C.F.; Vousden, K.H. P53 in Survival, Death and Metabolic Health: A Lifeguard with a Licence to Kill. Nat. Rev. Mol. Cell Biol. 2015, 16, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Cao, J.; Topatana, W.; Juengpanich, S.; Li, S.; Zhang, B.; Shen, J.; Cai, L.; Cai, X.; Chen, M. Targeting Mutant P53 for Cancer Therapy: Direct and Indirect Strategies. J. Hematol. Oncol. 2021, 14, 157. [Google Scholar] [CrossRef]
- Domenico, F.D.; Cenini, G.; Sultana, R.; Perluigi, M.; Uberti, D.; Memo, M.; Butterfield, D.A. Glutathionylation of the Pro-Apoptotic Protein P53 in Alzheimer’s Disease Brain: Implications for AD Pathogenesis. Neurochem. Res. 2009, 34, 727–733. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Gorelenkova Miller, O.; Mieyal, J.J. Critical Roles of Glutaredoxin in Brain Cells—Implications for Parkinson’s Disease. Antioxid. Redox Signal. 2019, 30, 1352–1368. [Google Scholar] [CrossRef]
- Mazari, A.M.A.; Zhang, L.; Ye, Z.-W.; Zhang, J.; Tew, K.D.; Townsend, D.M. The Multifaceted Role of Glutathione S-Transferases in Health and Disease. Biomolecules 2023, 13, 688. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Deng, P.; Liu, S.; Bian, Y.; Xu, Y.; Zhang, Q.; Wang, H.; Pi, J. Is Nuclear Factor Erythroid 2-Related Factor 2 a Target for the Intervention of Cytokine Storms? Antioxidants 2023, 12, 172. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.N.; Marques, C.; Guedes, R.C.; Castro-Caldas, M.; Rodrigues, E.; Van Horssen, J.; Gama, M.J. S-Glutathionylation of Keap1: A New Role for Glutathione S-Transferase Pi in Neuronal Protection. FEBS Lett. 2016, 590, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Dagnino-Subiabre, A.; Cassels, B.K.; Baez, S.; Johansson, A.-S.; Mannervik, B.; Segura-Aguilar, J. Glutathione Transferase M2-2 Catalyzes Conjugation of Dopamine and Dopa o-Quinones. Biochem. Biophys. Res. Commun. 2000, 274, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Badillo-Ramírez, I.; Saniger, J.M.; Rivas-Arancibia, S. 5-S-Cysteinyl-Dopamine, a Neurotoxic Endogenous Metabolite of Dopamine: Implications for Parkinson’s Disease. Neurochem. Int. 2019, 129, 104514. [Google Scholar] [CrossRef] [PubMed]
- Badillo-Ramírez, I.; Landeros-Rivera, B.; Saniger, J.M.; Popp, J.; Cialla-May, D. SERS-Based Detection of 5- S -Cysteinyl-Dopamine as a Novel Biomarker of Parkinson’s Disease in Artificial Biofluids. Analyst 2023, 148, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- McColgan, P.; Tabrizi, S.J. Huntington’s Disease: A Clinical Review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef]
- Silajdžić, E.; Björkqvist, M. A Critical Evaluation of Wet Biomarkers for Huntington’s Disease: Current Status and Ways Forward. J. Huntington’s Dis. 2018, 7, 109–135. [Google Scholar] [CrossRef]
- Hong, C.; Seo, H.; Kwak, M.; Jeon, J.; Jang, J.; Jeong, E.M.; Myeong, J.; Hwang, Y.J.; Ha, K.; Kang, M.J.; et al. Increased TRPC5 Glutathionylation Contributes to Striatal Neuron Loss in Huntington’s Disease. Brain 2015, 138, 3030–3047. [Google Scholar] [CrossRef]
- Kawamata, H.; Ng, S.K.; Diaz, N.; Burstein, S.; Morel, L.; Osgood, A.; Sider, B.; Higashimori, H.; Haydon, P.G.; Manfredi, G.; et al. Abnormal Intracellular Calcium Signaling and SNARE-Dependent Exocytosis Contributes to SOD1G93A Astrocyte-Mediated Toxicity in Amyotrophic Lateral Sclerosis. J. Neurosci. 2014, 34, 2331–2348. [Google Scholar] [CrossRef] [PubMed]
- Gudlur, A.; Zeraik, A.E.; Hirve, N.; Rajanikanth, V.; Bobkov, A.A.; Ma, G.; Zheng, S.; Wang, Y.; Zhou, Y.; Komives, E.A.; et al. Calcium Sensing by the STIM1 ER-Luminal Domain. Nat. Commun. 2018, 9, 4536. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Caro, C.; Berrocal, M.; Lopez-Guerrero, A.M.; Alvarez-Barrientos, A.; Pozo-Guisado, E.; Gutierrez-Merino, C.; Mata, A.M.; Martin-Romero, F.J. STIM1 Deficiency Is Linked to Alzheimer’s Disease and Triggers Cell Death in SH-SY5Y Cells by Upregulation of L-Type Voltage-Operated Ca2+ Entry. J. Mol. Med. 2018, 96, 1061–1079. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Caro, C.; Espinosa-Bermejo, N.; Pozo-Guisado, E.; Martin-Romero, F.J. Role of STIM1 in Neurodegeneration. World J. Biol. Chem. 2018, 9, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Hogan, P.G.; Rao, A. Store-Operated Calcium Entry: Mechanisms and Modulation. Biochem. Biophys. Res. Commun. 2015, 460, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Mielnicka, A.; Michaluk, P. Exocytosis in Astrocytes. Biomolecules 2021, 11, 1367. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.; Kukkuta Sarma, G.R.; Dsouza, D.S.; Muralidharan, M.; Srinivasan, K.; Mandal, A.K. Characterization of Residue-Specific Glutathionylation of CSF Proteins in Multiple Sclerosis—A MS-Based Approach. Anal. Biochem. 2019, 564–565, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Castro, I.H.; Pignataro, M.F.; Sewell, K.E.; Espeche, L.D.; Herrera, M.G.; Noguera, M.E.; Dain, L.; Nadra, A.D.; Aran, M.; Smal, C.; et al. Frataxin Structure and Function. In Macromolecular Protein Complexes II: Structure and Function; Harris, J.R., Marles-Wright, J., Eds.; Subcellular Biochemistry; Springer International Publishing: Cham, Switzerland, 2019; Volume 93, pp. 393–438. ISBN 978-3-030-28150-2. [Google Scholar]
- Maio, N.; Rouault, T.A. Outlining the Complex Pathway of Mammalian Fe-S Cluster Biogenesis. Trends Biochem. Sci. 2020, 45, 411–426. [Google Scholar] [CrossRef]
- Pastore, A.; Tozzi, G.; Gaeta, L.M.; Bertini, E.; Serafini, V.; Cesare, S.D.; Bonetto, V.; Casoni, F.; Carrozzo, R.; Federici, G.; et al. Actin Glutathionylation Increases in Fibroblasts of Patients with Friedreich’s Ataxia. J. Biol. Chem. 2003, 278, 42588–42595. [Google Scholar] [CrossRef]
- Piermarini, E.; Cartelli, D.; Pastore, A.; Tozzi, G.; Compagnucci, C.; Giorda, E.; D’Amico, J.; Petrini, S.; Bertini, E.; Cappelletti, G.; et al. Frataxin Silencing Alters Microtubule Stability in Motor Neurons: Implications for Friedreich’s Ataxia. Hum. Mol. Genet. 2016, 25, 4288–4301. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, W.; Zhang, J. A Richer and More Diverse Future for Microglia Phenotypes. Heliyon 2023, 9, e14713. [Google Scholar] [CrossRef]
- Butturini, E.; Boriero, D.; Carcereri De Prati, A.; Mariotto, S. STAT1 Drives M1 Microglia Activation and Neuroinflammation under Hypoxia. Arch. Biochem. Biophys. 2019, 669, 22–30. [Google Scholar] [CrossRef]
- Butturini, E.; Cozzolino, F.; Boriero, D.; Carcereri De Prati, A.; Monti, M.; Rossin, M.; Canetti, D.; Cellini, B.; Pucci, P.; Mariotto, S. S-Glutathionylation Exerts Opposing Roles in the Regulation of STAT1 and STAT3 Signaling in Reactive Microglia. Free Radic. Biol. Med. 2018, 117, 191–201. [Google Scholar] [CrossRef]
- Lenaers, G.; Beaulieu, C.; Charif, M.; Gerber, S.; Kaplan, J.; Rozet, J.-M. Autosomal Recessive Leber Hereditary Optic Neuropathy, a New Neuro-Ophthalmo-Genetic Paradigm. Brain 2023, 146, 3156–3161. [Google Scholar] [CrossRef]
- Zhou, L.; Chan, J.C.Y.; Chupin, S.; Gueguen, N.; Desquiret-Dumas, V.; Koh, S.K.; Li, J.; Gao, Y.; Deng, L.; Verma, C.; et al. Increased Protein S-Glutathionylation in Leber’s Hereditary Optic Neuropathy (LHON). Int. J. Mol. Sci. 2020, 21, 3027. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Rasheed, M.S.U.; Singh, M.P. Redox Modulation of Mitochondrial Proteins in the Neurotoxicant Models of Parkinson’s Disease. Antioxid. Redox Signal. 2023, 38, 824–852. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Yi, H.; Inaba, K.; Akao, Y.; Shamoto-Nagai, M. Mitochondria in Neurodegenerative Disorders: Regulation of the Redox State and Death Signaling Leading to Neuronal Death and Survival. J. Neural Transm. 2009, 116, 1371–1381. [Google Scholar] [CrossRef]
- Tamma, G.; Valenti, G. Evaluating the Oxidative Stress in Renal Diseases: What Is the Role for S-Glutathionylation? Antioxid. Redox Signal. 2016, 25, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Takayama, F.; Tsutsui, S.; Horie, M.; Shimokata, K.; Niwa, T. Glutathionyl Hemoglobin in Uremic Patients Undergoing Hemodialysis and Continuous Ambulatory Peritoneal Dialysis. Kidney Int. 2001, 59, S155–S158. [Google Scholar] [CrossRef]
- Jhee, K.-H.; Kruger, W.D. The Role of Cystathionine β-Synthase in Homocysteine Metabolism. Antioxid. Redox Signal. 2005, 7, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Roh, H.; Kwon, Y. Causes of Hyperhomocysteinemia and Its Pathological Significance. Arch. Pharm. Res. 2018, 41, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Feng, J.; Ji, P.; Liu, Y.; Wan, H.; Zhang, J. Association of Hyperhomocysteinemia and Chronic Kidney Disease in the General Population: A Systematic Review and Meta-Analysis. BMC Nephrol. 2023, 24, 247. [Google Scholar] [CrossRef] [PubMed]
- Kabil, O.; Motl, N.; Banerjee, R. H2S and Its Role in Redox Signaling. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2014, 1844, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.-N.; Yadav, P.K.; Adamec, J.; Banerjee, R. S-Glutathionylation Enhances Human Cystathionine β-Synthase Activity Under Oxidative Stress Conditions. Antioxid. Redox Signal. 2015, 22, 350–361. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.H.; Van Der Velden, J.L.J.; Lahue, K.G.; Qian, X.; Schneider, R.W.; Iberg, M.S.; Nolin, J.D.; Abdalla, S.; Casey, D.T.; Tew, K.D.; et al. Attenuation of Lung Fibrosis in Mice with a Clinically Relevant Inhibitor of Glutathione-S-Transferase π. J. Clin. Investig. Insight 2016, 1, e85717. [Google Scholar] [CrossRef] [PubMed]
- Anttila, S.; Hirvonen, A.; Vainio, H.; Husgafvel-Pursiainen, K.; Hayes, J.D.; Ketterer, B. Immunohistochemical Localization of Glutathione S-Transferases in Human Lung1. Cancer Res. 1993, 53, 5643–5648. [Google Scholar] [PubMed]
- Das, S.K.; Vasudevan, D.M. Alcohol-Induced Oxidative Stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef]
- Kelley, J.L.; Ozment, T.R.; Li, C.; Schweitzer, J.B.; Williams, D.L. Scavenger Receptor-A (CD204): A Two-Edged Sword in Health and Disease. Crit. Rev. Immunol. 2014, 34, 241–261. [Google Scholar] [CrossRef]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch Signaling Pathway: Architecture, Disease, and Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef]
- Li, L.; Luo, J.; Zhu, Z.; Wang, P.; Xu, Q.; Chang, B.; Wang, D.; Yu, L.; Lu, X.; Zhou, J.; et al. Macrophage-Expressed SRA Ameliorates Alcohol-Induced Liver Injury by Suppressing S-Glutathionylation of Notch1 via Recruiting Thioredoxin. J. Leukoc. Biol. 2024, 115, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Friedman, S.L. Mechanisms of Hepatic Stellate Cell Activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Li, Y.; Xu, P.; Li, S.; Liu, Z.; Tung, H.; Cai, X.; Wang, J.; Huang, H.; Wang, M.; et al. The Anti-Fibrotic Drug Pirfenidone Inhibits Liver Fibrosis by Targeting the Small Oxidoreductase Glutaredoxin-1. Sci. Adv. 2021, 7, eabg9241. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Rai, A.; Checker, R.; Patwardhan, R.S.; Singh, B.; Sharma, D.; Sandur, S.K. Role of Protein S-Glutathionylation in Cancer Progression and Development of Resistance to Anti-Cancer Drugs. Arch. Biochem. Biophys. 2021, 704, 108890. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF- B Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Zhang, L.; Ludden, C.M.; Cullen, A.J.; Tew, K.D.; Branco De Barros, A.L.; Townsend, D.M. Nuclear Factor Kappa B Expression in Non-Small Cell Lung Cancer. Biomed. Pharmacother. 2023, 167, 115459. [Google Scholar] [CrossRef]
- Aslam, N.; Alvi, F. Protein Kinase C Life Cycle: Explained Through Systems Biology Approach. Front. Physiol. 2022, 13, 818688. [Google Scholar] [CrossRef]
- Benavides, F.; Blando, J.; Perez, C.J.; Garg, R.; Conti, C.J.; DiGiovanni, J.; Kazanietz, M.G. Transgenic Overexpression of PKCε in the Mouse Prostate Induces Preneoplastic Lesions. Cell Cycle 2011, 10, 268–277. [Google Scholar] [CrossRef]
- Chu, F. PKC Isozyme S-Cysteinylation by Cystine Stimulates the pro-Apoptotic Isozyme PKCdelta and Inactivates the Oncogenic Isozyme PKCvarepsilon. Carcinogenesis 2003, 24, 317–325. [Google Scholar] [CrossRef]
- Ward, N.E.; Stewart, J.R.; Ioannides, C.G.; O’Brian, C.A. Oxidant-Induced S -Glutathiolation Inactivates Protein Kinase C-α (PKC-α): A Potential Mechanism of PKC Isozyme Regulation. Biochemistry 2000, 39, 10319–10329. [Google Scholar] [CrossRef]
- Ozaki, T.; Nakagawara, A. Role of P53 in Cell Death and Human Cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef]
- Velu, C.S.; Niture, S.K.; Doneanu, C.E.; Pattabiraman, N.; Srivenugopal, K.S. Human P53 Is Inhibited by Glutathionylation of Cysteines Present in the Proximal DNA-Binding Domain during Oxidative Stress. Biochemistry 2007, 46, 7765–7780. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.A.; Chuang, T.; Bhat, G.J.; Srivenugopal, K.S. Cys-141 Glutathionylation of Human P53: Studies Using Specific Polyclonal Antibodies in Cancer Samples and Cell Lines. Free Radic. Biol. Med. 2010, 49, 908–917. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, N.W.C.J.; Pawlyn, C.; Yong, K.L. Multiple Myeloma. Lancet 2021, 397, 410–427. [Google Scholar] [CrossRef]
- Adams, J. The Development of Proteasome Inhibitors as Anticancer Drugs. Cancer Cell 2004, 5, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ye, Z.; Chen, W.; Culpepper, J.; Jiang, H.; Ball, L.E.; Mehrotra, S.; Blumental-Perry, A.; Tew, K.D.; Townsend, D.M. Altered Redox Regulation and S-Glutathionylation of BiP Contribute to Bortezomib Resistance in Multiple Myeloma. Free Radic. Biol. Med. 2020, 160, 755–767. [Google Scholar] [CrossRef]
- Lind, C.; Gerdes, R.; Hamnell, Y.; Schuppe-Koistinen, I.; Von Löwenhielm, H.B.; Holmgren, A.; Cotgreave, I.A. Identification of S-Glutathionylated Cellular Proteins during Oxidative Stress and Constitutive Metabolism by Affinity Purification and Proteomic Analysis. Arch. Biochem. Biophys. 2002, 406, 229–240. [Google Scholar] [CrossRef]
- Hamnell-Pamment, Y.; Lind, C.; Palmberg, C.; Bergman, T.; Cotgreave, I.A. Determination of Site-Specificity of S-Glutathionylated Cellular Proteins. Biochem. Biophys. Res. Commun. 2005, 332, 362–369. [Google Scholar] [CrossRef]
- Giustarini, D.; Milzani, A.; Dalle-Donne, I.; Rossi, R. Measurement of S-Glutathionylated Proteins by HPLC. Amino Acids 2022, 54, 675–686. [Google Scholar] [CrossRef]
- Scirè, A.; Casari, G.; Romaldi, B.; De Bari, L.; Antognelli, C.; Armeni, T. Glutathionyl Hemoglobin and Its Emerging Role as a Clinical Biomarker of Chronic Oxidative Stress. Antioxidants 2023, 12, 1976. [Google Scholar] [CrossRef]
- Niwa, T.; Naito, C.; Mawjood, A.H.M.; Imai, K. Increased Glutathionyl Hemoglobin in Diabetes Mellitus and Hyperlipidemia Demonstrated by Liquid Chromatography/Electrospray Ionization-Mass Spectrometry. Clin. Chem. 2000, 46, 82–88. [Google Scholar] [CrossRef]
- Maria Rubino, F.; Della Noce, C.; Vigna, L.; De Giuseppe, R.; Novembrino, C.; De Liso, F.; Maiavacca, R.; Patrini, L.; Riboldi, L.; Bamonti, F. Measurement of Glutathionylated Haemoglobin by MAL-DI-ToF Mass Spectrometry as a Biomarker of Oxidative Stress in Heavy Smokers and in Occupational Obese Subjects. Int. J. Anal. Mass Spectrom. Chromatogr. 2013, 01, 22–30. [Google Scholar] [CrossRef]
- Li, R.; Kast, J. Biotin Switch Assays for Quantitation of Reversible Cysteine Oxidation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 585, pp. 269–284. ISBN 978-0-12-809742-7. [Google Scholar]
- Mailloux, R.J.; Xuan, J.Y.; McBride, S.; Maharsy, W.; Thorn, S.; Holterman, C.E.; Kennedy, C.R.J.; Rippstein, P.; deKemp, R.; Da Silva, J.; et al. Glutaredoxin-2 Is Required to Control Oxidative Phosphorylation in Cardiac Muscle by Mediating Deglutathionylation Reactions. J. Biol. Chem. 2014, 289, 14812–14828. [Google Scholar] [CrossRef]
- Potęga, A. Glutathione-Mediated Conjugation of Anticancer Drugs: An Overview of Reaction Mechanisms and Biological Significance for Drug Detoxification and Bioactivation. Molecules 2022, 27, 5252. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, Z.; Zhang, X.; Feng, J.; Ling, Y. N-Acetyl-S-(p-Chlorophenylcarbamoyl)Cysteine Induces Mitochondrial-Mediated Apoptosis and Suppresses Migration in Melanoma Cells. Oncol. Rep. 2015, 34, 2547–2556. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, C.H.; Pedersen, B.; Tønnesen, H. The Efficacy of Disulfiram for the Treatment of Alcohol Use Disorder: Disulfiram for alcohol use disorder. Alcohol. Clin. Exp. Res. 2011, 35, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, E.; Rohondia, S.; Khan, R.; Dou, Q.P. Repurposing Disulfiram as An Anti-Cancer Agent: Updated Review on Literature and Patents. Recent Pat. Anti-Cancer Drug Discov. 2019, 14, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.C.; Grossman, K.F.; Brittain-Blankenship, M.; Thorne, K.M.; Akerley, W.L.; Terrazas, M.C.; Kosak, K.M.; Boucher, K.M.; Buys, S.S.; McGregor, K.A.; et al. A Phase 1 Dose-Escalation Study of Disulfiram and Copper Gluconate in Patients with Advanced Solid Tumors Involving the Liver Using S-Glutathionylation as a Biomarker. BMC Cancer 2021, 21, 510. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Federici, L.; Masulli, M.; De Laurenzi, V.; Allocati, N. The Role of S-Glutathionylation in Health and Disease: A Bird’s Eye View. Nutrients 2024, 16, 2753. https://doi.org/10.3390/nu16162753
Federici L, Masulli M, De Laurenzi V, Allocati N. The Role of S-Glutathionylation in Health and Disease: A Bird’s Eye View. Nutrients. 2024; 16(16):2753. https://doi.org/10.3390/nu16162753
Chicago/Turabian StyleFederici, Luca, Michele Masulli, Vincenzo De Laurenzi, and Nerino Allocati. 2024. "The Role of S-Glutathionylation in Health and Disease: A Bird’s Eye View" Nutrients 16, no. 16: 2753. https://doi.org/10.3390/nu16162753
APA StyleFederici, L., Masulli, M., De Laurenzi, V., & Allocati, N. (2024). The Role of S-Glutathionylation in Health and Disease: A Bird’s Eye View. Nutrients, 16(16), 2753. https://doi.org/10.3390/nu16162753






