Body Composition Analysis of the Clinical Routine Using Air Displacement Plethysmography: Age-Group-Specific Feasibility Analysis among Preterm Infants
Abstract
1. Introduction
- The feasibility of weekly routine ADP assessments for preterm infants;
- Preterm infants’ readiness for first ADP testing across different gestational ages at birth and number of repeated tests during in-hospital routine;
- The workload of body composition measurements using ADP in clinical practice.
2. Materials and Methods
2.1. Study Design
2.1.1. Nutrition
2.1.2. Body Composition and Anthropometric Measurements
2.2. The Clinical Procedure at the Children’s University Hospital Nuremberg
2.2.1. Inclusion Criteria and Testing Procedure
2.2.2. Testing Workflow
- Screening: One day prior to the test day, the study nurse screened all neonates in the units. On the test day, eligibility for testing was evaluated using inclusion and exclusion criteria (see Testing Procedure). The attending physician confirmed clinical stability. A list of all infants to be tested on that day was provided to the unit to inform the bedside nurses which infants were being measured.
- Preparation: The PEAPOD operating nurse was switched on the PEAPOD at least two hours before the first body composition assessment on the day to allow for system warm-up and equilibration. When tests started early in the day, the PEAPOD system was switched on the night before the test day. Automated volume calibration was started before each volume measurement. Manual system calibration was performed at the beginning of each test day. The results from the quality control tests were reviewed once a month.
- Testing: The PEAPOD nurse transferred infants from the unit to the PEAPOD room after a final infant stability check-up was requested from the nurse at the unit. The infants were undressed prior to testing. Head circumference and length measurements were performed together by the PEAPOD operating nurse and the PEAPOD nurse. The PEAPOD measurements were coordinated and performed by the PEAPOD operating nurse. The detailed instructions for operating the PEAPOD device are described in the manual of the PEAPOD operator [27].
- Body composition data: The PEAPOD operating nurse was responsible for obtaining and printing the body composition data. The results were visualized on individual body composition graphs and added to the patient’s folder, which was accessible to the physicians, thus allowing interpretation of body composition data.
- Responsible physicians evaluated body composition tests: However, no standardized recommendations for individual interventions based on body composition results have been published.
2.3. Data Analysis
3. Results
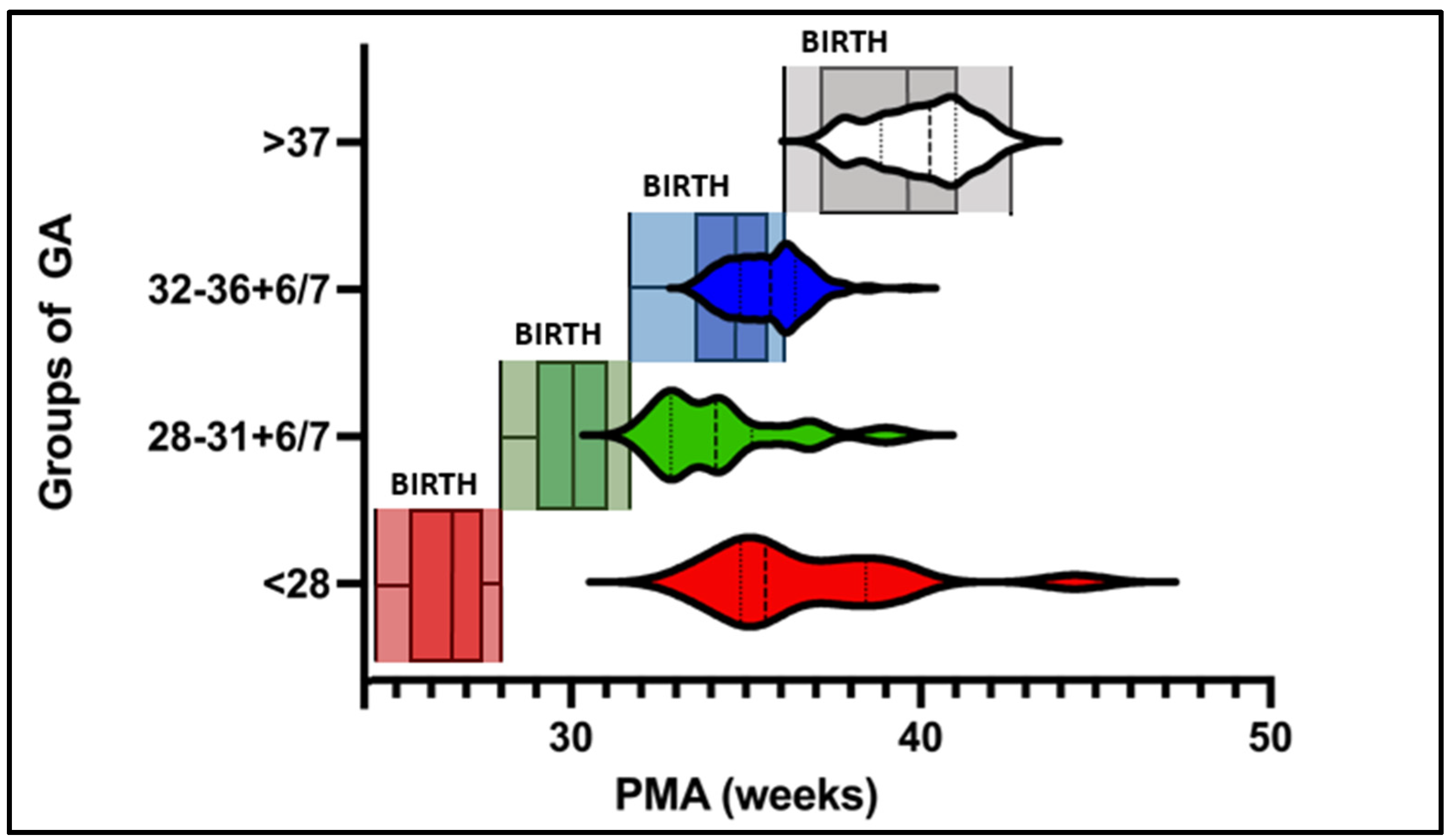
3.1. Feasility and Infants’ Readiness
3.2. Weekly Routine Testing
3.3. Personnel Requirements
4. Discussion
4.1. Feasibility of ADP Testing in Routine Clinical Practice
4.2. Routine Testing
4.2.1. Postnatal Age at the First Test
4.2.2. Repeated Testing
4.2.3. Frequency
4.2.4. Time and Personnel Requirements
4.2.5. Clinical Significance
4.3. Future Clinical Utility of Body Composition Data
4.4. Limitations of the ADP Method
4.5. Limitations and Strengths of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costeloe, K.L.; Hennessy, E.M.; Haider, S.; Stacey, F.; Marlow, N.; Draper, E.S. Short Term Outcomes after Extreme Preterm Birth in England: Comparison of Two Birth Cohorts in 1995 and 2006 (the EPICure Studies). BMJ 2012, 345, e7976. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M.; et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Hofman, P.L.; Regan, F.; Jackson, W.E.; Jefferies, C.; Knight, D.B.; Robinson, E.M.; Cutfield, W.S. Premature Birth and Later Insulin Resistance. N. Engl. J. Med. 2004, 351, 2179–2186. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.K.; Ahmed, M.L.; Emmett, P.M.; Preece, M.A.; Dunger, D.B. Association between Postnatal Catch-up Growth and Obesity in Childhood: Prospective Cohort Study. BMJ 2000, 320, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Carr, H.; Cnattingius, S.; Granath, F.; Ludvigsson, J.F.; Edstedt Bonamy, A.-K. Preterm Birth and Risk of Heart Failure up to Early Adulthood. J. Am. Coll. Cardiol. 2017, 69, 2634–2642. [Google Scholar] [CrossRef] [PubMed]
- Ramel, S.E.; Gray, H.L.; Christiansen, E.; Boys, C.; Georgieff, M.K.; Demerath, E.W. Greater Early Gains in Fat-Free Mass, but Not Fat Mass, Are Associated with Improved Neurodevelopment at 1 Year Corrected Age for Prematurity in Very Low Birth Weight Preterm Infants. J. Pediatr. 2016, 173, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Pfister, K.M.; Zhang, L.; Miller, N.C.; Ingolfsland, E.C.; Demerath, E.W.; Ramel, S.E. Early Body Composition Changes Are Associated with Neurodevelopmental and Metabolic Outcomes at 4 Years of Age in Very Preterm Infants. Pediatr. Res. 2018, 84, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Bua, J.; Risso, F.M.; Bin, M.; Vallon, F.; Travan, L.; Paviotti, G. Association between Body Composition at Term Equivalent Age and Bayley Scores at 2 Years in Preterm Infants. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2021, 41, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.A.; Ramel, S.E.; Robinson, D.T.; Wagner, C.L.; Scottoline, B.; Belfort, M.B. Body Composition Measurement for the Preterm Neonate: Using a Clinical Utility Framework to Translate Research Tools into Clinical Care. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2022, 42, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Dung, N.Q.; Fusch, G.; Armbrust, S.; Jochum, F.; Fusch, C. Body Composition of Preterm Infants Measured during the First Months of Life: Bioelectrical Impedance Provides Insignificant Additional Information Compared to Anthropometry Alone. Eur. J. Pediatr. 2007, 166, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Koo, W.W.K.; Walters, J.C.; Hockman, E.M. Body Composition in Neonates: Relationship Between Measured and Derived Anthropometry with Dual-Energy X-ray Absorptiometry Measurements. Pediatr. Res. 2004, 56, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Nagel, E.; Hickey, M.; Teigen, L.; Kuchnia, A.; Curran, K.; Soumekh, L.; Earthman, C.; Demerath, E.; Ramel, S. Clinical Application of Body Composition Methods in Premature Infants. J. Parenter. Enter. Nutr. 2020, 44, 785–795. [Google Scholar] [CrossRef]
- Roggero, P.; Giannì, M.L.; Amato, O.; Piemontese, P.; Morniroli, D.; Wong, W.W.; Mosca, F. Evaluation of Air-Displacement Plethysmography for Body Composition Assessment in Preterm Infants. Pediatr. Res. 2012, 72, 316–320. [Google Scholar] [CrossRef]
- Ma, G.S.; Yao, M.J.; Liu, Y.; Lin, A.W.; Zou, H.; Urlando, A.; Wong, W.W.; Nommsen-Rivers, L.; Dewey, K.G. Validation of a New Pediatric Air-Displacement Plethysmograph for Assessing Body Composition in Infants. Am. J. Clin. Nutr. 2004, 79, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Mazahery, H.; von Hurst, P.R.; McKinlay, C.J.D.; Cormack, B.E.; Conlon, C.A. Air Displacement Plethysmography (Pea Pod) in Full-Term and Pre-Term Infants: A Comprehensive Review of Accuracy, Reproducibility, and Practical Challenges. Matern. Health Neonatol. Perinatol. 2018, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.J.; Yao, M.; Shypailo, R.J.; Urlando, A.; Wong, W.W.; Heird, W.C. Body-Composition Assessment in Infancy: Air-Displacement Plethysmography Compared with a Reference 4-Compartment Model. Am. J. Clin. Nutr. 2007, 85, 90–95. [Google Scholar] [CrossRef]
- Frondas-Chauty, A.; Louveau, I.; Le Huërou-Luron, I.; Rozé, J.-C.; Darmaun, D. Air-Displacement Plethysmography for Determining Body Composition in Neonates: Validation Using Live Piglets. Pediatr. Res. 2012, 72, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.A.; Jerome, M.L.; Chandler-Laney, P.; Ambalavanan, N.; Carlo, W.A. Serial Assessment of Fat and Fat-Free Mass Accretion in Very Preterm Infants: A Randomized Trial. Pediatr. Res. 2020, 88, 733–738. [Google Scholar] [CrossRef]
- Alja’nini, Z.; McNelis, K.M.; Viswanathan, S.; Goddard, G.R.; Merlino-Barr, S.; Collin, M.; Groh-Wargo, S. Infant Body Composition Assessment in the Neonatal Intensive Care Unit (NICU) Using Air Displacement Plethysmography: Strategies for Implementation into Clinical Workflow. Clin. Nutr. ESPEN 2021, 43, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap Consortium: Building an International Community of Software Platform Partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral Nutrient Supply for Preterm Infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef]
- Urlando, A.; Dempster, P.; Aitkens, S. A New Air Displacement Plethysmograph for the Measurement of Body Composition in Infants. Pediatr. Res. 2003, 53, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Lücke, L.; Fusch, C.; Knab, K.; Schäfer, S.; Zimmermann, J.L.; Felderhoff-Müser, U.; Meis, A.; Lohmüller-Weiß, S.; Szakacs-Fusch, A.; Rochow, N. Reproducibility of Air Displacement Plethysmography in Term and Preterm Infants—A Study to Enhance Body Composition Analysis in Clinical Routine. Nutrients 2024, 16, 1810. [Google Scholar] [CrossRef] [PubMed]
- Fomon, S.J.; Haschke, F.; Ziegler, E.E.; Nelson, S.E. Body Composition of Reference Children from Birth to Age 10 Years. Am. J. Clin. Nutr. 1982, 35, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Hopkinson, J.M.; Wong, W.W.; Smith, E.O.; Ellis, K.J. Body Composition during the First 2 Years of Life: An Updated Reference. Pediatr. Res. 2000, 47, 578–585. [Google Scholar] [CrossRef] [PubMed]
- COSMED PEA POD® Infant Body Composition System Operator’s Manual 2019. Available online: https://www.bioclinicalservices.com.au/cosmed-srl/clinical/pea-pod-operators-manual-rev-n (accessed on 1 May 2024).
- Rochow, N.; Fusch, G.; Ali, A.; Bhatia, A.; Therefore, H.Y.; Iskander, R.; Chessell, L.; El Helou, S.; Fusch, C. Individualized Target Fortification of Breast Milk with Protein, Carbohydrates, and Fat for Preterm Infants: A Double-Blind Randomized Controlled Trial. Clin. Nutr. 2021, 40, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Moutquin, J.-M. Classification and Heterogeneity of Preterm Birth. BJOG Int. J. Obstet. Gynaecol. 2003, 110 (Suppl. 20), 30–33. [Google Scholar] [CrossRef]
- Bruckner, M.; Khan, Z.; Binder, C.; Morris, N.; Windisch, B.; Holasek, S.; Urlesberger, B. Extremely Preterm Infants Have a Higher Fat Mass Percentage in Comparison to Very Preterm Infants at Term-Equivalent Age. Front. Pediatr. 2020, 8, 61. [Google Scholar] [CrossRef]
- Murki, S.; Vardhelli, V.; Deshabhotla, S.; Sharma, D.; Pawale, D.; Kulkarni, D.; Kumar, P.; Kabra, N.S.; Sundaram, M.; Plakkal, N.; et al. Predictors of Length of Hospital Stay among Preterm Infants Admitted to Neonatal Intensive Care Unit: Data from a Multicentre Collaborative Network from India (INNC: Indian National Neonatal Collaborative). J. Paediatr. Child Health 2020, 56, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.D.; Yellin, S.A.; Ong, F.J.; Singh, N.P.; Konyer, N.; Noseworthy, M.D.; Schmidt, L.A.; Saigal, S.; Morrison, K.M. ELBW Survivors in Early Adulthood Have Higher Hepatic, Pancreatic and Subcutaneous Fat. Sci. Rep. 2016, 6, 31560. [Google Scholar] [CrossRef] [PubMed]
- Parlapani, E.; Agakidis, C.; Karagiozoglou-Lampoudi, T. Anthropometry and Body Composition of Preterm Neonates in the Light of Metabolic Programming. J. Am. Coll. Nutr. 2018, 37, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Hamatschek, C.; Yousuf, E.I.; Möllers, L.S.; Therefore, H.Y.; Morrison, K.M.; Fusch, C.; Rochow, N. Fat and Fat-Free Mass of Preterm and Term Infants from Birth to Six Months: A Review of Current Evidence. Nutrients 2020, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Norris, T.; Ramel, S.E.; Catalano, P.; Caoimh, C.N.; Roggero, P.; Murray, D.; Fields, D.A.; Demerath, E.W.; Johnson, W. New Charts for the Assessment of Body Composition, According to Air-Displacement Plethysmography, at Birth and across the First 6 Month of Life. Am. J. Clin. Nutr. 2019, 109, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Demerath, E.W.; Johnson, W.; Davern, B.A.; Anderson, C.G.; Shenberger, J.S.; Misra, S.; Ramel, S.E. New Body Composition Reference Charts for Preterm Infants. Am. J. Clin. Nutr. 2017, 105, 70–77. [Google Scholar] [CrossRef]
| Groups per GA | Extremely Preterm; <28 wk. | Very Preterm; 28 to 31 + 6/7 wk. | Moderate and Late Preterm; 32 to 36 + 6/7 wk. | Term Infants; ≥37 wk. | All Subjects |
|---|---|---|---|---|---|
| Number of subjects (m/f) | 14 (11/3) | 28 (19/9) | 143 (81/62) | 75 (48/27) | 260 (159/101) |
| Total number of tests | 42 | 65 | 244 | 78 | 429 |
| Mean GA (weeks) | 26.3 | 30 | 34.4 | 39 | 33.6 |
| Week of life at first PEAPOD test | 10.5 ± 3.2 * | 4.4 ± 2.4 * | 1 ± 0.7 * | 0.7 ± 0.6 * | 2.5 |
| PMA at first PEAPOD test | 36.6 | 34.2 | 35.3 | 39.9 | 35.5 |
| Number of tests before discharge | 3.1 ± 1.4 * | 2.4 ± 1.4 | 1.7 ± 1.1 | 1.1 ± 0.2 * | 1.65 ± 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lücke, L.A.; Rochow, N.; Knab, K.; Schäfer, S.; Zimmermann, J.L.; Meis, A.; Lohmüller-Weiß, S.; Szakacs-Fusch, A.; Felderhoff-Müser, U.; Fusch, C. Body Composition Analysis of the Clinical Routine Using Air Displacement Plethysmography: Age-Group-Specific Feasibility Analysis among Preterm Infants. Nutrients 2024, 16, 2694. https://doi.org/10.3390/nu16162694
Lücke LA, Rochow N, Knab K, Schäfer S, Zimmermann JL, Meis A, Lohmüller-Weiß S, Szakacs-Fusch A, Felderhoff-Müser U, Fusch C. Body Composition Analysis of the Clinical Routine Using Air Displacement Plethysmography: Age-Group-Specific Feasibility Analysis among Preterm Infants. Nutrients. 2024; 16(16):2694. https://doi.org/10.3390/nu16162694
Chicago/Turabian StyleLücke, Lennart A., Niels Rochow, Katja Knab, Stefan Schäfer, Jasper L. Zimmermann, Anastasia Meis, Stephanie Lohmüller-Weiß, Adel Szakacs-Fusch, Ursula Felderhoff-Müser, and Christoph Fusch. 2024. "Body Composition Analysis of the Clinical Routine Using Air Displacement Plethysmography: Age-Group-Specific Feasibility Analysis among Preterm Infants" Nutrients 16, no. 16: 2694. https://doi.org/10.3390/nu16162694
APA StyleLücke, L. A., Rochow, N., Knab, K., Schäfer, S., Zimmermann, J. L., Meis, A., Lohmüller-Weiß, S., Szakacs-Fusch, A., Felderhoff-Müser, U., & Fusch, C. (2024). Body Composition Analysis of the Clinical Routine Using Air Displacement Plethysmography: Age-Group-Specific Feasibility Analysis among Preterm Infants. Nutrients, 16(16), 2694. https://doi.org/10.3390/nu16162694








