Regulation of Intestinal Inflammation by Walnut-Derived Bioactive Compounds
Highlights
- Walnuts may function as dietary supplements to alleviate inflammatory bowel disease (IBD).
- Bioactive compounds in walnuts modulate inflammation, oxidative stress pathways, and gut microbiota.
- Exploring the chemical basis and dose of anti-inflammatory effects in walnuts could optimize their therapeutic potential.
- Optimizing isolation and purification methods is crucial for effectively extracting anti-inflammatory compounds from walnuts.
Abstract
1. Introduction
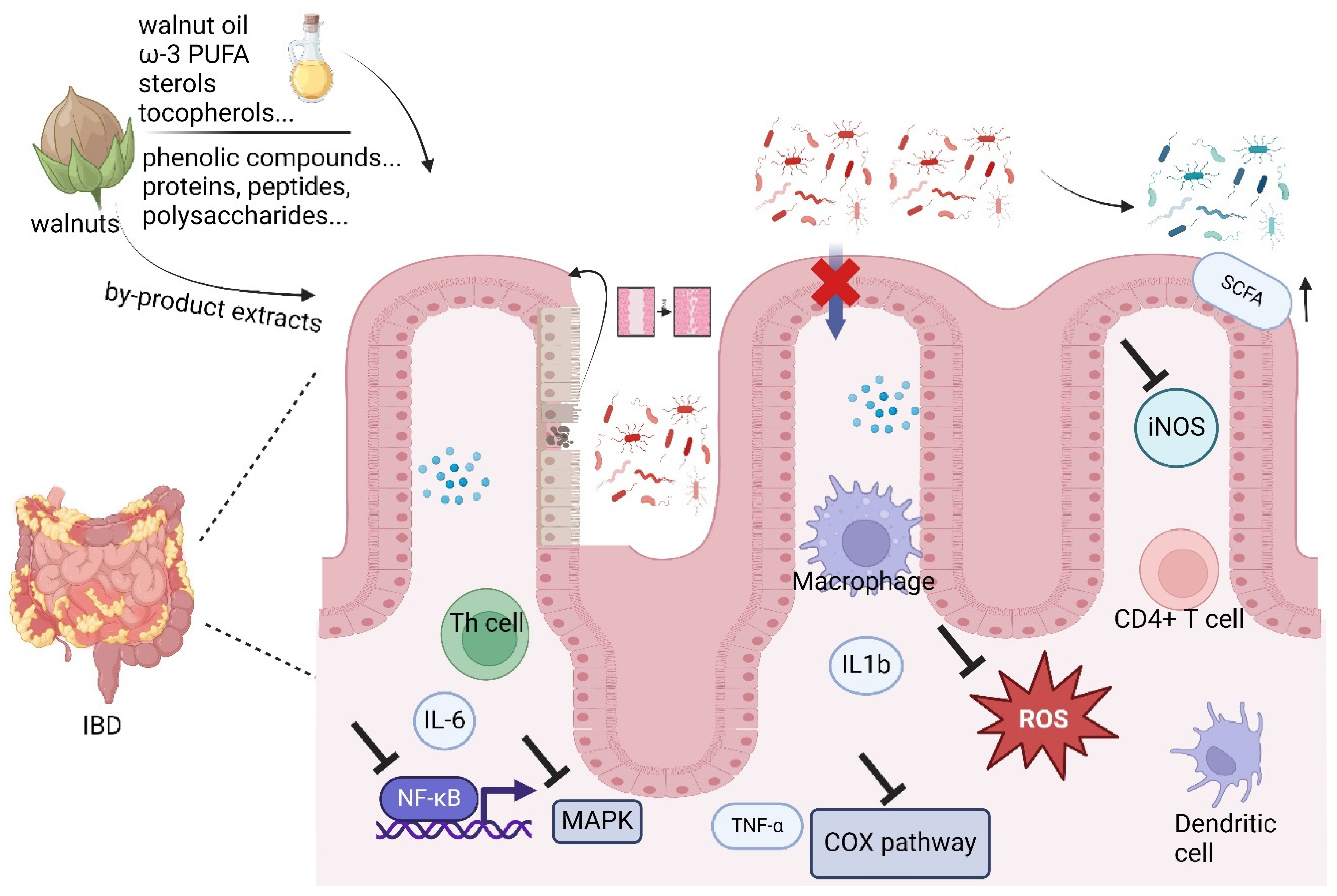
2. The Regulating Effects and Mechanisms of Walnuts and Its Derived Bioactive Compounds’ Roles in Intestinal Inflammation
2.1. Intestinal Mucosa Permeability
2.2. Oxidative Stress and ROS
2.3. NF-κB and Cytokines
2.4. COX/COX-2
2.5. MAPCK/MAPK
2.6. iNOS/NOS
2.7. Microbiome
2.8. Metabolic Markers
2.9. miRNA
2.10. Summary
3. Anti-Inflammatory Components in Walnuts
3.1. Lipids and Lipophilic Bioactive Compounds
3.1.1. Polyunsaturated Fatty Acids
3.1.2. Tocopherols
3.1.3. Phytosterols
3.1.4. Sphingolipids
3.1.5. Phospholipids
3.2. Phenolic Compounds
3.2.1. Phenolic Acids
3.2.2. Flavonoids
3.2.3. Tannins
3.2.4. Junglone
3.3. Proteins and Peptides
3.3.1. Proteins and Protein Hydrolyses
3.3.2. Bioactive Peptides
3.4. Polysaccharides
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Neurath, M.F. Strategies for targeting cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2024, 24, 559–576. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.K.; Heilmann, R.M.; Paital, B.; Patel, A.; Yadav, V.K.; Wong, D.; Jergens, A.E. Oxidative stress, hormones, and effects of natural antioxidants on intestinal inflammation in inflammatory bowel disease. Front. Endocrinol. 2023, 14, 1217165. [Google Scholar] [CrossRef] [PubMed]
- Vagianos, K.; Clara, I.; Carr, R.; Graff, L.A.; Walker, J.R.; Targownik, L.E.; Lix, L.M.; Rogala, L.; Miller, N.; Bernstein, C.N. What Are Adults with Inflammatory Bowel Disease (IBD) Eating? A Closer Look at the Dietary Habits of a Population-Based Canadian IBD Cohort. J. Parenter. Enter. Nutr. 2014, 40, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Zhang, C.; Yu, X.-F.; Zou, J.; Yu, Y.; Bao, Z.-J. Dietary risk factors for inflammatory bowel disease in Shanghai: A case-control study. Asia Pac. J. Clin. Nutr. 2022, 31, 405–414. [Google Scholar] [CrossRef]
- Preda, C.; Manuc, T.; Chifulescu, A.E.; Istratescu, D.; Louis, E.; Baicus, C.; Sandra, I.; Diculescu, M.; Reenaers, C.; Van Kemseke, C.; et al. Diet as an environmental trigger in inflammatory bowel disease: A retrospective comparative study in two European cohorts. Rev. Esp. Enfermedades Dig. 2020, 112, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.A.; Melton, S.L.; Yao, C.K.; Gibson, P.R.; Halmos, E.P. Dietary management of adults with IBD—The emerging role of dietary therapy. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 652–669. [Google Scholar] [CrossRef]
- Nakanishi, M.; Matz, A.; Klemashevich, C.; Rosenberg, D.W. Dietary Walnut Supplementation Alters Mucosal Metabolite Profiles During DSS-Induced Colonic Ulceration. Nutrients 2019, 11, 1118. [Google Scholar] [CrossRef]
- Ni, Z.-J.; Zhang, Y.-G.; Chen, S.-X.; Thakur, K.; Wang, S.; Zhang, J.-G.; Shang, Y.-F.; Wei, Z.-J. Exploration of walnut components and their association with health effects. Crit. Rev. Food Sci. Nutr. 2021, 62, 5113–5129. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Shan, C.; Ning, D. Walnut oil alleviates LPS-induced intestinal epithelial cells injury by inhibiting TLR4/MyD88/NF-kappaB pathway activation. J. Food Biochem. 2021, 45, e13955. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Shan, C.; Shah, S.A.; Akhtar, R.W.; Wang, X.; Ning, D. Effect of walnut (Juglans sigillata) oil on intestinal antioxidant, anti-inflammatory, immunity, and gut microbiota modulation in mice. J. Food Biochem. 2021, 45, e13567. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Shan, C.; Shah, S.A.H.; Akhtar, R.W.; Geng, S.; Ning, D.; Wang, X. The protective effect of walnut oil on lipopolysaccharide–induced acute intestinal injury in mice. Food Sci. Nutr. 2020, 9, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.-J.; Choi, Y.-I.; Kim, Y.; Kim, Y.-S.; Choi, S.W.; Kim, J.W.; Kim, B.G.; Lee, K.L. Walnut phenolic extract inhibits nuclear factor kappaB signaling in intestinal epithelial cells, and ameliorates experimental colitis and colitis-associated colon cancer in mice. Eur. J. Nutr. 2018, 58, 1603–1613. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, F.; Carpena, M.; Lourenço-Lopes, C.; Taofiq, O.; Otero, P.; Cao, H.; Xiao, J.; Simal-Gandara, J.; Prieto, M.A. By-Products of Walnut (Juglans regia) as a Source of Bioactive Compounds for the Formulation of Nutraceuticals and Functional Foods. Biol. Life Sci. Forum 2022, 12, 35. [Google Scholar] [CrossRef]
- Zhu, N.; Li, Y. Research Progress of Biological Activity of Walnut Peptide. Food Nutr. China 2018, 24, 58–62. [Google Scholar]
- Wang, G.; Yang, X.; Wang, J.; Zhong, D.; Zhang, R.; Zhang, Y.; Feng, L.; Zhang, Y. Walnut green husk polysaccharides prevent obesity, chronic inflammatory responses, nonalcoholic fatty liver disease and colonic tissue damage in high-fat diet fed rats. Int. J. Biol. Macromol. 2021, 182, 879–898. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Gervasi, T.; Rosenberg, D.W.; Lapsley, K.G.; Baer, D.J. Effect of Nuts on Gastrointestinal Health. Nutrients 2023, 15, 1733. [Google Scholar] [CrossRef] [PubMed]
- Bartoszek, A.; Makaro, A.; Bartoszek, A.; Kordek, R.; Fichna, J.; Salaga, M. Walnut Oil Alleviates Intestinal Inflammation and Restores Intestinal Barrier Function in Mice. Nutrients 2020, 12, 1302. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Shan, C.; Ma, T.; Geng, S.; Ning, D. Walnut oil alleviates DSS–induced colitis in mice by inhibiting NLRP3 inflammasome activation and regulating gut microbiota. Microb. Pathog. 2021, 154, 104866. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, J.; Zhao, J.; Chen, Y.; Ren, C.; Chen, Y. Antioxidant effects of compound walnut oil capsule in mice aging model induced by D-galactose. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef]
- Willis, L.M.; Bielinski, D.F.; Fisher, D.R.; Matthan, N.R.; Joseph, J.A. Walnut Extract Inhibits LPS-induced Activation of Bv-2 Microglia via Internalization of TLR4: Possible Involvement of Phospholipase D2. Inflammation 2010, 33, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, Z.; Nurmohammadi, F.; Majlesi, S.; Maghool, F. Protective effects of walnut extract against oxidative damage in acetic acid-induced experimental colitis rats. Physiol. Pharmacol. 2019, 23, 51–58. [Google Scholar]
- Chen, S.; Wu, X.; Yu, Z. Juglone Suppresses Inflammation and Oxidative Stress in Colitis Mice. Front. Immunol. 2021, 12, 674341. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, X.; Zhang, Y.; Leng, Y.; Liu, X.; Wang, X.; Wu, D.; Wang, J.; Min, W. Walnut-Derived Peptide Improves Cognitive Impairment in Colitis Mice Induced by Dextran Sodium Sulfate via the Microbiota–Gut–Brain Axis (MGBA). J. Agric. Food Chem. 2023, 71, 19501–19515. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yuan, C.; Wang, G.; Luo, J.; Ma, H.; Xu, L.; Mu, Y.; Li, Y.; Seeram, N.P.; Huang, X.; et al. Urolithins Attenuate LPS-Induced Neuroinflammation in BV2Microglia via MAPK, Akt, and NF-κB Signaling Pathways. J. Agric. Food Chem. 2018, 66, 571–580. [Google Scholar] [CrossRef]
- Alizadeh Nobakht, N.A.; Lashgari, N.A.; Momeni Roudsari, N.; Niknejad, A.; Khayatan, D.; Tavakoli, S.; Abdollahi, A.R.; Esmaealzadeh, N.; Momtaz, S.; Abdolghaffari, A.H. Juglone Mediates Inflammatory Bowel Disease Through Inhibition of TLR-4/NF KappaB Pathway in Acetic Acid-induced Colitis in Rats. Anti-Inflamm. Anti-Allergy Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Inflamm. Anti-Allergy Agents) 2023, 22, 92–103. [Google Scholar] [CrossRef]
- Shattuck-Brandt, R.L.; Varilek, G.W.; Radhika, A.; Yang, F.; Washington, M.K.; DuBois, R.N. Cyclooxygenase 2 expression is increased in the stroma of colon carcinomas from IL–10−/− mice. Gastroenterology 2000, 118, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, J.; Tan, S.; Xu, M.; Tao, J.; Jiang, J.; Liu, H.; Wu, B. COX-1/PGE2/EP4 alleviates mucosal injury by upregulating β-arr1-mediated Akt signaling in colitis. Sci. Rep. 2017, 7, 1055. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhi, T.; Han, P.; Li, S.; Xia, J.; Chen, Z.; Wang, C.; Wu, Y.; Jia, Y.; Ma, A. Potential anti-inflammatory activity of walnut protein derived peptide leucine-proline-phenylalanine in lipopolysaccharides-irritated RAW264.7 cells. Food Agric. Immunol. 2021, 32, 663–678. [Google Scholar] [CrossRef]
- Wang, D.; Sun, M.; Zhang, Y.; Chen, Z.; Zang, S.; Li, G.; Li, G.; Clark, A.R.; Huang, J.; Si, L. Enhanced therapeutic efficacy of a novel colon-specific nanosystem loading emodin on DSS-induced experimental colitis. Phytomedicine 2020, 78, 153293. [Google Scholar] [CrossRef] [PubMed]
- Coskun, M.; Olsen, J.; Seidelin, J.B.; Nielsen, O.H. MAP kinases in inflammatory bowel disease. Clin. Chim. Acta 2011, 412, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Amri, E.; Mohamed; Fitzgerald, U.; Schlosser, G. MAP kinases in inflammatory bowel disease+ MARCKS and MARCKS-like proteins in development and regeneration. J. Biomed. Sci. 2018, 25, 43. [Google Scholar]
- Broom, O.J.; Widjaya, B.; Troelsen, J.; Olsen, J.; Nielsen, O.H. Mitogen activated protein kinases: A role in inflammatory bowel disease? Clin. Exp. Immunol. 2009, 158, 272–280. [Google Scholar] [CrossRef]
- Hong, Z.; Shi, C.; Hu, X.; Chen, J.; Li, T.; Zhang, L.; Bai, Y.; Dai, J.; Sheng, J.; Xie, J.; et al. Walnut Protein Peptides Ameliorate DSS-Induced Ulcerative Colitis Damage in Mice: An in Silico Analysis and in Vivo Investigation. J. Agric. Food Chem. 2023, 71, 15604–15619. [Google Scholar] [CrossRef]
- Bisgaard, T.H.; Allin, K.H.; Keefer, L.; Ananthakrishnan, A.N.; Jess, T. Depression and anxiety in inflammatory bowel disease: Epidemiology, mechanisms and treatment. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 717–726. [Google Scholar] [CrossRef]
- Schwager, S.; Detmar, M. Inflammation and Lymphatic Function. Front. Immunol. 2019, 10, 308. [Google Scholar] [CrossRef]
- Kolios, G.; Valatas, V.; Ward, S.G. Nitric oxide in inflammatory bowel disease: A universal messenger in an unsolved puzzle. Immunology 2004, 113, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Mu, Y.; Dong, H.; Yan, H.; Hao, C.; Wang, X.; Zhang, L. Chemical Constituents of the Ethyl Acetate Extract from Diaphragma juglandis Fructus and Their Inhibitory Activity on Nitric Oxide Production In Vitro. Molecules 2017, 23, 72. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Sun, L.; Gonzalez, F.J. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 2022, 30, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Kudelka, M.R.; Stowell, S.R.; Cummings, R.D.; Neish, A.S. Intestinal epithelial glycosylation in homeostasis and gut microbiota interactions in IBD. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 597–617. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Kanai, T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015, 37, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Schofield, W.B.; Palm, N.W. Gut Microbiota: IgA Protects the Pioneers. Curr. Biol. 2018, 28, R1117–R1119. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Chen, D.; Guo, Y.; Zhang, X.; Ma, Y.; Zhao, S. Walnut Meal Extracts Rich In Polyphenols Mitigate Insulin Resistance and Modulate Gut Microbiota in High Fat Diet-Fed Rats. J. Med. Food 2022, 25, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Authier, H.; Bardot, V.; Berthomier, L.; Bertrand, B.; Blondeau, C.; Holowacz, S.; Coste, A. Synergistic Effects of Licorice Root and Walnut Leaf Extracts on Gastrointestinal Candidiasis, Inflammation and Gut Microbiota Composition in Mice. Microbiol. Spectr. 2022, 10, e0235521. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Liu, R.; Lu, M.; Guan, X.; Zhuang, S.; Tian, Y.; Zhang, Z.; Cui, L. Juglone regulates gut microbiota and Th17/Treg balance in DSS-induced ulcerative colitis. Int. Immunopharmacol. 2021, 97, 107683. [Google Scholar] [CrossRef]
- Thomas, S.; Dilbarov, N.; Kelly, J.; Mercogliano, G.; Prendergast, G.C. Diet effects on colonic health influence the efficacy of Bin1 mAb immunotherapy for ulcerative colitis. Sci. Rep. 2023, 13, 11802. [Google Scholar] [CrossRef]
- Li, L.; Wang, S.; Zhang, T.; Lv, B.; Jin, Y.; Wang, Y.; Chen, X.; Li, N.; Han, N.; Wu, Y.; et al. Walnut peptide alleviates obesity, inflammation and dyslipidemia in mice fed a high-fat diet by modulating the intestinal flora and metabolites. Front. Immunol. 2023, 14, 1305656. [Google Scholar] [CrossRef] [PubMed]
- Bamberger, C.; Rossmeier, A.; Lechner, K.; Wu, L.; Waldmann, E.; Fischer, S.; Stark, R.G.; Altenhofer, J.; Henze, K.; Parhofer, K.G. A Walnut-Enriched Diet Affects Gut Microbiome in Healthy Caucasian Subjects: A Randomized, Controlled Trial. Nutrients 2018, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Chandra, M.; See, J.R.; Leister, J.; Jafari, F.; Tindall, A.; Kris-Etherton, P.M.; Lamendella, R. Walnut consumption and gut microbial metabolism: Results of an exploratory analysis from a randomized, crossover, controlled-feeding study. Clin. Nutr. 2023, 42, 2258–2269. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Guetterman, H.M.; Swanson, K.S.; An, R.; Matthan, N.R.; Lichtenstein, A.H.; Novotny, J.A.; Baer, D.J. Walnut Consumption Alters the Gastrointestinal Microbiota, Microbially Derived Secondary Bile Acids, and Health Markers in Healthy Adults: A Randomized Controlled Trial. J. Nutr. 2018, 148, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Shin, P.-K.; Kim, Y.; Hong, C.P.; Choi, S.-W. Metabolic influence of walnut phenolic extract on mitochondria in a colon cancer stem cell model. Eur. J. Nutr. 2018, 58, 1635–1645. [Google Scholar] [CrossRef]
- Shin, P.-K.; Zoh, Y.; Choi, J.; Kim, M.-S.; Kim, Y.; Choi, S.-W. Walnut phenolic extracts reduce telomere length and telomerase activity in a colon cancer stem cell model. Nutr. Res. Pract. 2019, 13, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Tsoukas, M.A.; Ko, B.-J.; Witte, T.R.; Dincer, F.; Hardman, W.E.; Mantzoros, C.S. Dietary walnut suppression of colorectal cancer in mice: Mediation by miRNA patterns and fatty acid incorporation. J. Nutr. Biochem. 2015, 26, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Cong, Z.; Wang, C.; He, M.; Liu, C.; Gao, P. Research progress on Walnut oil: Bioactive compounds, health benefits, extraction methods, and medicinal uses. J. Food Biochem. 2022, 46, e14504. [Google Scholar] [CrossRef] [PubMed]
- Federica, U.; Federica, R.; Silvio, D.; D’Alessio, S. Actors and factors in the resolution of intestinal inflammation: Lipid mediators as a new approach to therapy in inflammatory bowel diseases. Front. Immunol. 2017, 8, 1331. [Google Scholar]
- Eleonora, S.; Elisa, L.; Andrea, B. The imbalance between n-6/n-3 polyunsaturated fatty acids and inflammatory bowel disease: A comprehensive review and future therapeutic perspectives. Int. J. Mol. Sci. 2017, 18, 2619. [Google Scholar] [CrossRef]
- Arjomand Fard, N.; Bording-Jorgensen, M.; Wine, E. A potential role for gut microbes in mediating effects of omega-3 fatty acids in inflammatory bowel diseases: A comprehensive review. Curr. Microbiol. 2023, 80, 363. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T.; Mousavi, S.M.; Hamedi, M.; Khodaiyan, F. Determination and characterization of kernel biochemical composition and functional compounds of Persian walnut oil. J. Food Sci. Technol. 2011, 51, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Crews, C.; Hough, P.; Godward, J.; Brereton, P.; Lees, M.; Guiet, S.; Winkelmann, W. Study of the Main Constituents of Some Authentic Hazelnut Oils. J. Agric. Food Chem. 2005, 53, 4843–4852. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Comparative study of chemical compositions and antioxidant capacities of oils obtained from two species of walnut: Juglans regia and Juglans sigillata. Food Chem. 2018, 279, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, W.; Li, Q.; Cui, M.; Shen, D.; Shu, J.; Mo, R.; Liu, Y. Evaluation of Lipid Quality in Fruit: Utilizing Lipidomic Approaches for Assessing the Impact of Biotic Stress on Pecans (Carya illinoinensis). Foods 2024, 13, 974. [Google Scholar] [CrossRef] [PubMed]
- Miraliakbari, H.; Shahidi, F. Antioxidant activity of minor components of tree nut oils. Food Chem. 2008, 111, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Peng, W.; Ouyang, H.; Liu, X.; Wang, P.; Yu, X.; Xie, T.; Li, S. Exploration of markers in oxidized rancidity walnut kernels based on lipidomics and volatolomics. Food Res. Int. 2024, 182, 114141. [Google Scholar] [CrossRef] [PubMed]
- Ion, T.; Sina, C. Total phenolic content, antioxidant capacity and individual phenolic compounds of defatted kernel from different cultivars of walnut. Erwerbs-Obstbau 2020, 62, 309–314. [Google Scholar]
- Liu, R.; Zhao, Z.; Dai, S.; Che, X.; Liu, W. Identification and Quantification of Bioactive Compounds in Diaphragma juglandis Fructus by UHPLC-Q-Orbitrap HRMS and UHPLC-MS/MS. J. Agric. Food Chem. 2019, 67, 3811–3825. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Kan, H.; Chen, S.X.; Thakur, K.; Wang, S.; Zhang, J.G.; Shang, Y.F.; Wei, Z.J. Comparison of phenolic compounds extracted from Diaphragma juglandis fructus, walnut pellicle, and flowers of Juglans regia using methanol, ultrasonic wave, and enzyme assisted-extraction. Food Chem. 2020, 321, 126672. [Google Scholar] [CrossRef]
- Luo, J.-J.; Yang, B.; Zeng, Y.; Li, C. Chemical constituents from the flower of Juglans regia. Zhong Yao Cai = Zhongyaocai = J. Chin. Med. Mater. 2012, 35, 1614–1616. [Google Scholar]
- Lu, Z.K.; Wu, Q.Z.; Zhang, J.; Mao, X.Y. Evaluation of phenolic content and in vitro antioxidant and antibacterial activity of extract from green walnut husks. Food Sci. 2023, 44, 79–87. [Google Scholar]
- Dandan, L.I.; Junsong, Z.H.; Zhang, X.; Min, C.H.; Yiting, G.U.; Haile, M.A. Nutritional Evaluation of Walnut Protein and Its Ameliorative Effect on DSS Induced Acute Colitis in Mice. Int. Syst. Agric. Sci. Technol. 2022, 43, 372–379. [Google Scholar]
- Wang, S.; Zheng, L.; Zhao, T.; Zhang, Q.; Liu, Y.; Sun, B.; Su, G.; Zhao, M. Inhibitory Effects of Walnut (Juglans regia) Peptides on Neuroinflammation and Oxidative Stress in Lipopolysaccharide-Induced Cognitive Impairment Mice. J. Agric. Food Chem. 2020, 68, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Wang, Y.; Chen, F.; Xiao, T.; Zhang, L. Polysaccharides from Diaphragma juglandis fructus: Extraction optimization, antitumor, and immune-enhancement effects. Int. J. Biol. Macromol. 2018, 115, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hou, D.; Chen, X.; Li, D.; Zhu, L.; Zhang, Y.; Li, J.; Bian, Z.; Liang, X.; Cai, X.; et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: Evidence of cross-kingdom regulation by microRNA. Cell Res. 2012, 22, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Kim, Y.S.; Lee, J.; Lee, J.H.; Choi, S.W.; Kim, Y. Compositional analysis of walnut lipid extracts and properties as an anti-cancer stem cell regulator via suppression of the self-renewal capacity. Food Sci. Biotechnol. 2016, 25, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Igarashi, K.; Uchihara, T.; Jishage, K.-I.; Tomita, H.; Inaba, A.; Li, Y.; Arita, M.; Suzuki, H.; Mizusawa, H.; et al. Delayed-onset ataxia in mice lacking α-tocopherol transfer protein: Model for neuronal degeneration caused by chronic oxidative stress. Proc. Natl. Acad. Sci. USA 2001, 98, 15185–15190. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R. Bioactivity of vitamin E. Nutr. Res. Rev. 2006, 19, 174–186. [Google Scholar] [CrossRef]
- Amaral, J.S.; Casal, S.; Pereira, J.A.; Seabra, R.M.; Oliveira, B.P. Determination of sterol and fatty acid compositions, oxidative stability, and nutritional value of six walnut (Juglans regia L.) cultivars grown in Portugal. J. Agric. Food Chem. 2003, 51, 7698–7702. [Google Scholar] [CrossRef]
- Zhang, J.J.; Gao, Y.; Xu, X.; Zhao, M.L.; Xi, B.N.; Shu, Y.; Li, C.; Shen, Y. In Situ Rapid Analysis of Squalene, Tocopherols, and Sterols in Walnut Oils Based on Supercritical Fluid Chromatography–Quadrupole Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2023, 71, 16371–16380. [Google Scholar] [CrossRef]
- Wen, S.; He, L.; Zhong, Z.; Zhao, R.; Weng, S.; Mi, H.; Liu, F. Stigmasterol restores the balance of Treg/Th17 cells by activating the butyrate-PPARγ axis in colitis. Front. Immunol. 2021, 12, 741934. [Google Scholar] [CrossRef]
- Lee, I.-A.; Kim, E.-J.; Kim, D.-H. Inhibitory Effect of β-Sitosterol on TNBS-Induced Colitis in Mice. Planta Medica 2012, 78, 896–898. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Dai, Z.; Liu, A.; Wang, H.; Chen, J.; Luo, Z.; Yang, C.S. β-Sitosterol and stigmasterol ameliorate dextran sulfate sodium-induced colitis in mice fed a high fat Western-style diet. Food Funct. 2017, 8, 4179–4186. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Wang, L.; Belwal, T.; Li, L.; Luo, Z. Phytosterols extraction from hickory (Carya cathayensis Sarg.) husk with a green direct citric acid hydrolysis extraction method. Food Chem. 2020, 315, 126217. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Cheong, L.-Z.; Wang, H.; Man, Q.-Q.; Pang, S.-J.; Li, Y.-Q.; Ren, B.; Wang, Z.; Zhang, J. Characterization of phospholipid profiles in six kinds of nut using HILIC-ESI-IT-TOF-MS system. Food Chem. 2018, 240, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap–Orbitrap mass spectrometry. Food Chem. 2013, 152, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Piwowarski, J.P.; Kiss, A.K.; Granica, S.; Moeslinger, T. Urolithins, gut microbiota-derived metabolites of ellagitannins, inhibit LPS-induced inflammation in RAW 264.7 murine macrophages. Mol. Nutr. Food Res. 2015, 59, 2168–2177. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Li, X.F.; Gao, H.Y.; Fang, X.J. The Analysis of Chemical Composition and Antioxidant Activities of Phenolic Compounds from Carya (Carya Cathayensis) Kernel. J. Nucl. Agric. Sci. 2013, 27, 0061–0067. [Google Scholar]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Yan, H.P.; Yu, L.Y.; Chen, X.H.; Zhong, Q.L. The anti-inflammatory, antifibrotic and antibacterial function of Amentoflavone in radiation proctitis via NF-κB pathway. Chin. J. Gastroenterol. Hepatol. 2023, 32, 367–373. [Google Scholar]
- Su, Y.; Miao, R.R.; Yang, C.; Zuo, G.X.; Gong, K.D.; Shi, C.; Dong, H.N.; Yang, L.T.; Yan, X.F.; Peng, X.Q.; et al. Study on Functional Recovery of Dandelion Flavonoid in Mice Induced by DSS. Shandong Chem. Ind. 2020, 53, 143–144. [Google Scholar]
- Jurd, L. Plant Polyphenols. III. The Isolation of a New Ellagitannin from the Pellicle of the Walnut. J. Am. Chem. Soc. 1958, 80, 2249–2252. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Salvatore, S.; Del Rio, D.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. Mol. Nutr. Food Res. 2006, 50, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.-P.; Cai, L.; Chen, Y.; Li, Y.; Wang, Y.-R.; Liu, C.-S.; Ding, Z.-T. Tannins and Antioxidant Activities of the Walnut (Juglans regia) Pellicle. Nat. Prod. Commun. 2015, 10, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Kiasalari, Z.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Azadi-Ahmadabadi, E.; Esmaeil-Jamaat, E.; Fahanik-Babaei, J.; Fakour, M.; Fereidouni, F.; Ghasemi-Tarie, R.; Jalalzade-Ogvar, S.; et al. Ellagic acid ameliorates neuroinflammation and demyelination in experimental autoimmune encephalomyelitis: Involvement of NLRP3 and pyroptosis. J. Chem. Neuroanat. 2020, 111, 101891. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Kulkarni, V.H.; Chakraborty, M.; Habbu, P.V.; Ray, A. Ellagic acid restored lead-induced nephrotoxicity by anti-inflammatory, anti-apoptotic and free radical scavenging activities. Heliyon 2021, 7, e05921. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.J.; Teuber, S.S.; Gobeille, A.; Cremin, P.; Waterhouse, A.L.; Steinberg, F.M. Walnut Polyphenolics Inhibit In Vitro Human Plasma and LDL Oxidation. J. Nutr. 2001, 131, 2837–2842. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Liu, R.Q.; Sun, E.; Zhong, R.L.; Wei, L.F. Preparation of mixed micelles of juglone F127/TPGS and their therapeutic effects on ulcerative colit. Northwest Pharm. J. 2024, 39, 70–76. [Google Scholar]
- Lu, Y.; Li, W.; Cui, J.W.; Xu, X.; Wang, G. Influence of juglone on adhesion and activities of matrix metalloproteinases in human colon carcinoma HCT-8 cells. J. Jilin Univ. (Med. Ed.) 2012, 38, 4. [Google Scholar]
- Mao, X.; Hua, Y. Composition, Structure and Functional Properties of Protein Concentrates and Isolates Produced from Walnut (Juglans regia L.). Int. J. Mol. Sci. 2012, 13, 1561–1581. [Google Scholar] [CrossRef]
- Martínez, M.L.; Labuckas, D.O.; Lamarque, A.L.; Maestri, D.M. Walnut (Juglans regia L.): Genetic resources, chemistry, by-products. J. Sci. Food Agric. 2010, 90, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- United States Dietary Guidelines Advisory Committee. Dietary Guidelines for Americans, 2010; US Department of Health and Human Services, US Department of Agriculture: Washington, DC, USA, 2010. [Google Scholar]
- Li, X.; Guo, M.; Chi, J.; Ma, J. Bioactive Peptides from Walnut Residue Protein. Molecules 2020, 25, 1285. [Google Scholar] [CrossRef] [PubMed]
- Grancieri, M.; Martino, H.S.; Gonzalez de Mejia, E. Protein digests and pure peptides from chia seed prevented adipogenesis and inflammation by inhibiting PPARγ and NF-κB pathways in 3T3L-1 adipocytes. Nutrients 2021, 13, 176. [Google Scholar] [CrossRef] [PubMed]
- Grancieri, M.; Martino, H.S.D.; de Mejia, E.G. Digested total protein and protein fractions from chia seed (Salvia hispanica L.) had high scavenging capacity and inhibited 5-LOX, COX-1-2, and iNOS enzymes. Food Chem. 2019, 289, 204–214. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Li, S.; Zhou, J.; Xu, J. Physico-chemical properties, antioxidant activities and antihypertensive effects of walnut protein and its hydrolysate. J. Sci. Food Agric. 2015, 96, 2579–2587. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, H.; Li, Y.; Jiang, L.; Zhou, Y. Study on Antioxdative Activities of Alcalase Hydrolysate of Walnut Proteins. Acta Agric. Boreali-Occident. Sin. 2010. [Google Scholar]
- Zhi, T.; Hong, D.; Zhang, Z.; Li, S.; Xia, J.; Wang, C.; Wu, Y.; Jia, Y.; Ma, A. Anti-inflammatory and gut microbiota regulatory effects of walnut protein derived peptide LPF in vivo. Food Res. Int. 2022, 152, 110875. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lin, L.; Li, C.; Zheng, J.; Chen, B.; Shen, Y.; Ren, D. Amelioration of walnut-derived novel peptides against d-galactose-induced cognitive impairment by modulating the gut microbiota composition. Food Funct. 2023, 14, 4228–4241. [Google Scholar] [CrossRef]
- Xia, W.; Gao, Y.; Fang, X.; Jin, L.; Liu, R.; Wang, L.-S.; Deng, Y.; Gao, J.; Yang, H.; Wu, W.; et al. Simulated gastrointestinal digestion of walnut protein yields anti-inflammatory peptides. Food Chem. 2024, 445, 138646. [Google Scholar] [CrossRef]
- Zhou, X.; Peng, X.; Pei, H.; Chen, Y.; Meng, H.; Yuan, J.; Xing, H.; Wu, Y. An overview of walnuts application as a plant-based. Front. Endocrinol. 2022, 13, 1083707. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Huang, G. Extraction, purification and antioxidant activity of Juglans regia shell polysaccharide. Chem. Biol. Technol. Agric. 2023, 10, 75. [Google Scholar] [CrossRef]
- Wang, G.; Yan, X.; Yang, X.; Feng, L.; Pang, H.; Zhang, R.; Zhang, Y. Structural characterization and immunomodulatory activity of an acidic polysaccharide from walnut green husk. J. Funct. Foods 2023, 110, 105877. [Google Scholar] [CrossRef]
- Xu, S.S.; Cao, K.L.; Liu, W.Y.; Lu, J.; Gu, R.Z.; Cai, M.Y. Research Progress of Synergistic Effects between Bioactive Peptides and Non-peptide Active Substances. Food Ind. 2024. [Google Scholar]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A Comprehensive Review on the Chemical Constituents and Functional Uses of Walnut (Juglans spp.) Husk. Int. J. Mol. Sci. 2019, 20, 3920. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lv, L.; Shi, S.; Cai, G.; Yu, L.; Xu, S.; Zhu, T.; Su, X.; Mao, N.; Zhang, Y.; et al. Polysaccharide from walnut green husk alleviates liver inflammation and gluconeogenesis dysfunction by altering gut microbiota in ochratoxin A-induced mice. Carbohydr. Polym. 2023, 322, 121362. [Google Scholar] [CrossRef] [PubMed]
- Barekat, S.; Nasirpour, A.; Keramat, J.; Dinari, M.; Meziane-Kaci, M.; Paris, C.; Desobry, S. Phytochemical composition, antimicrobial, anticancer properties, and antioxidant potential of green husk from several walnut varieties (Juglans regia L.). Antioxidants 2022, 12, 52. [Google Scholar] [CrossRef]
- Ventura, G.; Mesto, D.; Blasi, D.; Cataldi, T.R.I.; Calvano, C.D. The Effect of Milling on the Ethanolic Extract Composition of Dried Walnut (Juglans regia L.) Shells. Int. J. Mol. Sci. 2023, 24, 13059. [Google Scholar] [CrossRef]
- Zhang, D.; Ye, N.; Li, M.; Dai, G.; Ma, Y.; Wang, Y.; Liu, C.; Ma, H. Walnut green husk extract enhances the effect of chlorine dioxide on kernel quality and antioxidant properties of fresh-eating walnuts during their shelf life. Food Chem. 2023, 428, 136797. [Google Scholar] [CrossRef]
- Maguire, L.S.; O’sullivan, S.M.; Galvin, K.; O’connor, T.P.; O’brien, N.M. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int. J. Food Sci. Nutr. 2004, 55, 171–178. [Google Scholar] [CrossRef]
| Anti-Inflammatory Classification | Compound Name | Content | References | |
|---|---|---|---|---|
| Lipids and lipophilic bioactive compounds | Polyunsaturated fatty acids | ω-3 PUFA α-linolenic acid | 10–18% | [20] |
| ω-9 monounsaturated fatty acid (MUFA) oleic acid | 11.26–25.09% | |||
| Tocopherols | γ-tocopherol | 315.3–351.2 mg/kg | [62] | |
| α-tocopherol | 25.5–40.3 mg/kg | |||
| δ-tocopherol | 16.3–25.1 mg/kg | |||
| β-tocopherol | 2.1–4.05 mg/kg | |||
| Phytosterols | β-sitosterol | 868.84–1385.18 mg/kg | [63,64] | |
| campesterol | 16.87–71.07 mg/kg | |||
| stigmasterol | 24.29–40.65 mg/kg | |||
| Sphingolipids | ceramides | - | [65,66] | |
| glycosphingolipids | - | |||
| hexosylceramides | - | |||
| Phospholipids | phosphatidylcholine | PC (34:2) 1103.4 μg/mL | [67] | |
| phosphatidylethanolamine | PE (34:2) 1713.7 μg/ML PE (36:4) 1023.5 μg/mL | |||
| phosphatidylglycerol | PG (34:2) 304.4 μg/mL | |||
| phosphatidylinositol | PI (34:2) 2164 μg/mL | |||
| phosphatidylserine | - | |||
| lysophosphatidylcholine | - | |||
| lysophosphatidylethanolamine | - | |||
| Phenolic compounds | Phenolic acids | cinnamic acid | 213.38 µg/g | [68] |
| tannic acid | 312.57 µg/g, 1023.9 µg/g | |||
| Flavonoids | 7-hydroxymethylcoumarin | 245.3 mg/g | [69] | |
| eugenol | 165.7 µg/g | |||
| apigenin | 74.21 µg/g | |||
| catechin | 44.21 µg/g | |||
| Tannins | HHDP-glucose Isomer | 259.17 µg/g | [70,71] | |
| bis-HHDP-glucose | 349.44 µg/g | |||
| Junglone | juglone | 283.4 µg/mg | [72] | |
| Proteins and peptides | Proteins and protein hydrolyses | albumin | [73] | |
| globulin | ||||
| protoprotein | ||||
| gluten | ||||
| Bioactive peptides | LPF | [74] | ||
| GVYY | ||||
| APTLW | ||||
| Polysaccharides | Polysaccharides | xylose, trehalose, and mannose | [75,76] | |
| rhamnose, arabinose, galactose, glucose, xylose, and galacturonic acid | 6.7%:16.5%:28.3%:11.2%:12.5%:24.8% | |||
| galacturonic acid, galactose, rhamnose, arabinose, glucose, glucuronic acid, xylose, fucose, fucose, and mannose | 69.47%:11.18%:8.67%:3.96%:2.21% :2.28%:0.83%:0.81%:0.59%:0.59% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, K.; Agarwal, N.; Rodriguez-Palacios, A.; Basson, A.R. Regulation of Intestinal Inflammation by Walnut-Derived Bioactive Compounds. Nutrients 2024, 16, 2643. https://doi.org/10.3390/nu16162643
Dai K, Agarwal N, Rodriguez-Palacios A, Basson AR. Regulation of Intestinal Inflammation by Walnut-Derived Bioactive Compounds. Nutrients. 2024; 16(16):2643. https://doi.org/10.3390/nu16162643
Chicago/Turabian StyleDai, Kexin, Neel Agarwal, Alexander Rodriguez-Palacios, and Abigail Raffner Basson. 2024. "Regulation of Intestinal Inflammation by Walnut-Derived Bioactive Compounds" Nutrients 16, no. 16: 2643. https://doi.org/10.3390/nu16162643
APA StyleDai, K., Agarwal, N., Rodriguez-Palacios, A., & Basson, A. R. (2024). Regulation of Intestinal Inflammation by Walnut-Derived Bioactive Compounds. Nutrients, 16(16), 2643. https://doi.org/10.3390/nu16162643






