Target Values for 25-Hydroxy and 1,25-Dihydroxy Vitamin D Based on Their Associations with Inflammation and Calcium-Phosphate Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Laboratory Parameters
2.3. Statistical Analyses
2.4. Potential Bias
3. Results
4. Discussion
4.1. Vitamin D and Inflammation
4.2. Calcium-Phosphate Metabolism
4.3. Out of Africa Hypothesis of Humans and Vitamin D
4.4. Mortality and Vitamin D Levels
4.5. Study Strengths and Limitations
4.6. Target Range for 1,25(OH)2D and 25(OH)D
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heaney, R.P. Vitamin D in health and disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1535–1541. [Google Scholar] [CrossRef]
- Ringe, J.D. Plain vitamin D or active vitamin D in the treatment of osteoporosis: Where do we stand today? Arch. Osteoporos. 2020, 15, 182. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Li, S.; De La Cruz, J.; Shroyer, N.F.; Criss, Z.K.; Verzi, M.P.; Fleet, J.C. Vitamin D and the intestine: Review and update. J. Steroid Biochem. Mol. Biol. 2020, 196, 105501. [Google Scholar] [CrossRef]
- Zappulo, F.; Cappuccilli, M.; Cingolani, A.; Scrivo, A.; Chiocchini, A.L.C.; Nunzio, M.D.; Donadei, C.; Napoli, M.; Tondolo, F.; Cianciolo, G.; et al. Vitamin D and the Kidney: Two Players, One Console. Int. J. Mol. Sci. 2022, 23, 9135. [Google Scholar] [CrossRef]
- Bover, J.; Massó, E.; Gifre, L.; Alfieri, C.; Soler-Majoral, J.; Fusaro, M.; Calabia, J.; Rodríguez-Pena, R.; Rodríguez-Chitiva, N.; López-Báez, V. Vitamin D and Chronic Kidney Disease Association with Mineral and Bone Disorder: An Appraisal of Tangled Guidelines. Nutrients 2023, 15, 1576. [Google Scholar] [CrossRef] [PubMed]
- Akimbekov, N.S.; Digel, I.; Sherelkhan, D.K.; Razzaque, M.S. Vitamin D and phosphate interactions in health and disease. In Phosphate Metabolism: From Physiology to Toxicity; Springer: Berlin/Heidelberg, Germany, 2022; pp. 37–46. [Google Scholar]
- Kumar, R.; Tebben, P.J.; Thompson, J.R. Vitamin D and the kidney. Arch. Biochem. Biophys. 2012, 523, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An update on the effects of vitamin D on the immune system and autoimmune diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef] [PubMed]
- Bui, L.; Zhu, Z.; Hawkins, S.; Cortez-Resendiz, A.; Bellon, A. Vitamin D regulation of the immune system and its implications for COVID-19: A mini review. SAGE Open Med. 2021, 9, 20503121211014073. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Chou, N.D.; Nielsen, O.H.; Moss, A.C. Systematic review with meta-analysis: Association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2019, 50, 1146–1158. [Google Scholar] [CrossRef]
- van der Post, S.; Jabbar, K.S.; Birchenough, G.; Arike, L.; Akhtar, N.; Sjovall, H.; Johansson, M.E.V.; Hansson, G.C. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 2019, 68, 2142–2151. [Google Scholar] [CrossRef]
- Mangin, M.; Sinha, R.; Fincher, K. Inflammation and vitamin D: The infection connection. Inflamm. Res. 2014, 63, 803–819. [Google Scholar] [CrossRef]
- de Oliveira, C.; Biddulph, J.P.; Hirani, V.; Schneider, I.J.C. Vitamin D and inflammatory markers: Cross-sectional analyses using data from the English Longitudinal Study of Ageing (ELSA). J. Nutr. Sci. 2017, 6, e1. [Google Scholar] [CrossRef] [PubMed]
- Laird, E.; McNulty, H.; Ward, M.; Hoey, L.; McSorley, E.; Wallace, J.; Carson, E.; Molloy, A.; Healy, M.; Casey, M. Vitamin D deficiency is associated with inflammation in older Irish adults. J. Clin. Endocrinol. Metab. 2014, 99, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.K.; Booth, S.L.; Massaro, J.M.; Jacques, P.F.; D’Agostino Sr, R.B.; Dawson-Hughes, B.; Ordovas, J.M.; O’Donnell, C.J.; Kathiresan, S.; Keaney Jr, J.F. Vitamin K and vitamin D status: Associations with inflammatory markers in the Framingham Offspring Study. Am. J. Epidemiol. 2008, 167, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Michos, E.D.; Streeten, E.A.; Ryan, K.A.; Rampersaud, E.; Peyser, P.A.; Bielak, L.F.; Shuldiner, A.R.; Mitchell, B.D.; Post, W. Serum 25-hydroxyvitamin d levels are not associated with subclinical vascular disease or C-reactive protein in the old order amish. Calcif. Tissue Int. 2009, 84, 195–202. [Google Scholar] [CrossRef] [PubMed]
- de la Guía-Galipienso, F.; Martínez-Ferran, M.; Vallecillo, N.; Lavie, C.J.; Sanchis-Gomar, F.; Pareja-Galeano, H. Vitamin D and cardiovascular health. Clin. Nutr. 2021, 40, 2946–2957. [Google Scholar] [CrossRef]
- Cui, X.; Gooch, H.; Groves, N.J.; Sah, P.; Burne, T.H.; Eyles, D.W.; McGrath, J.J. Vitamin D and the brain: Key questions for future research. J. Steroid Biochem. Mol. Biol. 2015, 148, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D epidemic and its health consequences. J. Nutr. 2005, 135, 2739S–2748S. [Google Scholar] [CrossRef]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef]
- Norman, A.W.; Bouillon, R. Vitamin D nutritional policy needs a vision for the future. Exp. Biol. Med. 2010, 235, 1034–1045. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, F.R.; Brincat, M.; Erel, C.T.; Tremollieres, F.; Gambacciani, M.; Lambrinoudaki, I.; Moen, M.H.; Schenck-Gustafsson, K.; Vujovic, S.; Rozenberg, S. EMAS position statement: Vitamin D and postmenopausal health. Maturitas 2012, 71, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Boonen, S.; Brandi, M.-L.; Bruyère, O.; Cooper, C.; Kanis, J.A.; Kaufman, J.-M.; Ringe, J.; Weryha, G.; Reginster, J.-Y. Vitamin D supplementation in elderly or postmenopausal women: A 2013 update of the 2008 recommendations from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Curr. Med. Res. Opin. 2013, 29, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; de Beur, S.J.; LeBoff, M.; Lewiecki, E.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O. Global consensus recommendations on prevention and management of nutritional rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bouillon, R.; Ebeling, P.R.; Lazaretti-Castro, M.; Marcocci, C.; Rizzoli, R.; Sempos, C.T.; Bilezikian, J.P. Controversies in vitamin D: Summary statement from an international conference. J. Clin. Endocrinol. Metab. 2019, 104, 234–240. [Google Scholar] [CrossRef]
- Bours, P.; Wielders, J.; Vermeijden, J.; Van De Wiel, A. Seasonal variation of serum 25-hydroxyvitamin D levels in adult patients with inflammatory bowel disease. Osteoporos. Int. 2011, 22, 2857–2867. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Mazidi, M.; Rezaie, P.; Vatanparast, H. Impact of vitamin D supplementation on C-reactive protein; a systematic review and meta-analysis of randomized controlled trials. BMC Nutr. 2018, 4, 1. [Google Scholar] [CrossRef]
- Mousa, A.; Misso, M.; Teede, H.; Scragg, R.; De Courten, B. Effect of vitamin D supplementation on inflammation: Protocol for a systematic review. BMJ Open 2016, 6, e010804. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D in the new millennium. Curr. Osteoporos. Rep. 2012, 10, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Song, L.; Hu, L.; Lei, X.; Huang, Y.; Lv, Y.; Yu, S. The role of systemic inflammation in the association between serum 25-hydroxyvitamin D and type 2 diabetes mellitus. Clin. Nutr. 2021, 40, 3661–3667. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, C. Vitamin d merging into immune system-skeletal muscle network: Effects on human health. Appl. Sci. 2020, 10, 5592. [Google Scholar] [CrossRef]
- Lind, L.; Zanetti, D.; Högman, M.; Sundman, L.; Ingelsson, E. Commonly used clinical chemistry tests as mortality predictors: Results from two large cohort studies. PLoS ONE 2020, 15, e0241558. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, S. Leukocyte count and the risk of adverse outcomes in patients with HFpEF. BMC Cardiovasc. Disord. 2021, 21, 333. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto Jr, A.M.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Luo, F.; Xing, J.C.; Zhang, F.; Xu, J.Z.; Zhang, Z.H. 1,25(OH)2D3 inhibited Th17 cells differentiation via regulating the NF-κB activity and expression of IL-17. Cell Prolif. 2018, 51, e12461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, G.; Li, M.; Li, Y.; Luo, X.; Liu, Y.; Zhang, X.; Hocher, J.-G.; Krämer, B.K.; Hocher, B.; et al. Both partial inactivation as well as activation of NF-κB signaling lead to hypertension and chronic kidney disease. Nephrol. Dial. Transplant. 2024, gfae090. [Google Scholar] [CrossRef]
- Grønhøj, M.H.; Gerke, O.; Mickley, H.; Steffensen, F.H.; Lambrechtsen, J.; Sand, N.P.R.; Rasmussen, L.M.; Olsen, M.H.; Diederichsen, A. Associations between calcium-phosphate metabolism and coronary artery calcification; a cross sectional study of a middle-aged general population. Atherosclerosis 2016, 251, 101–108. [Google Scholar] [CrossRef]
- Li, J.-W.; Xu, C.; Fan, Y.; Wang, Y.; Xiao, Y.-B. Can serum levels of alkaline phosphatase and phosphate predict cardiovascular diseases and total mortality in individuals with preserved renal function? A systemic review and meta-analysis. PLoS ONE 2014, 9, e102276. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Cheungpasitporn, W.; Mao, M.A.; Erickson, S.B. Calcium-phosphate product and its impact on mortality in hospitalized patients. Nephrology 2020, 25, 22–28. [Google Scholar] [CrossRef]
- Chen, X.; Chu, C.; Doebis, C.; Xiong, Y.; Cao, Y.; Krämer, B.K.; Von Baehr, V.; Hocher, B. Vitamin D status and its association with parathyroid hormone in 23,134 outpatients. J. Steroid Biochem. Mol. Biol. 2022, 220, 106101. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Wang, K.; Sun, H.; Wang, K.; Zhou, Y.; Cong, Y.; Deng, X.; Mao, Y. Threshold of 25 (OH) D and consequently adjusted parathyroid hormone reference intervals: Data mining for relationship between vitamin D and parathyroid hormone. J. Endocrinol. Investig. 2023, 46, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liao, L.; Li, J.; Wang, L.; Xie, Z. Older age is associated with decreased levels of VDR, CYP27B1, and CYP24A1 and increased levels of PTH in human parathyroid glands. Int. J. Endocrinol. 2020, 2020, 7257913. [Google Scholar] [CrossRef]
- Vieth, R.; Kessler, M.J.; Pritzker, K.P. Serum concentrations of vitamin D metabolites in Cayo Santiago rhesus macaques. J. Med. Primatol. 1987, 16, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Marx, S.J.; Jones, G.; Weinstein, R.S.; Chrousos, G.P.; Renquist, D.M. Differences in mineral metabolism among nonhuman primates receiving diets with only vitamin D3 or only vitamin D2. J. Clin. Endocrinol. Metab. 1989, 69, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Gacad, M.A.; Baker, A.J.; Gonzales, B.; Rude, R.K. Serum concentrations of 1, 25-dihydroxyvitamin D3 in Platyrrhini and Catarrhini: A phylogenetic appraisal. Am. J. Primatol. 1985, 9, 219–224. [Google Scholar] [CrossRef]
- Kewenig, S.; Schneider, T.; Hohloch, K.; Lampe–Dreyer, K.; Ullrich, R.; Stolte, N.; Stahl–Hennig, C.; Kaup, F.J.; Stallmach, A.; Zeitz, M. Rapid mucosal CD4+ T-cell depletion and enteropathy in simian immunodeficiency virus–infected rhesus macaques. Gastroenterology 1999, 116, 1115–1123. [Google Scholar] [CrossRef]
- Vieth, R. What is the optimal vitamin D status for health? Prog. Biophys. Mol. Biol. 2006, 92, 26–32. [Google Scholar] [CrossRef]
- Sempos, C.T.; Durazo-Arvizu, R.A.; Dawson-Hughes, B.; Yetley, E.A.; Looker, A.C.; Schleicher, R.L.; Cao, G.; Burt, V.; Kramer, H.; Bailey, R.L.; et al. Is there a reverse J-shaped association between 25-hydroxyvitamin D and all-cause mortality? Results from the U.S. nationally representative NHANES. J. Clin. Endocrinol. Metab. 2013, 98, 3001–3009. [Google Scholar] [CrossRef] [PubMed]
- Durazo-Arvizu, R.A.; Dawson-Hughes, B.; Kramer, H.; Cao, G.; Merkel, J.; Coates, P.M.; Sempos, C.T. The Reverse J-Shaped Association Between Serum Total 25-Hydroxyvitamin D Concentration and All-Cause Mortality: The Impact of Assay Standardization. Am. J. Epidemiol. 2017, 185, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Ginde, A.A.; Scragg, R.; Schwartz, R.S.; Camargo, C.A., Jr. Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J. Am. Geriatr. Soc. 2009, 57, 1595–1603. [Google Scholar] [CrossRef]
- Schlieper, G.; Schurgers, L.; Brandenburg, V.; Reutelingsperger, C.; Floege, J. Vascular calcification in chronic kidney disease: An update. Nephrol. Dial. Transplant. 2016, 31, 31–39. [Google Scholar] [CrossRef]
- Tsuprykov, O.; Chen, X.; Hocher, C.-F.; Skoblo, R.; Yin, L.; Hocher, B. Why should we measure free 25 (OH) vitamin D? J. Steroid Biochem. Mol. Biol. 2018, 180, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
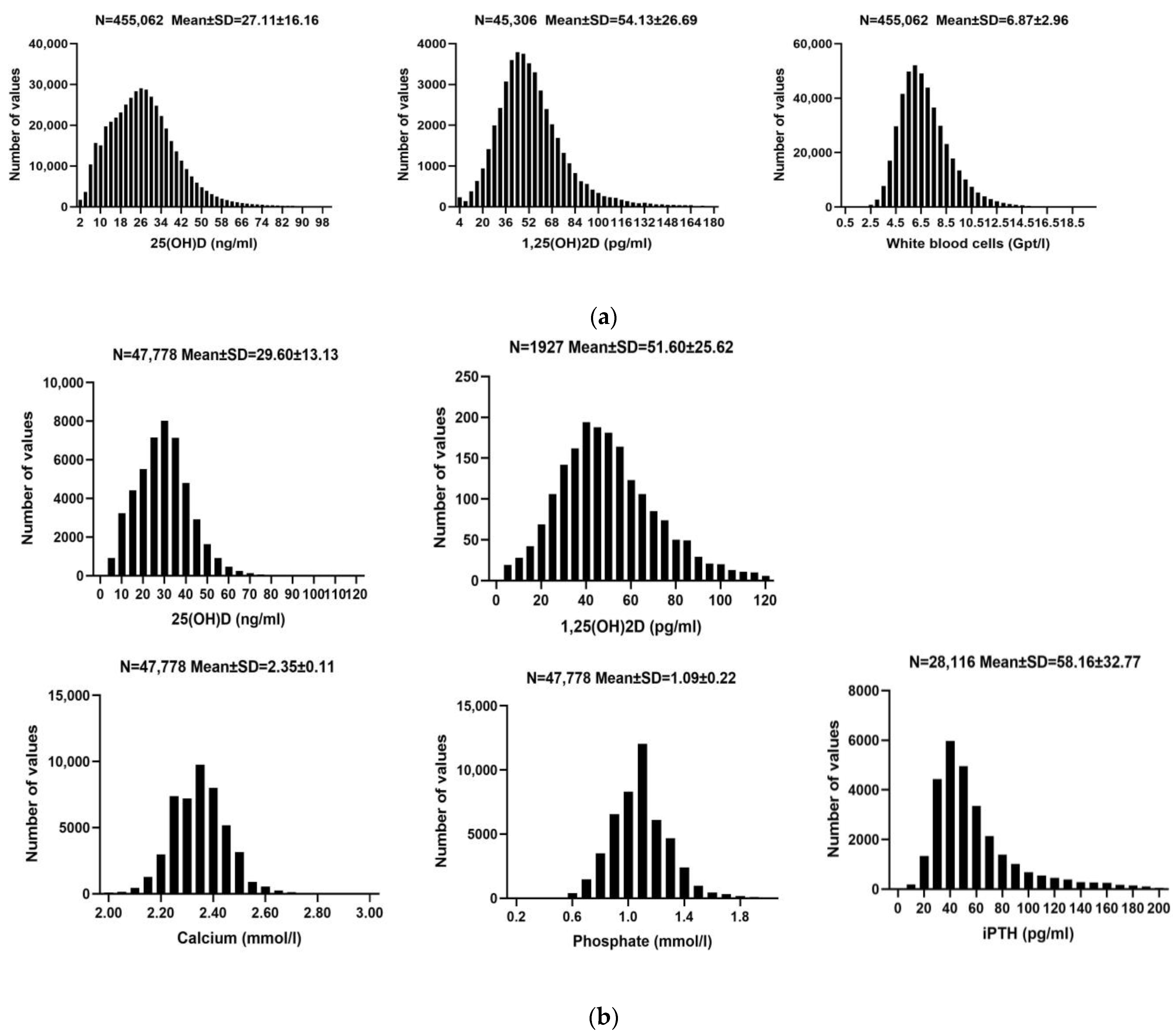
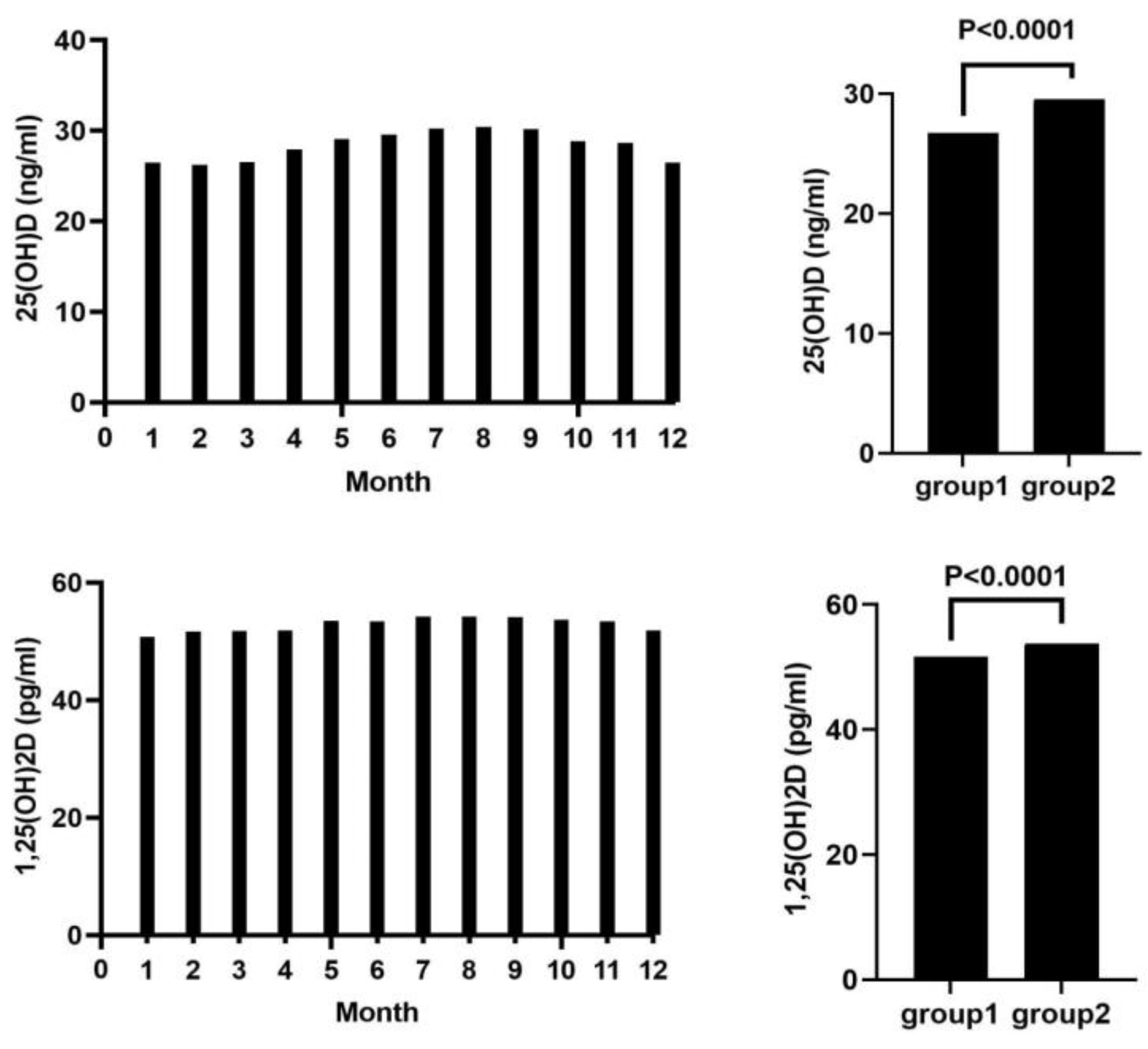
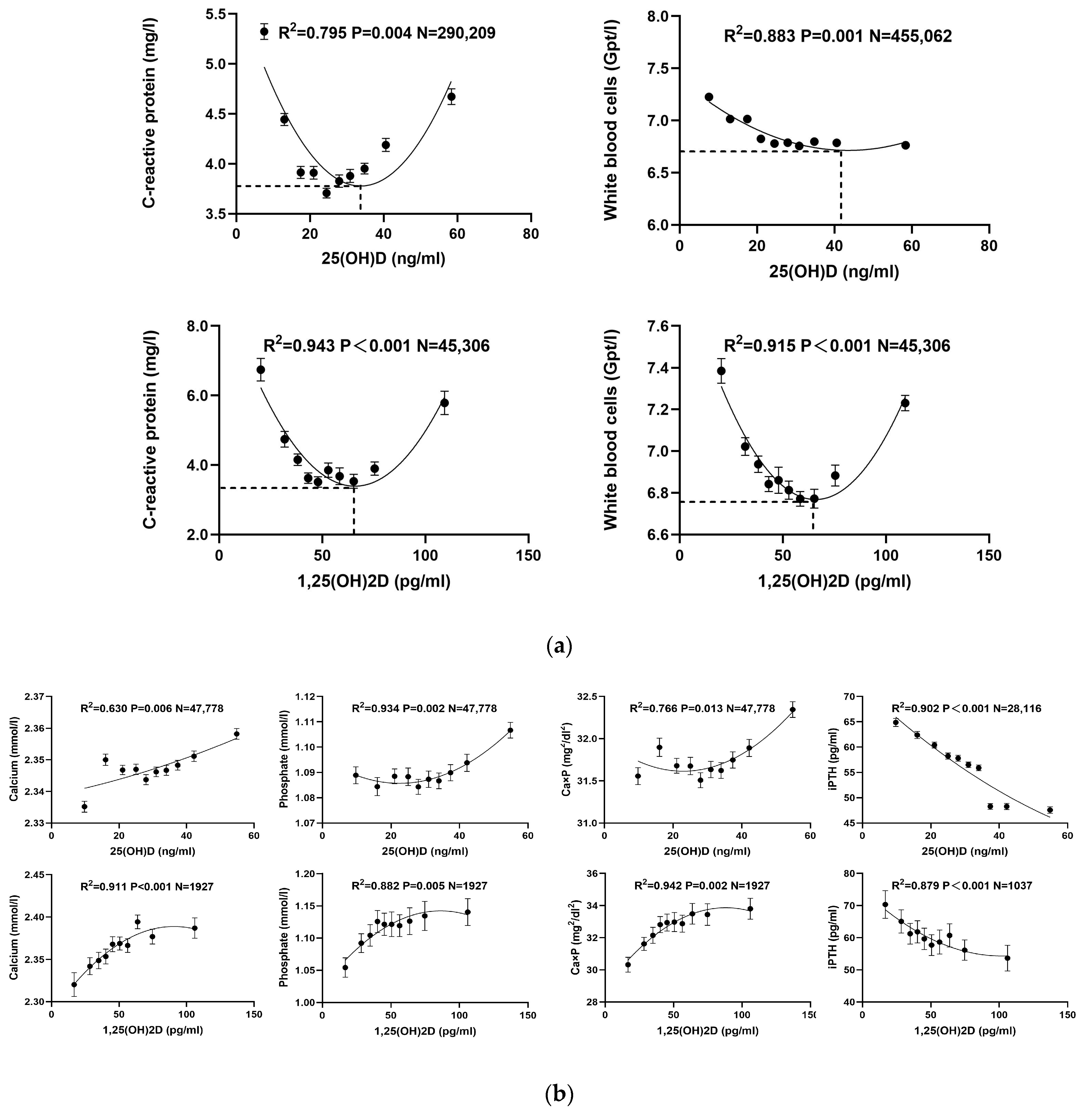
| Year | Target Disease | Concentration (ng/mL) | Explanation | |
|---|---|---|---|---|
| Holick [19] | 2004 | General health care | 30–50 | optimal range |
| ≥150 | toxicity | |||
| Holick [20] | 2005 | Bone health | <32 | deficiency |
| ≥150 | toxicity | |||
| Wang et al. [21] | 2008 | Cardiovascular Disease | <10 | significant risk |
| >30 | relative safety | |||
| Norman et al. [22] | 2010 | General health care | <5 | severe deficiency |
| 5–10 | deficiency | |||
| 10–30 | insufficiency | |||
| 30–100 | optimal range | |||
| 100–150 | probable toxicity | |||
| ≥300 | apparent toxicity | |||
| Ross et al. [23] | 2010 | General health care | 20–50 | optimal range |
| Pérez-López et al. [24] | 2012 | General health care | <20 | deficiency |
| <30 | insufficiency | |||
| Rizzoli et al. [25] | 2013 | General health care | <10 | deficiency |
| <20 | insufficiency | |||
| Cosman et al. [26] | 2014 | Osteoporosis | <30 | insufficiency |
| Munns et al. [27] | 2016 | General health care | <12 | deficiency |
| <20 | insufficiency | |||
| ≥100 | toxicity | |||
| Giustina et al. [28] | 2019 | Bone health | <12 | deficiency |
| <20 | insufficiency |
| (a) Cohort A | |||
| N (N = 455,062) | Mean ± SD | Reference Range | |
| Sex | Males: 162,019 (35.6%) | - | |
| Females: 293,043 (64.4%) | - | ||
| Age (years) | 455,062 | 53.67 ± 19.80 | - |
| 25(OH)D (ng/mL) | 455,062 | 27.11 ± 16.16 | 30.00–100.00 |
| 1,25(OH)2D (pg/mL) | 45,306 | 54.13 ± 26.69 | 19.90–79.30 |
| WBC (Gpt/L) | 455,062 | 6.87 ± 2.96 | 3.6–28.2 |
| CRP (mg/L) | 290,209 | 4.17 ± 10.83 | <5 |
| Granulocytes (Gpt/L) | 198,928 | 0.03 ± 0.01 | - |
| Neutrophils (Gpt/L) | 205,121 | 3.87 ± 1.65 | 1.3–22.3 |
| Eosinophils (Gpt/L) | 203,224 | 0.18 ± 0.06 | 0.02–1.10 |
| Basophilic granulocytes (Gpt/L) | 204,587 | 0.05 ± 0.03 | <0.35 |
| Lymphocytes (Gpt/L) | 205,120 | 2.00 ± 0.75 | 1.1–13.6 |
| Mononuclear cells (Gpt/L) | 205,120 | 0.55 ± 0.19 | 0.10–2.70 |
| (b) Cohort B | |||
| N (N = 47,778) | Mean ± SD | Reference Range | |
| Sex | Males: 22,161 (46.4%) | - | |
| Females: 25,617 (53.6%) | - | ||
| Age (years) | 47,778 | 59.00 ± 19.51 | - |
| 25(OH)D (ng/mL) | 47,778 | 29.60 ± 13.13 | 30.00–100.00 |
| 1,25(OH)2D (pg/mL) | 1927 | 51.60 ± 25.62 | 19.90–79.30 |
| Calcium (mmol/L) | 47,778 | 2.35 ± 0.11 | 1.90–2.75 |
| Phosphate (mmol/L) | 47,778 | 1.09 ± 0.22 | 0.81–2.42 |
| Ca × P (mg2/dL2) | 47,778 | 31.75 ± 6.72 | - |
| iPTH (pg/mL) | 28,116 | 58.16 ± 32.77 | 15.00–65.00 |
| Creatinine (mg/dL) | 40,496 | 0.88 ± 0.42 | 0.16–1.95 |
| LDL (mg/dL) | 32,074 | 114.42 ± 40.20 | <115.00 |
| HDL (mg/dL) | 31,311 | 55.23 ± 17.73 | >45.00 |
| TC (mg/dL) | 30,844 | 187.99 ± 45.52 | <200.00 |
| (a) Cohort A | ||||||
| 25(OH)D | ||||||
| Parameters | Linear | Exponential | Quadratic | |||
| R2 | p | R2 | p | R2 | p | |
| WBC (Gpt/L) | 0.512 | 0.020 | 0.523 | 0.019 | 0.883 | 0.001 |
| CRP (mg/L) | 0.021 | 0.731 | 0.012 | 0.785 | 0.795 | 0.004 |
| Granulocytes (Gpt/L) | 0.177 | 0.236 | 0.165 | 0.244 | 0.515 | 0.079 |
| Neutrophils (Gpt/L) | 0.243 | 0.148 | 0.242 | 0.150 | 0.658 | 0.024 |
| Eosinophils (Gpt/L) | 0.863 | <0.001 | 0.878 | <0.001 | 0.992 | <0.001 |
| Basophilic granulocytes (Gpt/L) | 0.549 | 0.014 | 0.553 | 0.014 | 0.852 | 0.001 |
| Lymphocytes (Gpt/L) | 0.934 | <0.001 | 0.943 | <0.001 | 0.995 | <0.001 |
| Mononuclear cells (Gpt/L) | 0.652 | 0.005 | 0.652 | 0.005 | 0.827 | 0.002 |
| 1,25(OH)2D | ||||||
| WBC (Gpt/L) | 0.004 | 0.861 | 0.004 | 0.865 | 0.943 | <0.001 |
| CRP (mg/L) | 0.004 | 0.864 | 0.002 | 0.906 | 0.915 | <0.001 |
| Granulocytes (Gpt/L) | 0.006 | 0.828 | 0.004 | 0.869 | 0.991 | <0.001 |
| Neutrophils (Gpt/L) | 0.031 | 0.627 | 0.028 | 0.641 | 0.971 | <0.001 |
| Eosinophils (Gpt/L) | 0.927 | <0.001 | 0.944 | <0.001 | 0.948 | <0.001 |
| Basophilic granulocytes (Gpt/L) | 0.870 | <0.001 | 0.888 | <0.001 | 0.970 | <0.001 |
| Lymphocytes (Gpt/L) | 0.065 | 0.476 | 0.068 | 0.469 | 0.619 | 0.034 |
| Mononuclear cells (Gpt/L) | 0.282 | 0.116 | 0.277 | 0.119 | 0.970 | <0.001 |
| (b) Cohort B | ||||||
| 25(OH)D | ||||||
| Parameters | Linear | Exponential | Quadratic | |||
| R2 | p | R2 | p | R2 | p | |
| Calcium (mmol/L) | 0.624 | 0.007 | 0.604 | 0.019 | 0.630 | 0.006 |
| Phosphate (mmol/L) | 0.565 | 0.012 | 0.646 | 0.010 | 0.934 | 0.002 |
| Ca × P (mg2/dL2) | 0.447 | 0.035 | 0.602 | 0.022 | 0.766 | 0.013 |
| iPTH (pg/mL) | 0.892 | <0.001 | 0.883 | <0.001 | 0.902 | <0.001 |
| Creatinine (mg/dL) | 0.983 | <0.001 | 0.980 | <0.001 | 0.983 | <0.001 |
| LDL (mg/dL) | 0.795 | <0.001 | 0.794 | <0.001 | 0.795 | <0.001 |
| HDL (mg/dL) | 0.923 | <0.001 | 0.953 | <0.001 | 0.965 | <0.001 |
| TC (mg/dL) | 0.785 | <0.001 | 0.794 | <0.001 | 0.806 | <0.001 |
| Hemoglobin (g/dL) | 0.787 | <0.001 | 0.817 | <0.001 | 0.835 | <0.001 |
| 1,25(OH)2D | ||||||
| Calcium (mmol/L) | 0.721 | 0.002 | 0.876 | 0.001 | 0.911 | <0.001 |
| Phosphate (mmol/L) | 0.648 | 0.005 | 0.723 | 0.005 | 0.882 | 0.005 |
| Ca × P (mg2/dL2) | 0.718 | 0.002 | 0.827 | 0.002 | 0.942 | 0.002 |
| iPTH (pg/mL) | 0.774 | <0.001 | 0.854 | <0.001 | 0.879 | <0.001 |
| Creatinine(mg/dL) | 0.614 | 0.007 | 0.856 | 0.005 | 0.977 | 0.003 |
| LDL (mg/dL) | 0.200 | 0.195 | 0.696 | 0.010 | 0.727 | 0.007 |
| HDL (mg/dL) | 0.720 | 0.002 | 0.803 | 0.002 | 0.835 | 0.001 |
| TC (mg/dL) | 0.264 | 0.128 | 0.333 | 0.104 | 0.473 | 0.092 |
| Hemoglobin(g/dL) | 0.157 | 0.257 | 0.344 | 0.131 | 0.654 | 0.071 |
| (a) | ||||||||||||
| WBC | CRP | |||||||||||
| p | B # | 95%CI | p | B # | 95%CI | |||||||
| Constant | 0.000 | 9.029 | 8.705~9.353 | <0.001 | 10.162 | 8.643~11.681 | ||||||
| Gender | 0.009 | −0.082 | −0.144~−0.020 | 0.031 | −0.324 | −0.619~−0.029 | ||||||
| Age (years) | 0.364 | 0.001 | −0.001~0.002 | <0.001 | 0.046 | 0.038~0.053 | ||||||
| Seasonal group | 0.900 | 0.004 | −0.054~0.062 | 0.042 | 0.293 | 0.011~0.576 | ||||||
| 25(OH)D (ng/mL) | 0.033 | 0.004 | 0.001~0.007 | <0.001 | 0.038 | 0.018~0.059 | ||||||
| Sqrt-25(OH)D | <0.001 | −0.157 | −0.210~−0.104 | <0.001 | −0.716 | −0.977~−0.456 | ||||||
| 1,25(OH)2D (pg/mL) | <0.001 | 0.027 | 0.022~0.031 | <0.001 | 0.104 | 0.083~0.124 | ||||||
| Sqrt-1,25(OH)2D | <0.001 | −0.383 | −0.453~−0.313 | <0.001 | −1.557 | −1.886~−1.229 | ||||||
| (b) | ||||||||||||
| Calcium | Phosphate | iPTH | ||||||||||
| p | B# | 95%CI | p | B# | 95%CI | p | B# | 95%CI | ||||
| Constant | 0.000 | 2.307 | 2.247~2.367 | <0.001 | 1.432 | 1.330~1.535 | <0.001 | 210.799 | 161.571~260.027 | |||
| Gender | 0.005 | 0.020 | 0.006~0.034 | <0.001 | 0.108 | 0.085~0.132 | <0.001 | −24.417 | −35.729~−13.105 | |||
| Age (years) | <0.001 | −0.001 | −0.001~0.000 | <0.001 | −0.005 | −0.005~−0.004 | 0.710 | 0.057 | −0.246~0.360 | |||
| Seasonal group | 0.941 | −0.001 | −0.014~0.013 | 0.074 | −0.021 | −0.044~0.002 | 0.307 | −5.673 | −16.560~5.215 | |||
| 25(OH)D (ng/mL) | 0.004 | 0.001 | 0.000~0.002 | <0.001 | −0.002 | −0.003~−0.001 | 0.007 | −0.558 | −0.961~−0.155 | |||
| Sqrt-25(OH)D | 0.008 | 0.010 | 0.003~0.017 | <0.001 | 0.054 | 0.037~0.070 | <0.001 | 13.071 | 5.446~20.696 | |||
| 1,25(OH)2D (pg/mL) | 0.045 | 0.002 | 0.001~0.003 | <0.001 | 0.004 | 0.003~0.005 | <0.001 | 0.965 | 0.550~1.379 | |||
| Sqrt-1,25(OH)2D | 0.012 | 0.014 | 0.003~0.025 | <0.001 | −0.083 | −0.102~−0.065 | <0.001 | −27.254 | −35.016~−19.493 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, Y.; Chen, X.; Reichetzeder, C.; Elitok, S.; Krämer, B.K.; Hocher, B. Target Values for 25-Hydroxy and 1,25-Dihydroxy Vitamin D Based on Their Associations with Inflammation and Calcium-Phosphate Metabolism. Nutrients 2024, 16, 2679. https://doi.org/10.3390/nu16162679
Li X, Liu Y, Chen X, Reichetzeder C, Elitok S, Krämer BK, Hocher B. Target Values for 25-Hydroxy and 1,25-Dihydroxy Vitamin D Based on Their Associations with Inflammation and Calcium-Phosphate Metabolism. Nutrients. 2024; 16(16):2679. https://doi.org/10.3390/nu16162679
Chicago/Turabian StyleLi, Xitong, Yvonne Liu, Xin Chen, Christoph Reichetzeder, Saban Elitok, Bernhard K. Krämer, and Berthold Hocher. 2024. "Target Values for 25-Hydroxy and 1,25-Dihydroxy Vitamin D Based on Their Associations with Inflammation and Calcium-Phosphate Metabolism" Nutrients 16, no. 16: 2679. https://doi.org/10.3390/nu16162679
APA StyleLi, X., Liu, Y., Chen, X., Reichetzeder, C., Elitok, S., Krämer, B. K., & Hocher, B. (2024). Target Values for 25-Hydroxy and 1,25-Dihydroxy Vitamin D Based on Their Associations with Inflammation and Calcium-Phosphate Metabolism. Nutrients, 16(16), 2679. https://doi.org/10.3390/nu16162679






