Association between Bisphenol A and Prostate-Specific Antigen (PSA) among U.S. Older Males: National Health and Nutrition Examination Survey (NHANES), 2003–2012
Abstract
1. Introduction
2. Materials and Method
2.1. Data Availability
2.2. Study Population
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Selected Participants Subsection
3.2. The Connection between BPA Concentrations and PSA
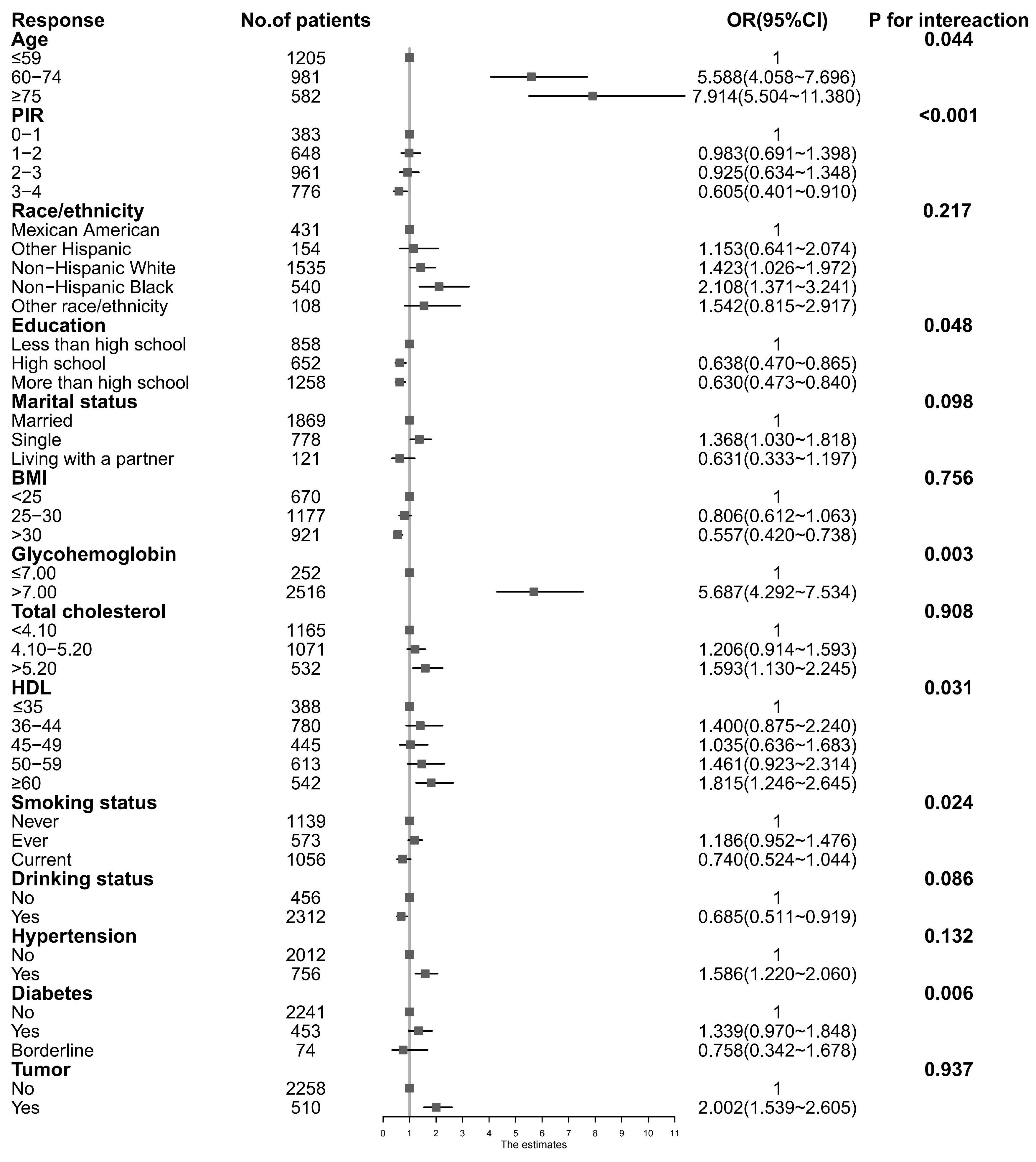
3.3. Stratified Associations between BPA Concentrations and PSA
3.4. Identification of Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhou, X.; Ren, X.; Zhang, X.; Wu, J.; Wang, S.; Wang, Z. Animal Toxicology Studies on the Male Reproductive Effects of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin: Data Analysis and Health Effects Evaluation. Front. Endocrinol. 2021, 12, 696106. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, I.; Fiory, F.; Perruolo, G.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. Int. J. Mol. Sci. 2020, 21, 5761. [Google Scholar] [CrossRef] [PubMed]
- Belluti, S.; Imbriano, C.; Casarini, L. Nuclear Estrogen Receptors in Prostate Cancer: From Genes to Function. Cancers 2023, 15, 4653. [Google Scholar] [CrossRef] [PubMed]
- Abbott, D.A.; Mancini, M.G.; Bolt, M.J.; Szafran, A.T.; Neugebauer, K.A.; Stossi, F.; Gorelick, D.A.; Mancini, M.A. A novel ERβ high throughput microscopy platform for testing endocrine disrupting chemicals. Heliyon 2023, 10, e23119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Exploring the Therapeutic Effects of Bisphenol-A on Prostate Cancer. Int. J. Res. Eng. 2018, 5, 475–479. [Google Scholar] [CrossRef]
- Li, X.; Ni, M.; Yang, Z.; Chen, X.; Zhang, L.; Chen, J. Bioinformatics analysis and quantitative weight of evidence assessment to map the potential mode of actions of bisphenol A. Environ. Pollut. 2021, 273, 116469. [Google Scholar] [CrossRef]
- Guan, T.; Sun, Y.; Wang, Y.; Li, Z.; Li, T.; Hou, L. Multi-Residue Method for the Analysis of Stilbene Estrogens in Milk. Int. J. Mol. Sci. 2019, 20, 744. [Google Scholar] [CrossRef]
- Fritschi, L.; Glass, D.C.; Tabrizi, J.S.; Leavy, J.E.; Ambrosini, G.L. Occupational risk factors for prostate cancer and benign prostatic hyperplasia: A case-control study in Western Australia. Occup. Environ. Med. 2007, 64, 60–65. [Google Scholar] [CrossRef]
- Grossman, D.C.; Curry, S.J.; Owens, D.K.; Bibbins-Domingo, K.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Ebell, M.; Epling, J.W., Jr.; Kemper, A.R.; et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 319, 1901–1913. [Google Scholar]
- Ballentine Carter, H. Prostate-Specific Antigen (PSA) Screening for Prostate Cancer. JAMA 2018, 319, 1866–1868. [Google Scholar] [CrossRef] [PubMed]
- Tarafdar, A.; Sirohi, R.; Balakumaran, P.A.; Reshmy, R.; Madhavan, A.; Sindhu, R.; Binod, P.; Kumar, Y.; Kumar, D.; Sim, S.J. The hazardous threat of Bisphenol A: Toxicity, detection and remediation. J. Hazard. Mater. 2022, 423 Pt A, 127097. [Google Scholar] [CrossRef]
- Di Donato, M.; Cernera, G.; Giovannelli, P.; Galasso, G.; Bilancio, A.; Migliaccio, A.; Castoria, G. Recent advances on bisphenol-A and endocrine disruptor effects on human prostate cancer. Mol. Cell. Endocrinol. 2017, 457, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.A. Prostate Cancer Incidence and Survival, by Stage and Race/Ethnicity-United States, 2001–2017. Morb. Mortal. Wkly. Rep. 2020, 69, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Shafei, A.; Ramzy, M.M.; Hegazy, A.I.; Husseny, A.K.; El-Hadary, U.G.; Taha, M.M.; Mosa, A.A. The molecular mechanisms of action of the endocrine disrupting chemical bisphenol A in the development of cancer. Gene 2018, 647, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Gupta, L.; Thakur, H.; Sobti, R.C.; Seth, A.; Singh, S.K. Role of genetic polymorphism of estrogen receptor-α gene and risk of prostate cancer in north Indian population. Mol. Cell. Biochem. 2009, 335, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Eyrich, N.W.; Morgan, T.M.; Tosoian, J.J. Biomarkers for detection of clinically significant prostate cancer: Contemporary clinical data and future directions. Transl. Androl. Urol. 2021, 10, 3091–3103. [Google Scholar] [CrossRef] [PubMed]
- Hond, E.D.; Tournaye, H.; De Sutter, P.; Ombelet, W.; Baeyens, W.; Covaci, A.; Cox, B.; Nawrot, T.S.; Van Larebeke, N.; D’Hooghe, T. Human exposure to endocrine disrupting chemicals and fertility: A case–control study in male subfertility patients. Environ. Int. 2015, 84, 154–160. [Google Scholar] [CrossRef]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. ESMO Guidelines Committee. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef]
- Discacciati, A.; Orsini, N.; Wolk, A. Body mass index and incidence of localized and advanced prostate cancer―A dose-response meta-analysis of prospective studies. Ann. Oncol. 2012, 23, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, H.; Yang, W.; Lee, J.H. Urinary concentrations of bisphenol A and triclosan and associations with demographic factors in the Korean population. Environ. Res. 2011, 111, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Mandrup, K.; Boberg, J.; Isling, L.K.; Christiansen, S.; Hass, U. Low-dose effects of bisphenol a on mammary gland development in rats. Andrology 2016, 4, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Prins, G.S.; Hu, W.Y.; Shi, G.B.; Hu, D.P.; Majumdar, S.; Li, G.; Huang, K.; Nelles, J.L.; Ho, S.-M.; Walker, C.L.; et al. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology 2014, 155, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Terracciano, D.; La Civita, E.; Athanasiou, A.; Liotti, A.; Fiorenza, M.; Cennamo, M.; Crocetto, F.; Tennstedt, P.; Schiess, R.; Haese, A.; et al. New strategy for the identification of prostate cancer: The combination of Proclarix and the prostate health index. Prostate 2022, 82, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.; Zhang, C.; Zhu, Q.; Zhao, J.; Zong, H.; Deng, Q.; Zheng, J.; Wu, E.; Wu, R.; et al. Association Between Urinary Bisphenols and Body Composition Among American Adults: Cross-Sectional National Health and Nutrition Examination Survey Study. JMIR Public Health Surveill. 2023, 9, e49652. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Tian, L.; Jia, B.; Wang, M.; Xiong, M.; Hu, B.; Deng, C.; Hou, Y.; Hou, T.; Yang, X.; et al. Association between Serum Triglycerides and Prostate Specific Antigen (PSA) among U.S. Males: National Health and Nutrition Examination Survey (NHANES), 2003–2010. Nutrients 2022, 14, 1325. [Google Scholar] [CrossRef]
- Meeker, J.D.; Ehrlich, S.; Toth, T.L.; Wright, D.L.; Calafat, A.M.; Trisini, A.T.; Ye, X.; Hauser, R. Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod. Toxicol. 2010, 30, 532–539. [Google Scholar] [CrossRef]
- Gentile, F.; La Civita, E.; Della Ventura, B.; Ferro, M.; Cennamo, M.; Bruzzese, D.; Crocetto, F.; Velotta, R.; Terracciano, D. A Combinatorial Neural Network Analysis Reveals a Synergistic Behaviour of Multiparametric Magnetic Resonance and Prostate Health Index in the Identification of Clinically Significant Prostate Cancer. Clin. Genitourin. Cancer 2022, 20, e406–e410. [Google Scholar] [CrossRef]

| Urinary BPA Concentrations (ng/mL) | Overall | Q1 | Q2 | Q3 | Q4 | p-Value |
|---|---|---|---|---|---|---|
| N | 2768 | 703 | 712 | 667 | 686 | |
| PSA ng/mL | 3.21 ± 6.24 | 2.59 ± 3.53 | 2.80 ± 5.12 | 3.03 ± 6.72 | 4.46 ± 8.43 | <0.001 |
| Sociodemographic variables | ||||||
| Age, mean ± SD (years) | 62.70 ± 12.93 | 63.09 ± 12.60 | 62.64 ± 12.78 | 61.46 ± 13.14 | 63.59 ± 13.13 | 0.019 |
| PIR, mean ± SD (years) | 2.79 ± 1.55 | 2.80 ± 1.52 | 2.86 ± 1.54 | 2.81 ± 1.56 | 2.71 ± 1.56 | 0.348 |
| Race/ethnicity, n (%) | 0.006 | |||||
| Mexican American | 431 (15.6) | 128 (18.2) | 101 (14.2) | 97 (14.5) | 105 (15.5) | |
| Other Hispanic | 154 (5.6) | 34 (4.8) | 37 (5.2) | 46 (6.9) | 37 (5.4) | |
| Non-Hispanic white | 1535 (55.5) | 390 (55.5) | 399 (56.0) | 357 (53.5) | 389 (56.7) | |
| Non-Hispanic black | 540 (19.5) | 115 (16.4) | 135 (19.0) | 152 (22.8) | 138 (20.1) | |
| Other race/ethnicity | 108 (3.9) | 36 (5.1) | 40 (5.6) | 15 (2.2) | 17 (2.5) | |
| Education, n (%) | 0.658 | |||||
| Less than high school | 858 (31.0) | 229 (32.6) | 217 (30.5) | 199 (29.8) | 213 (31.0) | |
| High school | 652 (23.6) | 158 (22.5) | 174 (24.4) | 168 (25.2) | 152 (22.2) | |
| More than high school | 1258 (45.4) | 316 (45.0) | 321 (45.1) | 300 (45.0) | 321 (46.8) | |
| Marital status, n (%) | 0.101 | |||||
| Married | 1869 (67.5) | 496 (70.6) | 482 (67.7) | 452 (67.8) | 439 (64.0) | |
| Single | 778 (28.1) | 176 (25.0) | 203 (28.5) | 187 (28.0) | 212 (30.9) | |
| Living with a partner | 121 (4.4) | 31 (4.4) | 27 (3.8) | 28 (4.2) | 35 (5.1) | |
| Medical examination and personal life history | ||||||
| BMI, kg/m2 | 28.74 ± 5.81 | 28.24 ± 5.46 | 28.81 ± 5.67 | 29.20 ± 6.80 | 28.71 ± 5.20 | 0.033 |
| Variables of laboratory data | ||||||
| Glycohemoglobin (%) | 5.90 ± 1.11 | 5.90 ± 1.16 | 5.92 ± 1.10 | 5.89 ± 1.12 | 5.91 ± 1.05 | 0.496 |
| Total cholesterol, mmol/L | 5.06 ± 1.10 | 5.13 ± 1.14 | 5.04 ± 1.09 | 5.01 ± 1.05 | 5.05 ± 1.13 | 0.325 |
| High-density lipoprotein, mmol/L | 1.28 ± 0.39 | 1.32 ± 0.42 | 1.27 ± 0.38 | 1.27 ± 0.39 | 1.25 ± 0.36 | 0.080 |
| Comorbidities, n (%) | ||||||
| Smoking status | 0.241 | |||||
| Never | 1056 (38.2) | 247 (35.1) | 275 (38.6) | 265 (39.7) | 269 (39.2) | |
| Current | 573 (20.7) | 151 (21.5) | 148 (20.8) | 143 (21.4) | 131 (19.1) | |
| Ever | 1139 (41.1) | 305 (43.4) | 289 (40.6) | 259 (39.7) | 286 (41.7) | |
| Drinking status | 2312 (83.5) | 580 (82.5) | 593 (83.3) | 561 (84.1) | 578 (84.3) | 0.904 |
| Yes | 2312 (83.5) | 580 (82.5) | 593 (83.3) | 561 (84.1) | 578 (84.3) | |
| No | 456 (16.5) | 123 (17.5) | 119 (16.7) | 106 (15.9) | 108 (15.7) | |
| Hypertension | 0.087 | |||||
| Yes | 756 (27.3) | 210 (29.9) | 186 (26.1) | 165 (24.7) | 195 (28.4) | |
| No | 2012 (72.7) | 493 (70.1) | 526 (79.6) | 502 (75.3) | 491 (71.6) | |
| Diabetes | 0.287 | |||||
| Yes | 453 (16.4) | 116 (16.5) | 121 (17.0) | 102 (15.3) | 114 (16.6) | |
| No | 2241 (81.0) | 566 (80.5) | 567 (79.6) | 553 (82.9) | 555 (80.9) | |
| Borderline | 74 (2.7) | 21 (3.0) | 24 (3.4) | 12 (1.8) | 17 (2.5) |
| Exposure | Non-Adjusted Model | Minimally Adjusted Model | Fully Adjusted Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | p-Value | Estimate | 95% CI | p-Value | Estimate | 95% CI | p-Value | |
| Urinary BPA | 3.416 | (2.007, 4.825) | 0.018 | 2.706 | (1.442, 3.974) | 0.037 | 2.760 | (1.506, 4.014) | 0.034 |
| Urinary BPA | |||||||||
| Q1 | Ref. | Ref. | Ref. | ||||||
| Q2 | 0.073 | (−0.118, 0.265) | 0.703 | 0.135 | (−0.033, 0.304) | 0.425 | 0.165 | (−0.002, 0.332) | 0.328 |
| Q3 | 0.232 | (0.028, 0.436) | 0.259 | 0.413 | (0.204, 0.622) | 0.053 | 0.311 | (0.235, 0.638) | 0.036 |
| Q4 | 1.070 | (0.816, 1.324) | <0.001 | 1.025 | (0.821, 1.228) | <0.001 | 1.074 | (0.862, 1.286) | <0.001 |
| p for trend | <0.001 | <0.001 | 0.011 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Gao, S.; Zhang, Y.; Xie, Z.; Wang, L.; Li, Y.; Niu, Q.; Li, H.; Guo, H.; Ma, R.; et al. Association between Bisphenol A and Prostate-Specific Antigen (PSA) among U.S. Older Males: National Health and Nutrition Examination Survey (NHANES), 2003–2012. Nutrients 2024, 16, 2589. https://doi.org/10.3390/nu16162589
Li Q, Gao S, Zhang Y, Xie Z, Wang L, Li Y, Niu Q, Li H, Guo H, Ma R, et al. Association between Bisphenol A and Prostate-Specific Antigen (PSA) among U.S. Older Males: National Health and Nutrition Examination Survey (NHANES), 2003–2012. Nutrients. 2024; 16(16):2589. https://doi.org/10.3390/nu16162589
Chicago/Turabian StyleLi, Qingyuan, Shipeng Gao, Youxian Zhang, Zhanpeng Xie, Lu Wang, Yu Li, Qiang Niu, Haiyan Li, Heng Guo, Rulin Ma, and et al. 2024. "Association between Bisphenol A and Prostate-Specific Antigen (PSA) among U.S. Older Males: National Health and Nutrition Examination Survey (NHANES), 2003–2012" Nutrients 16, no. 16: 2589. https://doi.org/10.3390/nu16162589
APA StyleLi, Q., Gao, S., Zhang, Y., Xie, Z., Wang, L., Li, Y., Niu, Q., Li, H., Guo, H., Ma, R., & He, J. (2024). Association between Bisphenol A and Prostate-Specific Antigen (PSA) among U.S. Older Males: National Health and Nutrition Examination Survey (NHANES), 2003–2012. Nutrients, 16(16), 2589. https://doi.org/10.3390/nu16162589






