Vigeo Promotes Myotube Differentiation and Protects Dexamethasone-Induced Skeletal Muscle Atrophy via Regulating the Protein Degradation, AKT/mTOR, and AMPK/Sirt-1/PGC1α Signaling Pathway In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Vigeo
2.2. Animals
2.3. C2C12 Cell Line Culture and Differentiation
2.4. C2C12 Cell Cytotoxicity
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. Western Blot
2.7. Immunofluorescence
2.8. Micro-CT (mCT)
2.9. Tissue Staining
2.10. Statistical Analysis
3. Results
3.1. Vigeo Ameliorates Muscle Wasting in a Dex-Induced Muscle Atrophy In Vivo
3.2. Administration of Vigeo Protects Dex-Induced GA Muscle Damage In Vivo
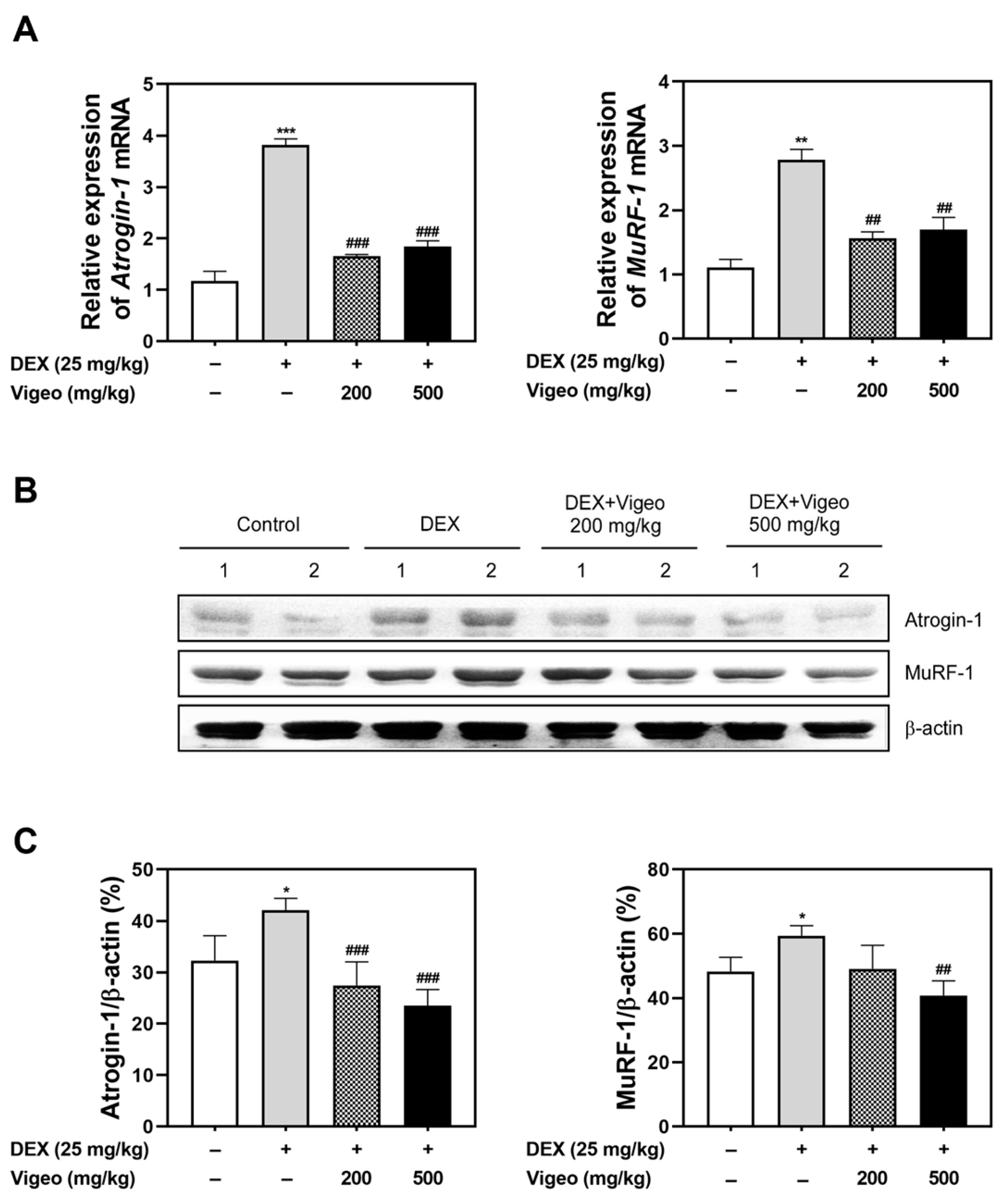
3.3. Vigeo Suppresses the Protein Degradation Pathway Stimulated by Dex In Vivo
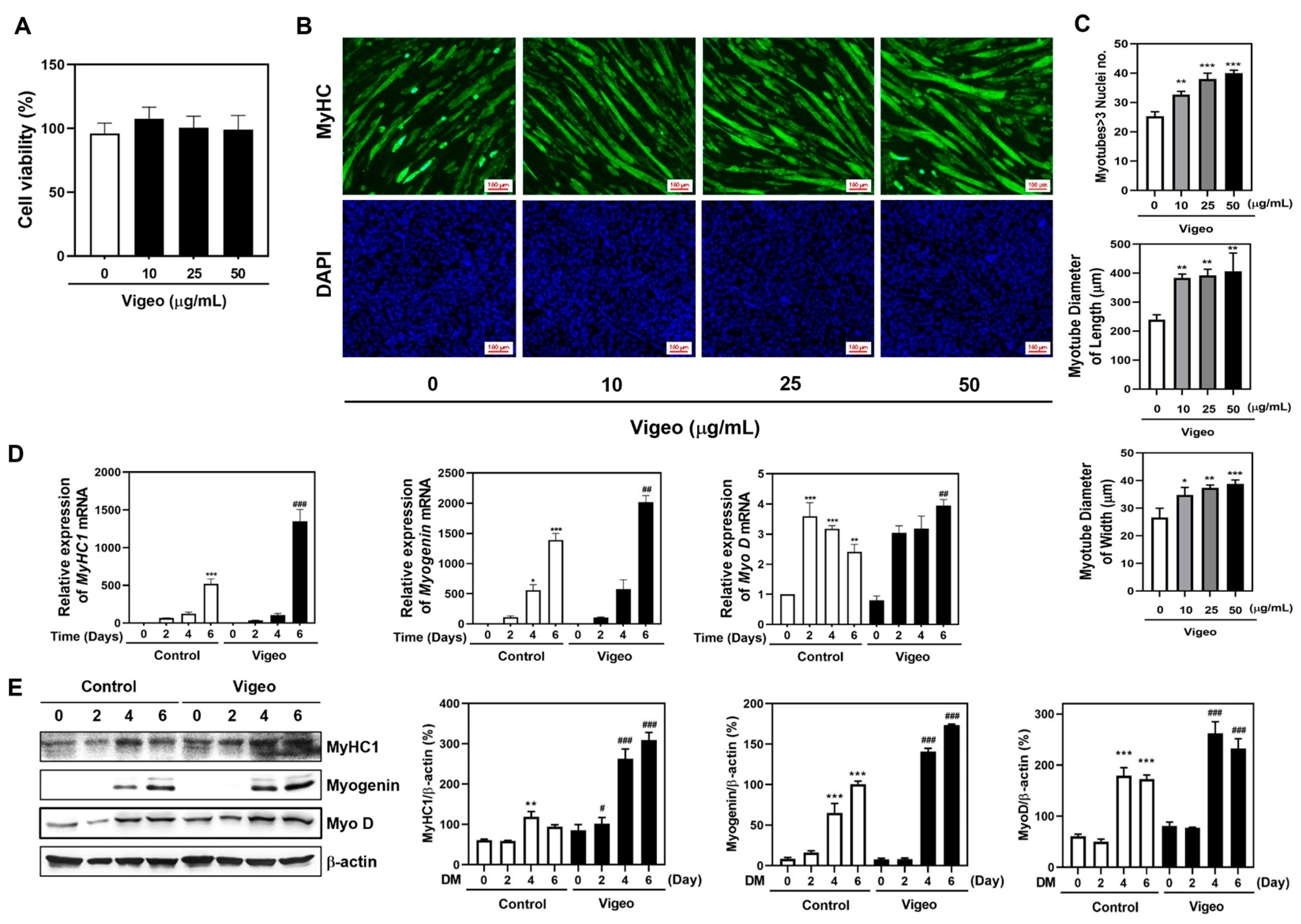
3.4. Vigeo Improves Myoblast Differentiation by Stimulating the Expression of Myogenic Marker Genes in C2C12 Myoblast Cell In Vitro
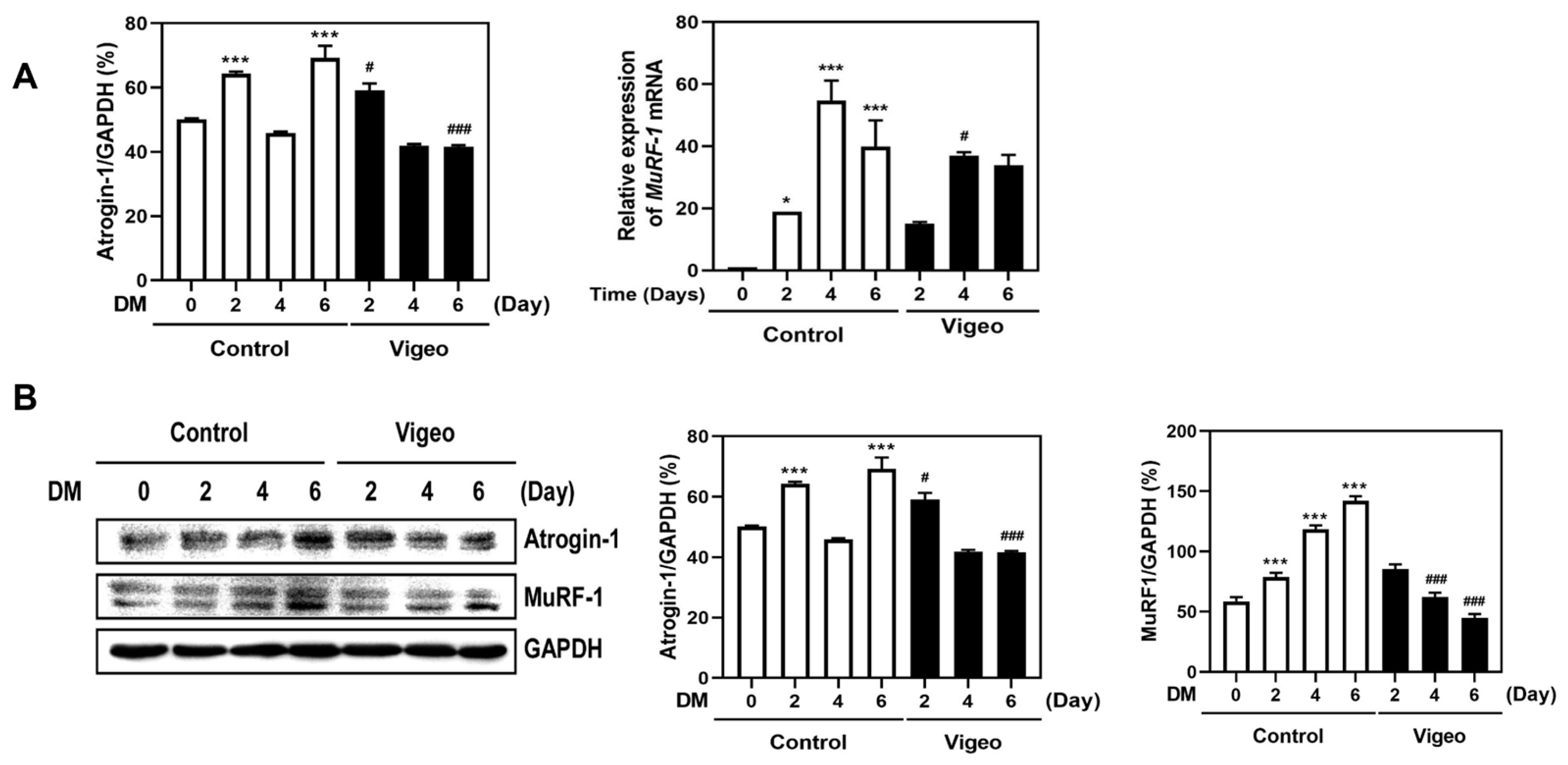
3.5. Vigeo Up-Regulates Myoblast Differentiation by Suppressing Proteolysis Markers
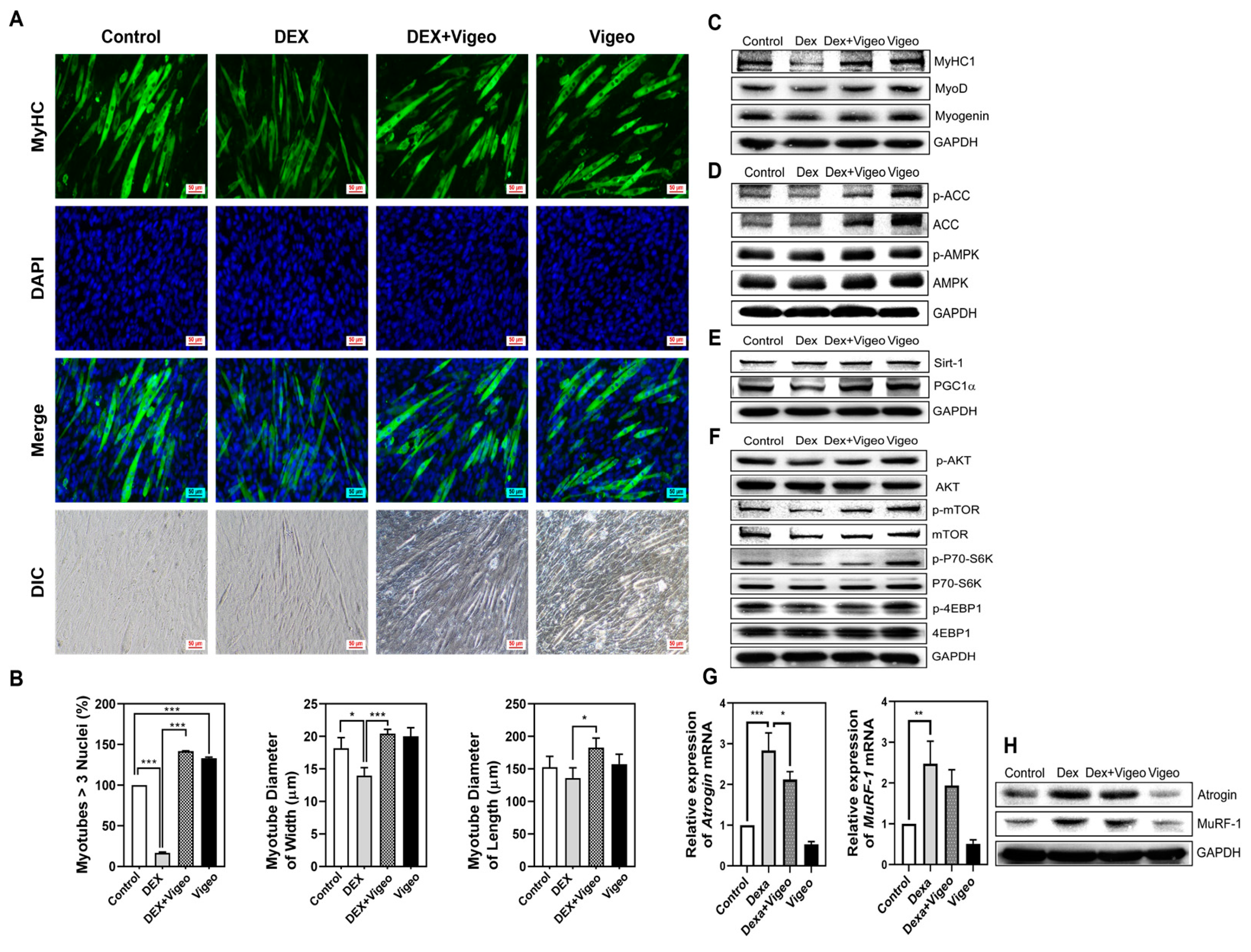
3.6. Vigeo Attenuates Dex-Induced Muscle Atrophy through AMPK/Sirt-1/PGC1α and Akt/mTOR Signaling in C2C12 Myoblast Cells in Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedersen, B.K. Muscle as a secretory organ. Compr. Physiol. 2013, 3, 1337–1362. [Google Scholar] [PubMed]
- Mercuri, E.; Muntoni, F. Muscular dystrophies. Lancet 2013, 381, 845–860. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, J.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Evans, W.J. Skeletal muscle loss: Cachexia, sarcopenia, and inactivity. Am. J. Clin. Nutr. 2010, 91, 1123S–1127S. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.D. Sarcopenia in older adults. Curr. Opin. Rhematol. 2012, 24, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, M.; Rigby, P.W.J. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell 2014, 28, 225–238. [Google Scholar] [CrossRef]
- Devlin, R.B.; Emerson Jr, C.P. Coordinate regulation of contractile protein synthesis during myoblast differentiation. Cell 1978, 13, 599–611. [Google Scholar] [CrossRef]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarjan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Wang, B.Y.H.; Hsiao, A.W.T.; Wong, N.; Chen, Y.F.; Lee, C.W.; Lee, W.Y.W. Is dexamethasone-induced muscle atrophy an alternative model for naturally aged sarcopenia model? J. Orthop. Translat. 2022, 39, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.E.; So, H.K.; Vuong, T.A.; Na, M.W.; Anh, S.; Lee, H.K.; Kim, K.H.; Kang, J.S.; Bae, G.U.; Lee, S.J. Aronia upregulates myogenic differentiation and augments muscle mass and function through muscle metabolism. Front. Nutr. 2021, 23, 753643. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, J.H.; Hwang, S.H.; Yeon, S.H.; Ryu, J.H. A lignan from alnus japonica activates myogenesis and alleviates dexamethasone-induced myotube atrophy. Planta Med. 2023, 89, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Eun, S.Y.; Cheon, Y.H.; Park, G.D.; Chung, C.H.; Lee, C.H.; Kim, J.Y.; Lee, M.S. Anti-osteoporosis effects of the Eleutherococcus senticosus, Achyranthes japonica, and Atractylodes japonica mixed extract fermented with nuruk. Nutrients 2021, 13, 3904. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zeng, J.; Zhang, S.; Xu, X.; Chen, L.; Yang, Z.; Wu, W.; Hu, C.; Zhao, Y.T. Remedial effects of tilapia skin peptides against dexamethasone-induced muscle atrophy in mice by modulation of AKT/FOXO3a and Sirt1/PGC-1 signaling pathways. J. Funct. Foods 2024, 119, 106315. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hasselgren, P.O. Glucocorticoids and muscle catabolism. Curr. Opin. Clin. Nutr Metab. Care 1999, 2, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.L.; Wang, X.; Price, S.R. The balance between glucocorticoids and insulin regulates muscle proteolysis via the ubiquitin-proteasome pathway. Miner. Electrolyte. Metab. 1999, 25, 220–223. [Google Scholar] [CrossRef]
- Wenz, T. Mitochondria and PGC-1α in aging and age-associated diseases. J. Aging Res. 2011, 2011, 810619. [Google Scholar] [CrossRef]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef]
- Amat, R.; Planavila, A.; Chen, S.L.; Lglesias, R.; Girait, M.; Villarroya, F. SIRT1 controls the transcription of the peroxisome proliferator-activated receptor-gamma Co-activator-1alpha (PGC-1alpha) gene in skeletal muscle through the PGC-1alpha autoregulatory loop and interaction with MyoD. J. Biol. Chem. 2009, 284, 21872–21880. [Google Scholar] [CrossRef]
- Yochida, T.; Delafontaine, P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef]
- Glass, D.J. Molecular mechanisms modulating muscle mass. Trends Mol. Med. 2003, 9, 344–350. [Google Scholar] [CrossRef]
- Marabita, M.; Baraldo, M.; Solagna, F.; Ceelen, J.J.M.; Sartori, R.; Nolte, H.; Nemazanyy, I.; Pyronnet, S.; Kruger, M.; Pende, M.; et al. S6K1 is required for increasing skeletal muscle force during hypertrophy. Cell Rep. 2016, 17, 501–513. [Google Scholar] [CrossRef]
- Castillero, E.; Alamdari, N.; Lecker, S.H.; Hasselgren, P.O. Suppression of atrogin-1 and MuRF1 prevents dexamethasone-induced atrophy of cultured myotubes. Metabolism 2013, 62, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Bagherniya, M.; Mahdavi, A.; Shokri-Mashhadi, N.; Banach, M.; Haehling, S.V.; Johnston, T.P.; Sahebker, A. The beneficial therapeutic effects of plant-derived natural products for the treatment of sarcopenia. J. Cachexia Sarcopenia Muscle 2022, 13, 2772–2790. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Zhou, S.; Li, P.; Liu, C.; Yuan, B.; Zhang, S.; Liu, A. Natural products and skeletal muscle health. J. Nutr. Biochem. 2021, 93, 108619. [Google Scholar] [CrossRef]
- Nagao, K.; Ueda, S.; Kanda, M.; Oohata, N.; Yamashita, M.; Hino, M. Drug development from natural fermentation products: Establishing a manufacturing process which maximizes the potential of microorganisms. Yakugaku Zasshi 2010, 130, 1471–1478. [Google Scholar] [CrossRef]
- Pettit, R.K. Mixed fermentation for natural product drug discovery. Appl. Microbiol. Biotechnol. 2009, 83, 19–25. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, E.J.; Park, W.D.; Kim, J.B.; Kim, E.O.; Choi, S.W. Anti-inflammatory and anti-osteoarthritis effects of fermented Achyranthes japonica Nakai. J. Ethnopharmacol. 2012, 142, 634–641. [Google Scholar] [CrossRef]
- Hwang, Y.C.; Jeong, I.K.; Ahn, K.J.; Chung, H.Y. The effects of Acanthopanax senticosus extract on bone turnover and bone mineral density in Korean postmenopausal women. J. Bone Miner. Metab. 2009, 27, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chang, Z.; Ma, R.; Guo, H.; Zhao, Q.; Wang, X.; Kong, L.; Hao, D. Eleutherococcus senticosus inhibits RANKL-induced osteoclast formation by attenuating the NF-κB and MAPKs signaling pathway. Int. J. Clin. Exp. Pathol. 2017, 10, 4514–4521. [Google Scholar]
- Kim, D.; Park, K.K.; Lee, S.K.; Lee, S.E.; Hwang, J.K. Cornus kousa F. Buerger ex Miquel increases glucose uptake through activation of peroxisome proliferator-activated receptor γ and insulin sensitization. J. Ethnopharmacol. 2011, 133, 803–809. [Google Scholar] [CrossRef]
- Woo, Y.M.; Kim, O.J.; Jo, E.S.; Kim, S.J.; Lee, Y.H.; Ahn, M.Y.; Lee, S.H.; Ha, J.M.; Kim, A. Anti-inflammatory effects of the combined extracts of Achyranthes japonica nakai and Aralia continentalis kitagawa in vitro and in vivo. Data Brief 2019, 25, 104088. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Kim, D.; Suminda, G.G.D.; Min, Y.; Yang, J.; Kim, M.; Zhao, Y.; Chosh, M.; Son, Y.O. Inhibitory effects of IL-6-mediated matrix metalloproteinase-3 and -13 by Achyranthes japonica Nakai root in osteoarthritis and rheumatoid arthritis mice models. Pharmaceutical 2021, 14, 776. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, S.N.; Shin, D.; Hur, I.C.; Kim, I.S.; Jin, S.K. Antioxidant activities of Achyranthes japonica Nakai extract and its application to the pork sausages. Asian-Australas. J. Anim. Sci. 2013, 26, 287–294. [Google Scholar] [CrossRef]
- Hong, M.H.; Kim, J.H.; Bae, H.; Lee, N.Y.; Shin, Y.C.; Kim, S.H.; Ko, S.G. Atractylodes japonica koidzumi inhibits the production of proinflammatory cytokines through inhibition of the NF-kappaB/IkappaB signal pathway in HMC-1 human mast cells. Arch. Pharm. Res. 2010, 33, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.T.; Chen, L.G.; Chou, D.S.; Liang, W.L.; Wang, C.C. Anti-oxidative abilities of essential oils from Atractylodes ovate rhizome. Evid. Based Complement. Altern. Med. 2011, 2011, 204892. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jung, H.W.; Park, Y.K. The roots of Atractylodes japonica Koidzumi promote adipogenic differentiation via activation of the insulin signaling pathway in 3T3-L1 cells. BMC Complement. Altern. Med. 2012, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Cheon, Y.H.; Lee, C.H.; Eun, S.Y.; Park, G.D.; Chung, C.H.; Kim, J.Y.; Lee, M.S. Vigeo attenuates cartilage and bone destruction in a collagen-induced arthritis mouse model by reducing production of pro-inflammatory cytokines. Exp. Ther. Med. 2024, 27, 208. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wu, D.; Sun, Y.; Zhao, H.; Wang, Y.; Zhang, W.; Su, F.; Yang, B.; Wang, Q.; Kuang, H. Comprehensive analysis of Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. leaves based on UPLC-MS/MS: Separation and rapid qualitative and quantitative analysis. Front. Pharmacol. 2022, 13, 865586. [Google Scholar]
- Ghimire, B.K.; Seo, J.W.; Kim, S.H.; Chimire, B.; Lee, J.G.; Yu, C.Y.; Chung, I.M. Influence of harvesting time on phenolic and mineral profiles and their association with the antioxidant and cytotoxic effects of Atractylodes japonica Koidz. Agronomy 2021, 11, 1327. [Google Scholar] [CrossRef]
- Asfour, H.A.; Allouh, M.Z.; Said, R.S. Myogenic regulatory factors: The orchestrators of myogenesis after 30 years of discovery. Exp. Biol. Med. 2018, 243, 118–128. [Google Scholar] [CrossRef]
- Bergstrom, D.A.; Penn, B.H.; Strand, A.; Perry, R.L.S.; Rudnicki, M.A.; Tapscott, S.J. Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol. Cell 2002, 9, 587–600. [Google Scholar] [CrossRef]
- Rudnicki, M.A.; Schnegelsberg, P.N.; Stead, R.H.; Braun, T.; Arnold, H.H.; Jaenisch, R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 1993, 75, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Hasty, P.; Bradley, A.; Morris, J.H.; Edmondson, D.G.; Venuti, J.M.; Olson, E.N.; Klein, W.H. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 1993, 364, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, J.M.; García-González, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 2013, 43, 12–21. [Google Scholar] [CrossRef]
- Furlow, J.D.; Watson, M.L.; Waddell, D.S.; Neff, E.S.; Baehr, L.M.; Ross, A.P.; Bodine, S.C. Altered gene expression patterns in muscle ring finger 1 null mice during denervation- and dexamethasone-induced muscle atrophy. Physiol. Genomics 2023, 45, 1168–1185. [Google Scholar] [CrossRef]
- Chen, X.; Ji, Y.; Liu, R.; Zhu, X.; Wang, K.; Yang, X.; Liu, B.; Gao, Z.; Huang, Y.; Shen, Y.; et al. Mitochondrial dysfunction: Roles in skeletal muscle atrophy. J. Transl. Med. 2023, 21, 503. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Cao, W.; Yue, R.; Yuan, Y.; Guo, X.; Qin, D.; Xing, J.; Wang, X. Pretreatment with Tilianin improves mitochondrial energy metabolism and oxidative stress in rats with myocardial ischemia/reperfusion injury via AMPK/SIRT1/PGC-1 alpha signaling pathway. J. Pharmacol. Sci. 2019, 139, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Sartorelli, V.; Fulco, M. Molecular and cellular determinants of skeletal muscle atrophy and hypertrophy. Sci. STKE 2004, 2004, re11. [Google Scholar] [CrossRef] [PubMed]
- Kandarian, S.C.; Jackman, R.W. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve 2006, 33, 155–165. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheon, Y.-H.; Lee, C.-H.; Chung, C.-H.; Kim, J.-Y.; Lee, M.-S. Vigeo Promotes Myotube Differentiation and Protects Dexamethasone-Induced Skeletal Muscle Atrophy via Regulating the Protein Degradation, AKT/mTOR, and AMPK/Sirt-1/PGC1α Signaling Pathway In Vitro and In Vivo. Nutrients 2024, 16, 2687. https://doi.org/10.3390/nu16162687
Cheon Y-H, Lee C-H, Chung C-H, Kim J-Y, Lee M-S. Vigeo Promotes Myotube Differentiation and Protects Dexamethasone-Induced Skeletal Muscle Atrophy via Regulating the Protein Degradation, AKT/mTOR, and AMPK/Sirt-1/PGC1α Signaling Pathway In Vitro and In Vivo. Nutrients. 2024; 16(16):2687. https://doi.org/10.3390/nu16162687
Chicago/Turabian StyleCheon, Yoon-Hee, Chang-Hoon Lee, Chong-Hyuk Chung, Ju-Young Kim, and Myeung-Su Lee. 2024. "Vigeo Promotes Myotube Differentiation and Protects Dexamethasone-Induced Skeletal Muscle Atrophy via Regulating the Protein Degradation, AKT/mTOR, and AMPK/Sirt-1/PGC1α Signaling Pathway In Vitro and In Vivo" Nutrients 16, no. 16: 2687. https://doi.org/10.3390/nu16162687
APA StyleCheon, Y.-H., Lee, C.-H., Chung, C.-H., Kim, J.-Y., & Lee, M.-S. (2024). Vigeo Promotes Myotube Differentiation and Protects Dexamethasone-Induced Skeletal Muscle Atrophy via Regulating the Protein Degradation, AKT/mTOR, and AMPK/Sirt-1/PGC1α Signaling Pathway In Vitro and In Vivo. Nutrients, 16(16), 2687. https://doi.org/10.3390/nu16162687





