Iron Deficiency and Sleep/Wake Behaviors: A Scoping Review of Clinical Practice Guidelines—How to Overcome the Current Conundrum?
Abstract
1. Introduction
| Author | Study Population | Method | Results | ||
|---|---|---|---|---|---|
| Condition | Participant Number | Age (Y): Range Mean | |||
| Adisetiyo et al., 2014 [15] | ADHD | n = 22 ADHD n = 27 controls | 8–18 12.7 13.3 | MRI imaging relaxation rates (R2, R2*, R2′) and magnetic field correlation (MFC) in the globus pallidus, putamen, caudate nucleus, and thalamus -R2, R2*, R2′ -MFC | -No difference in R values -Lower MFC in ADHD (lower brain iron) |
| Adisetiyo et al., 2019 [16] | ADHD | n = 30 ADHD n = 29 controls | 8–18 14.0 13.9 | -R2* -MFC | -No difference in R2* or MFC -Increased R2* and MFC (increased brain iron) with psychostimulant use duration in ADHD more than with age |
| Allen et al., 2001 [17] | RLS | n = 5 RLS n = 5 controls | 66.2 66.4 | -R2′ | -Lower R2′(lower brain iron) in RLS and in proportion to RLS severity |
| Astrakas et al., 2008 [18] | RLS | n = 25 RLS n = 12 controls | 55–82 66.5 54–89 65.7 | -T2 | -Higher T2 (lower brain iron) in RLS |
| Beliveau et al., 2022 [19] | RLS | n = 72 RLS n = 72 controls | 46–59 51.9 (median) 51.0 (median) | -R2, R2′, and R2* -QSM | -Higher R and QSM values (increased iron brain iron) in RLS |
| Cortese et al., 2012 [20] | ADHD | n = 18 ADHD n = 9 controls n = 9 psychiatric controls | 118.8 mo 120.8 mo 123.5 mo | -T2* | -Higher T2* (lower brain iron) in ADHD -SF and T2* values did not correlate significantly in most regions |
| Earley et al., 2006 [21] | RLS | n = 22 early-onset RLS n = 19 late-onset RLS n = 39 controls | 57.1 67.4 60.5 | -R2′ | -Lower R2′ (lower brain iron) in early-onset RLS symptoms, but not late-onset RLS |
| Godau et al., 2008 [22] | RLS | n = 6 RLS n = 19 controls | 47–68 60 59 | -T2 | -Higher T2 (lower brain iron) in RLS |
| Hasaneen et al., 2017 [23] | ADHD | n = 17 ADHD n = 18 controls | 6–15 8.4 8.5 | -R2* | -Lower R2* (lower brain iron) in ADHD which correlated with ADHD type but not with ADHD severity |
| Knake et al., 2010 [24] | RLS | n = 12 RLS n = 12 controls | 43–46 58.5 41–74 56.8 | -T2 | -No difference in T2 values |
| Li et al., 2016 [25] | RLS | n = 39 RLS n = 29 controls | 58.4 57.9 | -QSM | -Lower magnetic susceptibility (lower brain iron) in RLS and possible connection to PLMS |
| Margariti et al., 2012 [26] | RLS | n = 11 RLS n = 11 controls | 48–70 55.3 42–73 56.1 | -T2 | -Lower T2 (higher brain iron) in RLS |
| Moon et al., 2014 [27] | RLS | n = 37 RLS n = 20 early-onset RLS n = 17 late-onset RLS n = 40 RLS controls n = 20 early-onset controls n = 20 late-onset controls | 50.3 58.1 47.0 59.4 | -T2 | -Higher T2 (lower brain iron) in late-onset RLS, but not early-onset RLS |
| Moon et al., 2015 [28] | RLS | n = 37 RLS n = 40 controls | 53.8 53.2 | -R2, R2*, and R2′ | -Relaxometry and ROI determination methods significantly influenced the outcome of brain iron estimates |
| Rizzo et al., 2013 [29] | RLS | n = 15 RLS n = 15 controls | 51.0 51.0 | -Phase from gradient-echo scan | -Higher phase values (lower brain iron) in RLS |
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Data Analysis
- 1
- Population defined by age, pregnancy status, and medical conditions. Guidelines were organized into three categories: (1) general ID, (2) ID in pregnancy, and (3) disease-specific ID.General ID guidelines were defined as those guidelines which could be applied to a general population and which may have included specific subpopulations within the guideline.Disease-specific ID guidelines were defined as those guidelines which dealt with only a specific population, namely chronic disease populations, in which the diagnosis and management of ID is different from general ID guidelines.
- 1.1
- Year and country of publication.
- 2
- Associated clinical presentations, conditions, diagnoses, and risk factors for ID.
- 2.1
- If ADHD and/or RLS were included as either signs/symptoms or as being associated with ID. Guidelines that used broad terminology such as “behavioral disturbances” or “sleep disturbances” without specifying the aforementioned conditions were not classified as having included ADHD and/or RLS.
- 3.
- Suggested cutoff values for SF, taking into account age- and sex-specific cutoff values.
- 3.1
- Additional iron and hematologic biomarkers included in the guidelines. Examples of iron biomarkers (other than SF) are serum iron and transferrin saturation, while hematologic biomarkers include hemoglobin and mean corpuscular volume/mean corpuscular hemoglobin.
3. Results
3.1. Guideline Development Methodology
3.2. Types of Iron Deficiency
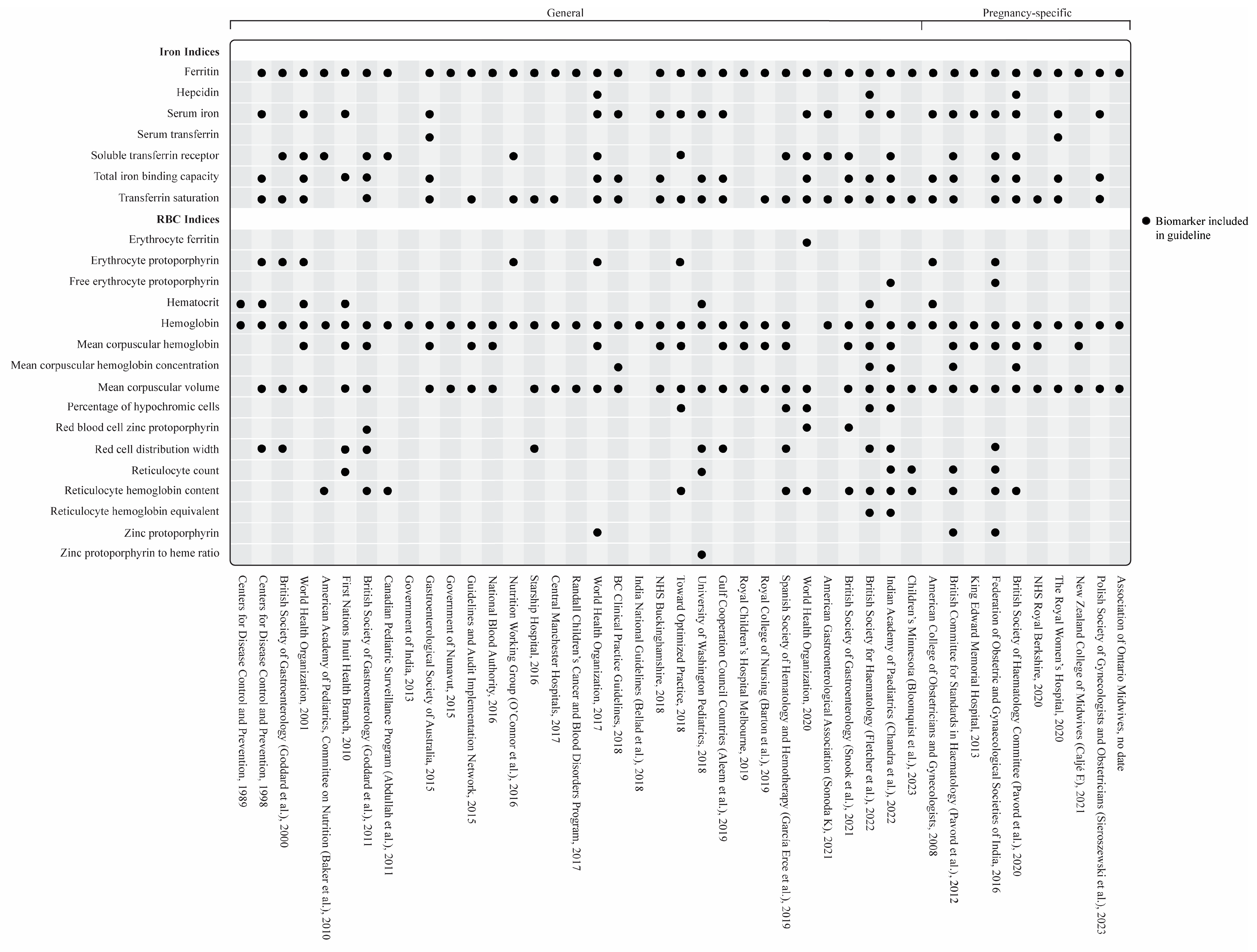
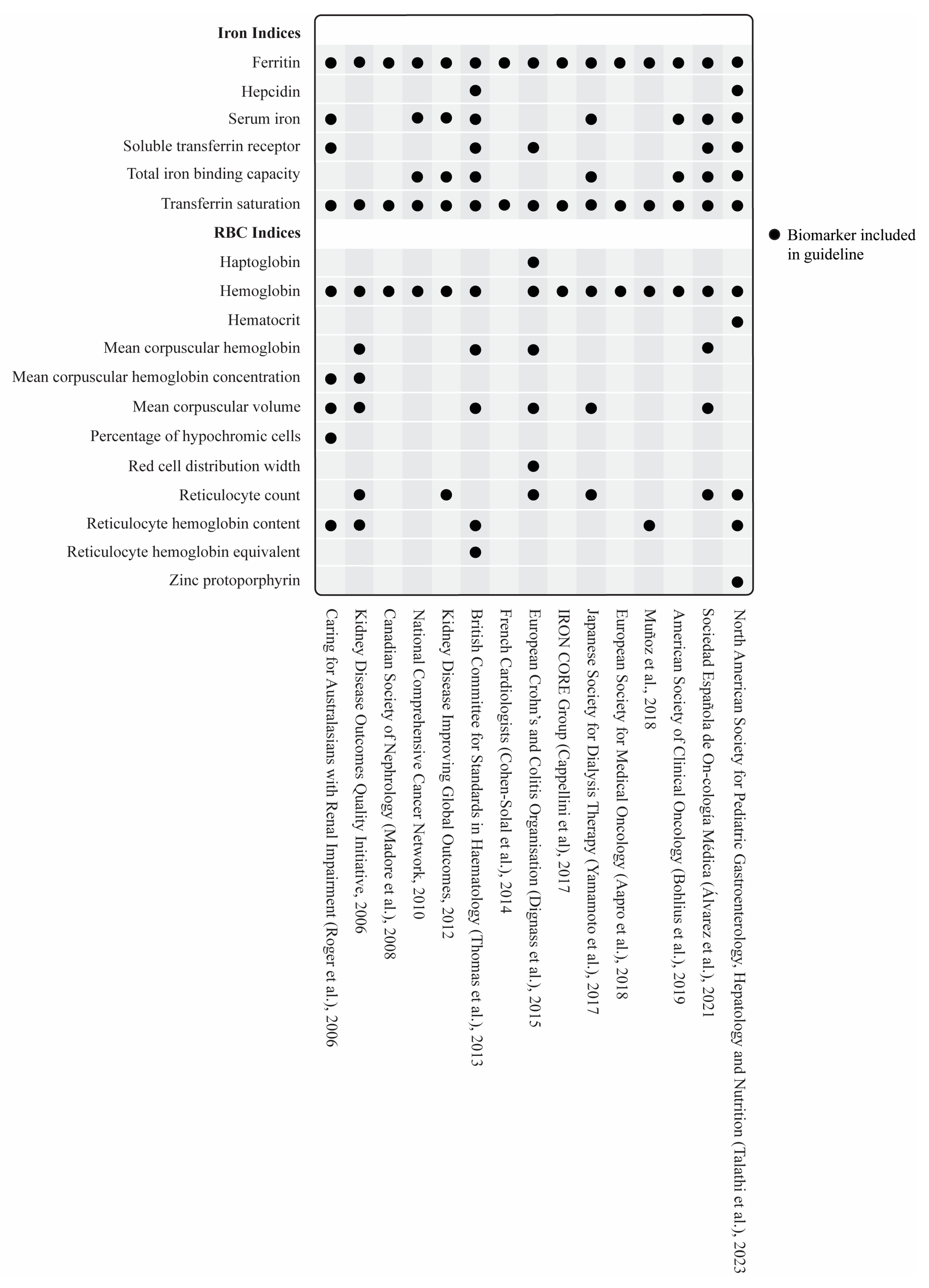
3.3. Biomarkers
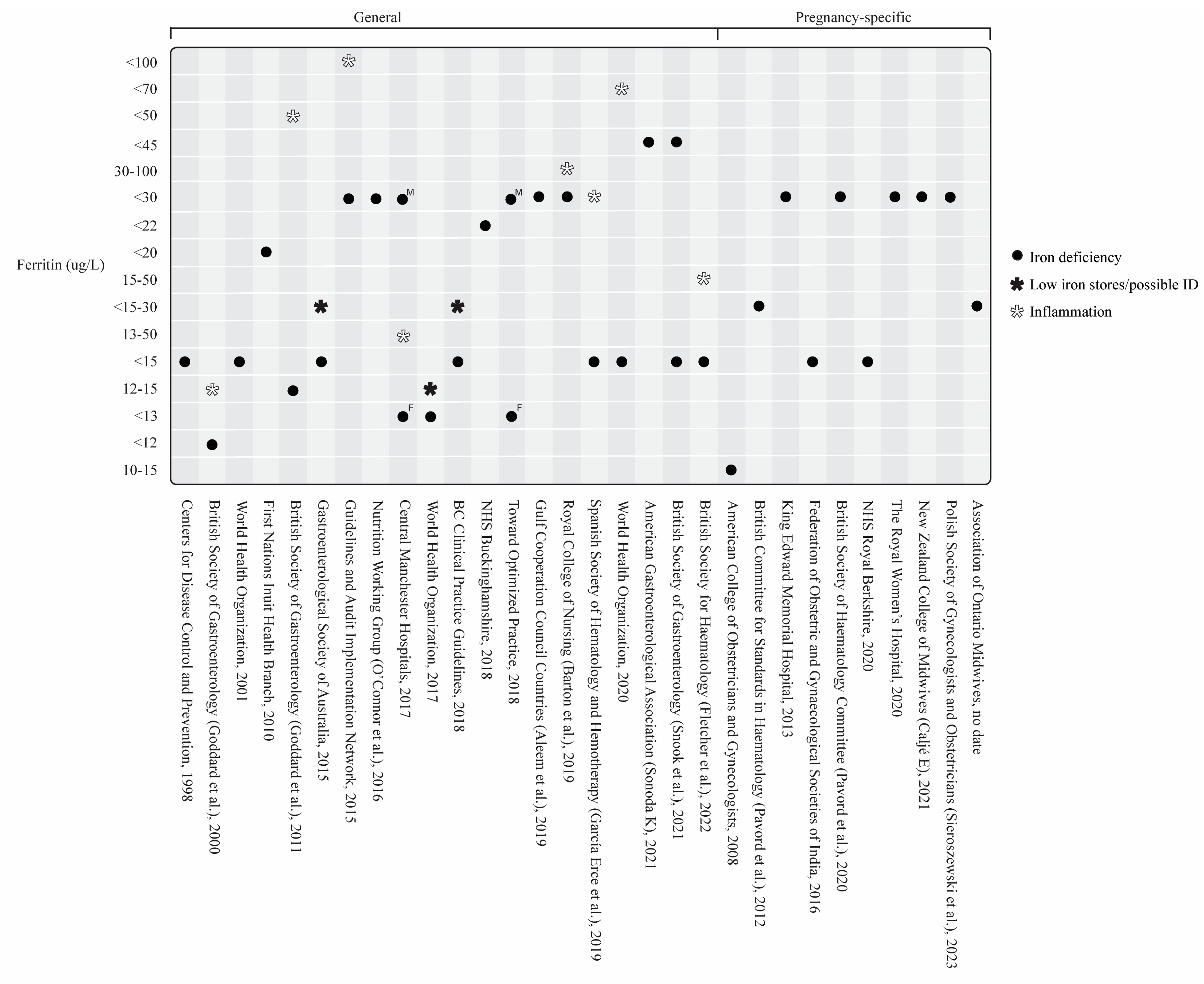
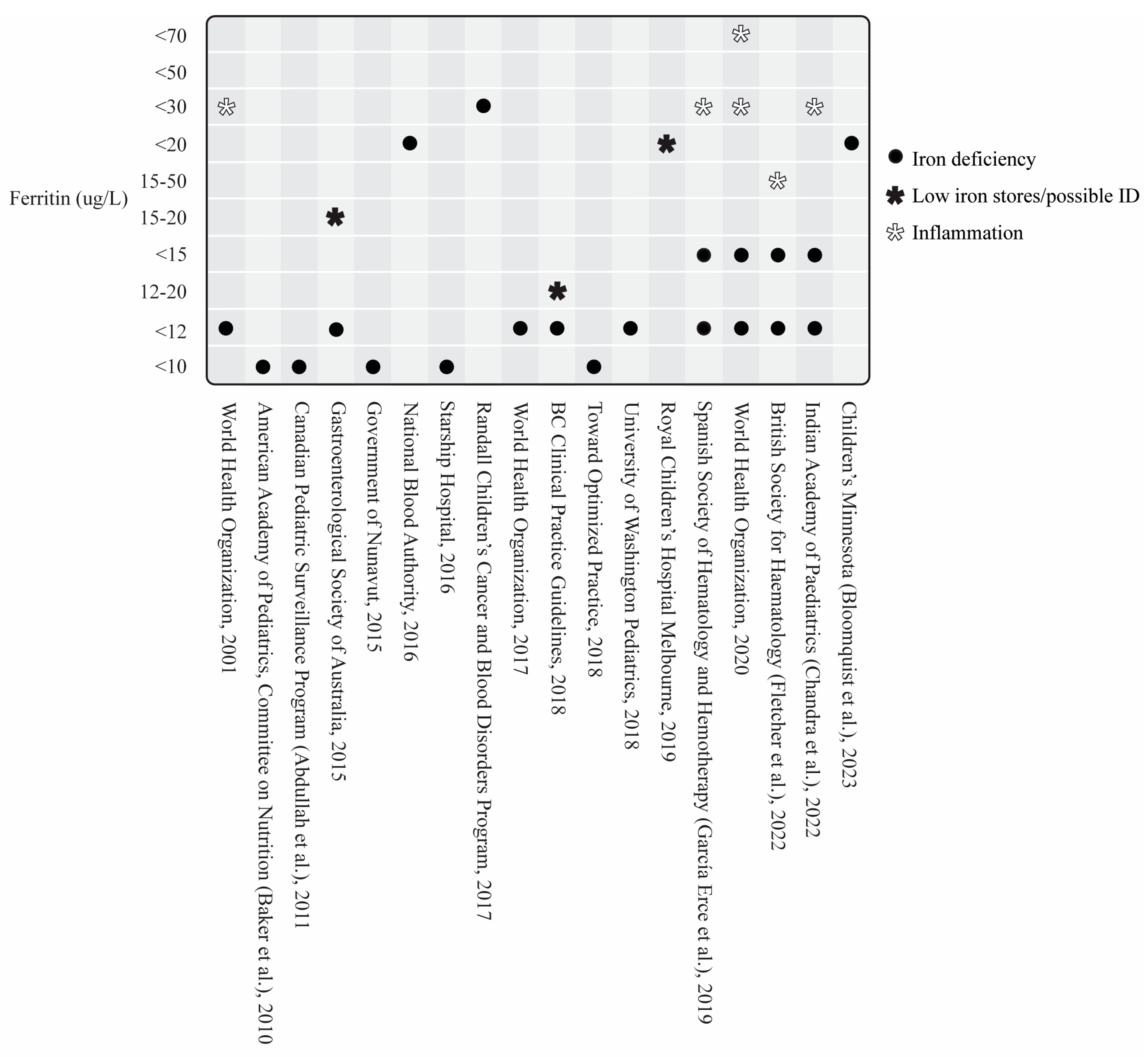
3.3.1. Serum Ferritin and Other Iron Biomarkers
General Iron Deficiency Guidelines
Iron Deficiency in Pregnancy
Iron Deficiency in Disease-Specific States
3.3.2. Hematologic Biomarkers
3.3.3. Biomarkers of Inflammation
3.4. Iron Deficiency, ADHD, and RLS
4. Discussion
4.1. Iron Deficiency
4.2. Serum Ferritin
4.3. ID-Associated Conditions
4.4. Brain Iron Imaging
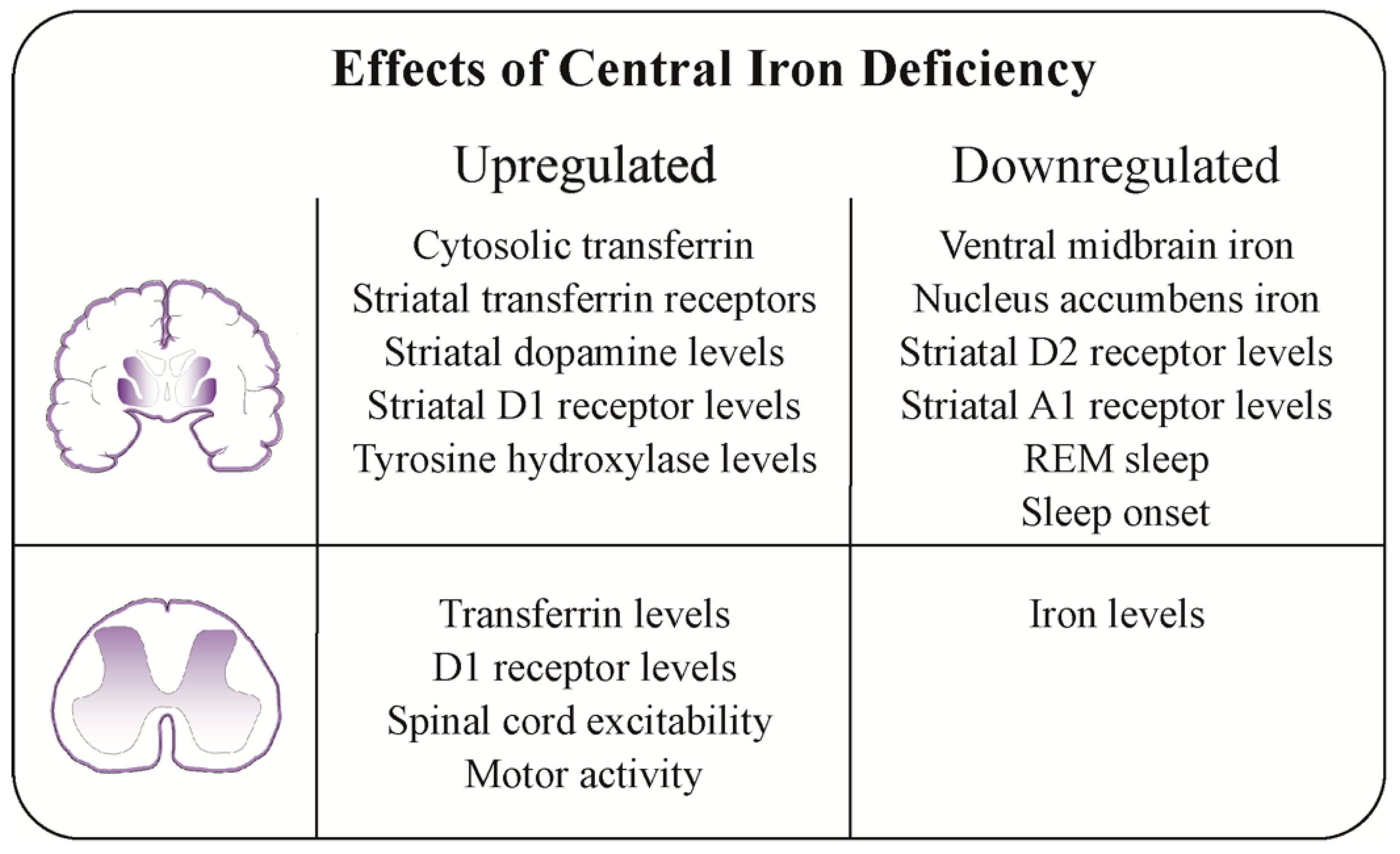
4.5. Possible Pathophysiological Mechanisms of Central Iron Deficiency
4.6. Clinical Perspectives and Future Research
5. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Section | Item | PRISMA-ScR Checklist Item | Reported on Page # |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a scoping review. | 1 |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary that includes (as applicable): background, objectives, eligibility criteria, sources of evidence, charting methods, results, and conclusions that relate to the review questions and objectives. | 1 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. Explain why the review questions/objectives lend themselves to a scoping review approach. | 2,3,5 |
| Objectives | 4 | Provide an explicit statement of the questions and objectives being addressed with reference to their key elements (e.g., population or participants, concepts, and context) or other relevant key elements used to conceptualize the review questions and/or objectives. | 5 |
| Methods | |||
| Protocol and registration | 5 | Indicate whether a review protocol exists; state if and where it can be accessed (e.g., a Web address); and if available, provide registration information, including the registration number. | 5 |
| Eligibility criteria | 6 | Specify characteristics of the sources of evidence used as eligibility criteria (e.g., years considered, language, and publication status), and provide a rationale. | 6 |
| Information sources * | 7 | Describe all information sources in the search (e.g., databases with dates of coverage and contact with authors to identify additional sources), as well as the date the most recent search was executed. | 5,6 |
| Search | 8 | Present the full electronic search strategy for at least 1 database, including any limits used, such that it could be repeated. | Table 2 |
| Selection of sources of evidence † | 9 | State the process for selecting sources of evidence (i.e., screening and eligibility) included in the scoping review. | 5,6 |
| Data charting process ‡ | 10 | Describe the methods of charting data from the included sources of evidence (e.g., calibrated forms or forms that have been tested by the team before their use, and whether data charting was carried out independently or in duplicate) and any processes for obtaining and confirming data from investigators. | 6 |
| Data items | 11 | List and define all variables for which data were sought and any assumptions and simplifications made. | 6,7 |
| Critical appraisal of individual sources of evidence § | 12 | If performed, provide a rationale for conducting a critical appraisal of included sources of evidence; describe the methods used and how this information was used in any data synthesis (if appropriate). | N/A |
| Synthesis of results | 13 | Describe the methods of handling and summarizing the data that were charted. | 6,7 |
| Results | |||
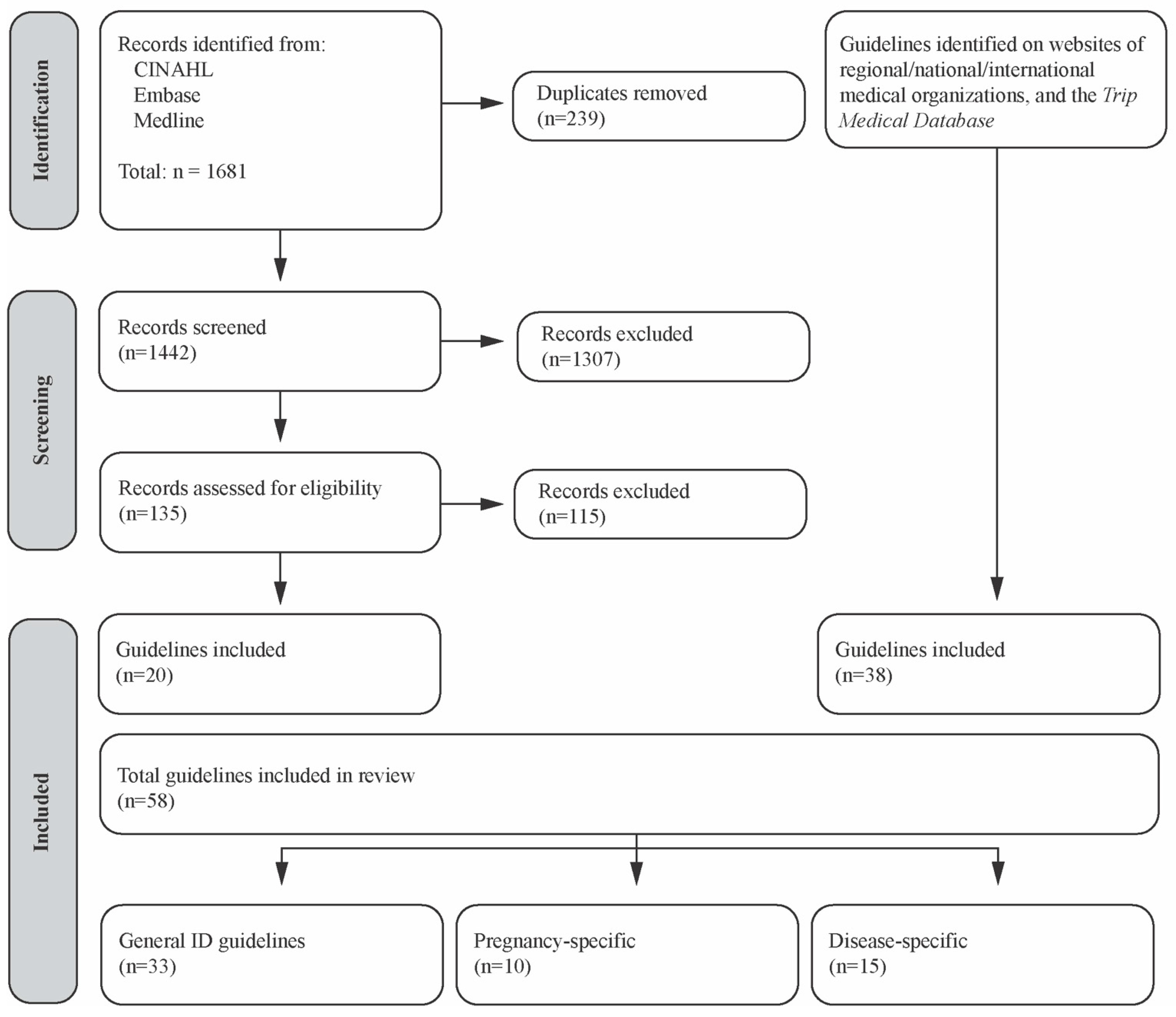
| Selection of sources of evidence | 14 | Give numbers of sources of evidence screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally using a flow diagram. | 7, Figure 2 |
| Characteristics of sources of evidence | 15 | For each source of evidence, present characteristics for which data were charted and provide the citations. | 7,8,9,11,12,13, Table A2, Table A3 and Table A4 |
| Critical appraisal within sources of evidence | 16 | If performed, present data on critical appraisal of included sources of evidence (see item 12). | N/A |
| Results of individual sources of evidence | 17 | For each included source of evidence, present the relevant data that were charted that relate to the review questions and objectives. | 7,8,9,11,12,13, Table A2, Table A3 and Table A4, Figure 3, Figure 4, Figure 5 and Figure 6 |
| Synthesis of results | 18 | Summarize and/or present the charting results as they relate to the review questions and objectives. | 7,8,9,11,12,13, |
| Discussion | |||
| Summary of evidence | 19 | Summarize the main results (including an overview of concepts, themes, and types of evidence available), link to the review questions and objectives, and consider the relevance to key groups. | 13,14,15,16,17 |
| Limitations | 20 | Discuss the limitations of the scoping review process. | 17 |
| Conclusions | 21 | Provide a general interpretation of the results with respect to the review questions and objectives, as well as potential implications and/or next steps. | 17 |
| Funding | |||
| Funding | 22 | Describe sources of funding for the included sources of evidence, as well as sources of funding for the scoping review. Describe the role of the funders of the scoping review. | 17 |
| Guideline | Country/Region | Type of ID | Population | C R P | A D H D | R L S | Serum Ferritin (ug/L) | Additional Biomarkers | Risk Factors for ID/IDA |
|---|---|---|---|---|---|---|---|---|---|
| Centers for Disease Control and Prevention, 1989 [86] | USA | IDA | Children and females | ⨯ | ⨯ | ⨯ | N/A | -Hb -Hct | -Infants/ children -Females of child-bearing age -Pregnancy |
| Centers for Disease Control and Prevention, 1998 [85] | USA | IDA | Children and adults | ⨯ * | ⨯ | ⨯ | >6 months: ≤15 | -EP -Hb -Hct -MCV -RDW -SI -TIBC -TSAT | -Infants/ children -Females of child-bearing age -Infant diet -Diet -Infection/ inflammation/ comorbidities -Pregnancy -Prematurity/ LBW -Medications -Low SES -Race/ ethnicity |
| British Society of Gastroenterology (Goddard et al.), 2000 [81] | UK | IDA | Adults | ⨯ * | ⨯ | ⨯ | <12 12–15 in the presence of inflammation | -EP -Hb -MCV -RDW -sTfR -TSAT | -Menstruation -Infection/ inflammation/ comorbidities |
| World Health Organization, 2001 [30] | Intl | IDA | Children and adults | ⨯ * | ⨯ | ⨯ | <5 years: <12 <30 in the presence of infection >5 years: <15 | -EP -Hb -Hct -MCH -MCV -SI -sTfR -TIBC -TSAT | -Menstruation -Pregnancy -Infection/ inflammation/ comorbidities -Diet -Low SES -Infant diet |
| American Academy of Pediatrics, Committee on Nutrition (Baker et al.), 2010 [64] | USA | IDA NAID | Infants and children 0–3 years | ✓ | ⨯ | ⨯ | 0–3 years: <10 | -CHr -Hb -STfR | -Prematurity/ LBW -Infant diet -Lead exposure -Poor growth -Low SES |
| First Nations and Inuit Health Branch, 2010 [34] | Canada | IDA | Adults | ⨯ * | ⨯ | ⨯ | <20 | -Hb -Hct -MCH -MCV -RDW -Retic. count -SI -TIBC | -Females of child- bearing age -Infants/ children, -Adolescents -Advanced age -Infection/ inflammation/ comorbidities -Race/ ethnicity -Menstruation |
| British Society of Gastroenterology (Goddard et al.), 2011 [33] | UK | IDA | Adults | ⨯ * | ⨯ | ⨯ | <12–15 <50 in the presence of inflammation | -CHr -Hb -MCH -MCV -rbZP -RDW -sTfR -TIBC -TSAT | -Infection/ inflammation/ comorbidities |
| Canadian Pediatric Surveillance Program (Abdullah et al.), 2011 [48] | Canada | IDA NAID | Children | ✓ | ⨯ | ⨯ | <10 | -CHr -Hb -sTfR | -Low SES -Race/ ethnicity -Prematurity/ LBW -Infant diet -Infection/ inflammation/ comorbidities -Non-attendance to daycare -Overweight/obesity |
| Government of India, 2013 [36] | India | IDA | Adults and children | ⨯ | ⨯ | ⨯ | N/A | -Hb | -Females of childbearing age -Infants/ children -Infection/ inflammation/ comorbidities -Diet -Infant diet |
| Gastroenterol-gical Society of Australia, 2015 [35] | Australia | IDA NAID | Adults and children | ⨯ * | ✓ | ⨯ | Children: <12 diagnostic <15–20 low iron stores Adults: <15 diagnostic <15–30 low iron stores | -Hb -MCH -MCV -SI -TIBC -Serum transferrin -TSAT | -Pregnancy -Females of child-bearing age -Infants/ children -Infection/ inflammation/ comorbidities -Diet -Advanced age -Race/ ethnicity -Elite athletes -Overweight/obesity |
| Government of Nunavut, 2015 [37] | Canada | IDA | Infants and children 6 mo–3 years | ✓ | ⨯ | ⨯ | <10 | -Hb -MCV | -Low SES -Infant diet |
| Guidelines and Audit Implementati- on Network, 2015 [55] | UK | IDA | Adults | ✓ | ⨯ | ⨯ | CRP < 30 mg/L: <30 CRP > 30 mg/L: <100 | -Hb -MCH -MCV -TSAT | -Infection/ inflammation/ comorbidities -Pregnancy -Menstruation |
| National Blood Authority, 2016 [38] | Australia | IDA | Children | ✓ | ⨯ | ⨯ | <20 High risk populations/chronic disease: <50 | -Hb -MCH -MCV | -Race/ ethnicity -Maternal ID -Prematurity/ LBW -Infant diet -Infection/ inflammation/ comorbidities |
| Nutrition Working Group (O’Connor et al.), 2016 [44] | Canada | IDA NAID | Females (adolescence to menopause) | ⨯ * | ⨯ | ⨯ | <30 | -EP -Hb -sTfR -TSAT | -Diet -Low SES -Race/ ethnicity -Menstruation -Pregnancy -Overweight/ obesity |
| Starship Hospital, 2016 [41] | NZ | IDA NAID | Children age 1–5 years | ⨯ * | ⨯ | ⨯ | <10 | -Hb -TSAT -MCV -RDW | -Infants/ children -Race/ ethnicity -Prematurity/ LBW -Maternal ID -Overweight/ obesity -Adolescence -Diet -Infant diet -Menstruation |
| Central Manchester Hospitals, 2017 [54] | UK | IDA | Non- pregnant adults | ✓ | ⨯ | ⨯ | <13 (F) <30 (M) Elevated ESR/PV: 13–50 | -Hb -MCV -TSAT | -Infection/ inflammation/ comorbidities |
| Randall Children’s Cancer and Blood Disorders Program, 2017 [52] | USA | IDA | Children | ⨯ | ⨯ | ⨯ | <30 | -Hb -MCV | -Infection/ inflammation/ comorbidities |
| World Health Organization, 2017 [43] | Intl | IDA | Children and adults | ✓ | ⨯ | ⨯ | <5 years: <12 >5 years: <15 | -EP -Hb -Hepcidin -MCH -MCV -SI -sTfR -TIBC -TSAT -ZPP | -Infants/ children -Menstruation -Pregnancy -Advanced age |
| BC Clinical Practice Guidelines, 2018 [31] | Canada | IDA NAID | Adults and children | ✓ | ⨯ | ✓ | Children: <12 12–20 possible ID Adults: <15 15–30 probable ID | -Hb -MCHC -MCV -SI -TIBC -TSAT | -Pregnancy -Infants/ children - Low SES -Ethnicity/race -Diet -Infection/ inflammation/ comorbidities -Advanced age -Menstruation |
| India National Guidelines (Bellad et al.), 2018 [32] | India | IDA | Children aged 6 month–14 years, non-pregnant females age > 15, pregnant females | ⨯ | ⨯ | ⨯ | N/A | -Hb | -Pregnancy, -Infants/ children -Adolescents -Infection/ inflammation/ comorbidities |
| NHS Buckinghams-hire, 2018 [57] | UK | IDA | Adults | ✓ | ⨯ | ⨯ | <22 | -Hb -MCH -MCV -SI -TIBC -TSAT | -Menstruation -Infection inflammation/ comorbidities |
| Toward Optimized Practice Alberta, 2018 [42] | Canada | IDA | Children (≥5 years) and adults | ⨯ * | ✓ | ✓ | <12 years: <10 >12 years: <13 F <30 M | -CHr -EP -Hb -%HRC -MCH -MCV -SI -sTfR -TSAT | -Infants/ children -Adolescence -Menstruation -Pregnancy -Low SES -Diet -Advanced age -Infection/ inflammation/ comorbidities |
| University of Washington Pediatrics, 2018 [58] | USA | IDA | Children | ✓ | ⨯ | ✓ | <5 years: <12 ≥5 years: <15 | -Hb -Hct -MCV -RDW -Retic. count -SI -TIBC -TSAT -ZPPH | -Prematurity/ LBW -Poor growth/FTT -Infant diet -Diet -Lead exposure -Infection/ inflammation/ Comorbidities -Overweight/ obesity -Menstruation |
| Gulf Cooperation Council Countries (Aleem et al.), 2019 [46] | Gulf Coop Countries | IDA | Adults and children | ⨯ * | ⨯ | ⨯ | <30 (ID should still be considered in high-risk patients with SF > 30) | -Hb -MCH -MCV -RDW -SI -TIBC -TSAT | -Pregnancy -Infant feeding -Prematurity/ LBW -Overweight/ obesity |
| Royal Children’s Hospital Melbourne, 2019 [65] | Australia | IDA NAID | Children | ⨯ * | ⨯ | ⨯ | <20 | -Hb -MCH -MCV | -Maternal ID -Prematurity/ LBW -Pregnancy -Infant diet -Diet -Infection/ inflammation/ comorbidities -Menstruation -Extreme athletes |
| Royal College of Nursing (Barton et al.), 2019 [39] | UK | IDA | Adults | ✓ | ⨯ | ⨯ | <30 CRP > 5 mg/L: 30–100 | -Hb -MCH -MCV -TSAT | -Diet -Infection/ inflammation/ comorbidities |
| Spanish Society of Hematology and Hemotherapy (García Erce et al.), 2019 [40] | Spain | IDA | Children and adults | ⨯ * | ⨯ | ⨯ | ≤ 5 years: <12 >5 years: <15 <30 in the presence of inflammation Athletes > 15 years: <30 | -CHr -Hb -%HRC -MCH -MCV -RDW -sTfR -TSAT | -Pregnancy -Elite athletes -Infant diet -Adolescents |
| World Health Organization, 2020 [74] | Intl | IDA NAID | Children and adults | ✓ | ⨯ | ⨯ | <5 years: <12 <30 in the presence of infection/ inflammation 5–10 years, adolescents, adults, elderly, 1st trimester pregnancy: <15 <70 in the presence of infection/ inflammation | -CHr -Erythrocyte ferritin -%HRC -MCV -rbZP -SI -sTfR -TIBC -TSAT | N/A |
| American Gastroenterol-ogical Association (Sonoda K), 2021 [70] | USA | IDA | Adults | ✓ | ⨯ | ⨯ | <45 (with anemia) Ferritin threshold for ID without anemia is uncertain | -Hb -SI -sTfR -TSAT | -Females of child-bearing age -Infection/ inflammation/ comorbidities |
| British Society of Gastroenterol-ogy (Snook et al.), 2021 [71] | UK | IDA | Adults | ✓ | ⨯ | ✓ | <15 (highly specific for ID) <45 (respectable specificity for ID) | -CHr -Hb -MCH -MCV -rbZP -sTFR -TIBC -TSAT | -Menstruation -Infection/ inflammation/ comorbidities |
| British Society for Haematology (Fletcher et al.) 2022 [72] | UK | IDA NAID | Non-pregnant adults and children | ✓ | ⨯ | ⨯ | <5 years: <12 >5 years: <15 <15 or 15–50 in the presence of inflammation or raised CRP | -CHr -Hb -Hct -Hepcidin -%HRC -MCH -MCHC -MCV -RDW -Ret-HE -SI -TIBC -TSAT | -Menstruation -Advanced age |
| Indian Academy of Pediatrics (Chandra et al.), 2022 [76] | India | IDA | Children | ⨯ * | ⨯ | ⨯ | <5 years: <12 >5 years: <15 <30 in the presence of infection | -CHr -FEP -Hb -%HRC -MCH -MCHC -MCV -RDW -Ret-HE -Retic. count -SI -sTFR -TIBC -TSAT | -Prematurity /LBW -Infants/ children -Adolescents |
| Children’s Minnesota (Bloomquist et al.), 2023 [73] | USA | IDA | Children ≤ 5 years | ✓ | ⨯ | ⨯ | <12 (goal > 20) | -CHr -Hb -TSAT -MCV -Retic. count | -Diet -Infection/ inflammation/ comorbidities |
| Guideline | Country/ Region | Type of Iron Deficiency | C R P | A D H D | R L S | Serum Ferritin (ug/L) | Additional Biomarkers |
|---|---|---|---|---|---|---|---|
| American College of Obstetricians and Gynecologists, 2008 [80] | USA | IDA | ⨯ | ⨯ | ⨯ | <10–15 | -EP -Hb -Hct -MCV -TIBC -TSAT -SI |
| British Committee for Standards in Haematology (Pavord et al.), 2012 [45] | UK | IDA NAID | ✓ | ⨯ | ⨯ | <15–30 | -CHr -Hb -MCH -MCHC -MCV -Retic. count -SI -sTfR -TIBC -TSAT -ZPP |
| King Edward Memorial Hospital, 2013 [51] | Australia | IDA NAID | ✓ | ⨯ | ⨯ | <30 | -Hb -MCH -MCV -SI |
| The Federation of Obstetric and Gynaecological Societies of India, 2016 [53] | India | IDA | ⨯ * | ⨯ | ✓ | <15 (initiate treatment if <30) | -CHr -EP -FEP -Hb -MCH -MCV -RDW -Retic. count -SI -sTfR -TIBC -TSAT -ZPP |
| British Society of Hematology Committee (Pavord et al.), 2020 [68] | UK | IDA NAID | ⨯ * | ⨯ | ✓ | <30 | -CHr -Hb -Hepcidin -MCH -MCHC -MCV -TIBC -TSAT -SI -sTfR |
| NHS Royal Berkshire, 2020 [77] | UK | IDA | ⨯ | ⨯ | ⨯ | <15 (consider treatment if <30) | -Hb -MCH -MCV -TSAT |
| The Royal Women’s Hospital, 2020 [67] | Australia | IDA NAID | ⨯ * | ⨯ | ⨯ | <30 | -Hb -MCV -Serum transferrin -SI -TIBC -TSAT |
| New Zealand College of Midwives (Caljé E), 2021 [75] | New Zealand | IDA NAID | ✓ | ⨯ | ✓ | <30 | -Hb -MCH -MCV |
| Polish Society of Gynecologists and Obstetricians (Sieroszewski et al.), 2023 [78] | Poland | IDA NAID | ⨯ * | ⨯ | ⨯ | <30 (pre-latent) <12 (latent) Supplement when SF < 60 in pregnant females > 16 weeks of gestation | -Hb -MCV -SI -TIBC -TSAT |
| Association of Ontario Midwives, no date [87] | Canada | IDA | ⨯ * | ⨯ | ⨯ | <15–30 | -Hb -MCV |
| Guideline | Country/Region | C R P | A D H D | R L S | Condition | Serum Ferritin | Additional Biomarkers |
|---|---|---|---|---|---|---|---|
| Caring for Australasians with Renal Impairment (Roger et al.), 2006 [83] | Australia | ⨯ * | ⨯ | ⨯ | Kidney disease | <500 (commence IV iron in patients receiving ESA) | -CHr (research only) -Hb -% HRC -TSAT -MCHC -MCV -SI -sTfR |
| Kidney Disease Outcomes Quality Initiative, 2006 [84] | USA | ⨯ | ⨯ | ⨯ | Kidney disease | <200 (HD-CKD) <100 (non-HD-CKD) | -CHr -Hb -MCH -MCHC -MCV -Retic. count -TSAT |
| Canadian Society of Nephrology (Madore et al.), 2008 [82] | Canada | ⨯ | ⨯ | ⨯ | Kidney disease | <100 (non-dialysis) <200 (hemodialysis receiving ESA) | -Hb -TSAT |
| National Comprehensive Cancer Network, 2010 [66] | USA | ⨯ | ⨯ | ⨯ | Cancer | <30 (absolute ID) <800 (functional ID) | -Hb -SI -TIBC -TSAT |
| Kidney Disease Improving Global Outcomes, 2012 [50] | Intl | ✓ | ⨯ | ⨯ | Kidney disease | <500 (if being treated with ESA) | -Hb -Retic. count -SI -TIBC -TSAT |
| British Committee for Standards in Haematology (Thomas et al.), 2013 [60] | UK | ⨯ * | ⨯ | ⨯ | Functional ID | <12 (absent iron stores) <100 (non- hemodialysis pt) <200 (hemodialysis pt) | -CHr -Hb -Hepcidin (research investigation only) -MCH -MCV -Ret-He -SI -sTfR -TIBC -TSAT |
| French Cardiologists (Cohen-Solal et al.), 2014 [56] | France | ⨯ * | ⨯ | ⨯ | Heart failure | <100 Or 100–299 with TSAT < 20% | -TSAT |
| European Crohn’s and Colitis Organisation (Dignass et al.), 2015 [63] | Europe | ✓ | ✓ | ✓ | IBD | <30 (up to 100 in the presence of inflammation; >100 in anemia of chronic disease) | -Haptoglobin -Hb -MCH -MCV -RDW -Retic. count -sTfR -TSAT |
| IRON CORE Group (Cappellini et al.), 2017 [61] | Intl | ✓ | ⨯ | ✓ | Kidney disease CHF IBD | <100 (If SF 100–300, TSAT required for confirmation of ID) | -Hb -TSAT |
| Japanese Society for Dialysis Therapy (Yamamoto et al.), 2017 [59] | Japan | ⨯ * | ⨯ | ⨯ | Kidney disease | <50 (if not treated with ESA) <100 (if being treated with ESA) | -Hb -MCV -Retic. count -SI -TIBC -TSAT |
| European Society for Medical Oncology (Aapro et al.), 2018 [49] | Europe | ✓ | ⨯ | ⨯ | Cancer | <100 (for patients receiving chemotherapy) | -Hb -TSAT |
| Muñoz et al., 2018 [47] | Intl | ⨯ * | ⨯ | ⨯ | Post- operative | <100 Or 100–300 with TSAT < 20% | -CHr -Hb -TSAT |
| American Society of Clinical Oncology/American Society of Hematology (Bohlius et al.), 2019 [62] | USA | ⨯ | ⨯ | ⨯ | Cancer | No cutoff value (but recommend measuring ferritin) | -Hb -SI -TIBC -TSAT |
| Sociedad Española de Oncología Médica (Álvarez et al.), 2021 [69] | Spain | ✓ | ⨯ | ⨯ | Cancer | <30 30–100 (anemia of chronic disease with ID) | -Hb -MCH -MCV -Retic. count -SI -sTfR -TIBC -TSAT |
| North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (Talathi et al.), 2023 [79] | North America | ✓ | ⨯ | ⨯ | Intestinal rehabilitation (e.g., in children with intestinal failure or short bowel syndrome) | <30 or 30–100 with TSAT < 15% | -CHr -Hb -Hct -Hepcidin (research setting) -Retic. count -SI -sTfR -TIBC -TSAT -ZPP |
References
- Pasricha, S.R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Silvani, A.; Ghorayeb, I.; Manconi, M.; Li, Y.; Clemens, S. Putative animal models of restless legs syndrome: A systematic review and evaluation of their face and construct validity. Neurotherapeutics 2023, 20, 154–178. [Google Scholar] [CrossRef]
- Woods, S.; Basco, J.; Clemens, S. Effects of iron-deficient diet on sleep onset and spinal reflexes in a rodent model of restless legs syndrome. Front. Neurol. 2023, 14, 1160028. [Google Scholar] [CrossRef]
- McCann, S.; Perapoch Amadó, M.; Moore, S.E. The role of iron in brain development: A systematic review. Nutrients 2020, 12, 2001. [Google Scholar] [CrossRef]
- Means, R.T. Iron deficiency and iron deficiency anemia: Implications and impact in pregnancy, fetal development, and early childhood parameters. Nutrients 2020, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Wiegersma, A.M.; Dalman, C.; Lee, B.K.; Karlsson, H.; Gardner, R.M. Association of prenatal maternal anemia with neurodevelopmental disorders. JAMA Psychiatry 2019, 76, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, S.; Singh, I.; Leung, W.; Stockler, S.; Ipsiroglu, O.S. Iron deficiency and common neurodevelopmental disorders-a scoping review. PLoS ONE 2022, 17, e0273819. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Wu, T.T.; Wang, H.M.; Li, X.; Ni, L.Y.; Chen, T.J.; Qiu, M.; Shen, J.; Liu, T.; Ondo, W.G.; et al. Correlates of nonanemic iron deficiency in restless legs syndrome. Front. Neurol. 2020, 11, 298. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, L.; Zhang, L.; Qu, Y.; Mu, D. Iron Status in Attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0169145. [Google Scholar] [CrossRef]
- Leung, W.; Singh, I.; McWilliams, S.; Stockler, S.; Ipsiroglu, O.S. Iron deficiency and sleep—A scoping review. Sleep Med. Rev. 2020, 51, 101274. [Google Scholar] [CrossRef]
- DelRosso, L.M.; Mogavero, M.P.; Ferri, R. Restless sleep disorder, restless legs syndrome, and periodic limb movement disorder-sleep in motion! Pediatr. Pulmonol. 2022, 57, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Esteves, A.M.; Lopes, C.; Frussa-Filho, R.; Frank, M.K.; Cavagnolli, D.; Arida, R.M.; Tufik, S.; de Mello, M.T. Spontaneously hypertensive rats: Possible animal model of sleep-related movement disorders. J. Mot. Behav. 2013, 45, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.K.; Esteves, A.M.; Lopes, C.; Cavagnolli, D.A.; Tufik, S.; de Mello, M.T. The effects of physical exercise on the serum iron profile in spontaneously hypertensive rats. Biol. Trace Elem. Res. 2012, 145, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Lecendreux, M.; Bernardina, B.D.; Mouren, M.C.; Sbarbati, A.; Konofal, E. Attention-deficit/hyperactivity disorder, Tourette’s syndrome, and restless legs syndrome: The iron hypothesis. Med. Hypotheses 2008, 70, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Adisetiyo, V.; Jensen, J.H.; Tabesh, A.; Deardorff, R.L.; Fieremans, E.; Di Martino, A.; Gray, K.M.; Castellanos, F.X.; Helpern, J.A. Multimodal MR imaging of brain iron in attention deficit hyperactivity disorder: A noninvasive biomarker that responds to psychostimulant treatment? Radiology 2014, 272, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Adisetiyo, V.; Gray, K.M.; Jensen, J.H.; Helpern, J.A. Brain iron levels in attention-deficit/hyperactivity disorder normalize as a function of psychostimulant treatment duration. Neuroimage Clin. 2019, 24, 101993. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.P.; Barker, P.B.; Wehrl, F.W.; Song, H.K.; Earley, C.J. MRI measurement of brain iron in patients with restless legs syndrome. Neurology 2001, 56, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Astrakas, L.G.; Konitsiotis, S.; Margariti, P.; Tsouli, S.; Tzarouhi, L.; Argyropoulou, M.I. T2 relaxometry and fMRI of the brain in late-onset restless legs syndrome. Neurology 2008, 71, 911–916. [Google Scholar] [CrossRef]
- Beliveau, V.; Stefani, A.; Birkl, C.; Kremser, C.; Gizewski, E.R.; Högl, B.; Scherfler, C. Revisiting brain iron deficiency in restless legs syndrome using magnetic resonance imaging. Neuroimage Clin. 2022, 34, 103024. [Google Scholar] [CrossRef]
- Cortese, S.; Azoulay, R.; Castellanos, F.X.; Chalard, F.; Lecendreux, M.; Chechin, D.; Delorme, R.; Sebag, G.; Sbarbati, A.; Mouren, M.; et al. Brain iron levels in attention-deficit/hyperactivity disorder: A pilot MRI study. World J. Biol. Psychiatry 2012, 13, 223–231. [Google Scholar] [CrossRef]
- Earley, C.J.; Barker, P.B.; Horská, A.; Allen, R.P. MRI-determined regional brain iron concentrations in early- and late-onset restless legs syndrome. Sleep Med. 2006, 7, 458. [Google Scholar] [CrossRef] [PubMed]
- Godau, J.; Klose, U.; Di Santo, A.; Schweitzer, K.; Berg, D. Multiregional brain iron deficiency in restless legs syndrome. Mov. Disord. 2008, 23, 1184–1187. [Google Scholar] [CrossRef]
- Hasaneen, B.M.; Sarhan, M.; Samir, S.; ELAssmy, M.; Sakrana, A.A.; Ashamalla, G.A. T2 ∗ magnetic resonance imaging: A non-invasive biomarker of brain iron content in children with attention-deficit/hyperactivity disorder. Egypt. J. Radiol. Nucl. Med. 2017, 48, 161–167. [Google Scholar] [CrossRef]
- Knake, S.; Heverhagen, J.T.; Menzler, K.; Keil, B.; Oertel, W.H.; Stiasny-Kolster, K. Normal regional brain iron concentration in restless legs syndrome measured by MRI. Nat. Sci. Sleep 2010, 2, 19–22. [Google Scholar] [CrossRef]
- Li, X.; Allen, R.P.; Earley, C.J.; Liu, H.; Cruz, T.E.; Edden, R.A.E.; Barker, P.B.; van Zijl, P.C.M. Brain iron deficiency in idiopathic restless legs syndrome measured by quantitative magnetic susceptibility at 7 tesla. Sleep Med. 2016, 22, 75–82. [Google Scholar] [CrossRef]
- Margariti, P.N.; Astrakas, L.G.; Tsouli, S.G.; Hadjigeorgiou, G.M.; Konitsiotis, S.; Argyropoulou, M.I. Investigation of unmedicated early onset restless legs syndrome by voxel-based morphometry, T2 relaxometry, and functional MR imaging during the night-time hours. AJNR Am. J. Neuroradiol. 2012, 33, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Chang, Y.; Lee, Y.S.; Song, H.J.; Chang, H.W.; Ku, J.; Cho, Y.W. T2 relaxometry using 3.0-tesla magnetic resonance imaging of the brain in early- and late-onset restless legs syndrome. J. Clin. Neurol. 2014, 10, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Chang, Y.; Lee, Y.S.; Song, H.; Chang, H.W.; Ku, J.; Allen, R.P.; Earley, C.J.; Cho, Y.W. A comparison of MRI tissue relaxometry and ROI methods used to determine regional brain iron concentrations in restless legs syndrome. Med. Devices 2015, 8, 341–350. [Google Scholar] [CrossRef][Green Version]
- Rizzo, G.; Manners, D.; Testa, C.; Tonon, C.; Vetrugno, R.; Marconi, S.; Plazzi, G.; Pizza, F.; Provini, F.; Malucelli, E.; et al. Low brain iron content in idiopathic restless legs syndrome patients detected by phase imaging. Mov. Disord. 2013, 28, 1886–1890. [Google Scholar] [CrossRef]
- World Health Organization. Iron Deficiency Anaemia Assessment, Prevention and Control. Available online: https://www.who.int/publications/m/item/iron-children-6to23--archived-iron-deficiency-anaemia-assessment-prevention-and-control (accessed on 7 March 2021).
- Guidelines and Protocols Advisory Committee. Iron Deficiency—Diagnosis and Management. Available online: https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/iron-deficiency (accessed on 27 July 2024).
- Bellad, A.; Kapil, R.; Gupta, A. National guidelines for prevention and control of iron deficiency anemia in India. Indian J. Community Health 2018, 30, 89. Available online: http://search.ebscohost.com/login.aspx?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=22489509&AN=130317815&h=E4nzs%2B3k8C%2FZROu250unzRb%2FqoEq41cUPrf7tkSX9MGmORiqObEuqpa%2FdRRkQ3qTCyzb0vRujvvof4hdVvsOZw%3D%3D&crl=c (accessed on 7 March 2021). [CrossRef]
- Goddard, A.F.; James, M.W.; McIntyre, A.S.; Scott, B.B. British Society of Gastroenterology. Guidelines for the management of iron deficiency anaemia. Gut 2011, 60, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Chapter 10—Hematology, Metabolism and Endocrinology. In First Nations and Inuit Health Branch (FNIHB) Clinical Practice Guidelines for Nurses in Primary Care; 2010; p. 1. Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/fniah-spnia/alt_formats/pdf/services/nurs-infirm/clini/adult/hemo-endo-immuno-eng.pdf (accessed on 7 March 2021).
- Gastroenterological Society of Australia. Iron Deficiency; 2015. Available online: https://www.gesa.org.au/public/13/files/Education%20%26%20Resources/Clinical%20Practice%20Resources/Iron%20Deficiency/Iron_Deficiency_2015.pdf (accessed on 23 March 2021).
- Ministry of Health & Family Welfare Government of India. Guidelines for Control of Iron Deficiency Anaemia; Ministry of Health & Family Welfare Government of India: New Delhi, India, 2013. [Google Scholar]
- Nunavut Department of Health. Protocol for Identifying and Treating Iron Deficiency Anemia in Infants and Young Children. Available online: https://www.gov.nu.ca/sites/default/files/documents/2023-10/nunavut_protocol_for_ida_approved_2015_may_20_0.pdf (accessed on 7 March 2021).
- National Blood Authority. Neonatal and Paediatrics. In Patient Blood Management Guidelines: Module 6; National Blood Authority: Lyneham, Australia, 2016. Available online: https://blood.gov.au/system/files/14523_NBA-Module-6-Neonat_Paediatrics_internals_5_updated_14_May_2020.pdf (accessed on 7 March 2021).
- Barton, C.; Cowan, K.; Faulds, J.; Holloway, D.; Johnston, S.; Mason, I.; McMahon, A. Royal College of Nursing. Iron Deficiency and Anaemia in Adults. Available online: https://www.rcn.org.uk/Professional-Development/publications/pub-007460 (accessed on 7 March 2021).
- García Erce, J.A.; Altés, A.; López Rubio, M.; Remacha, A.F.; en representación del Grupo Español de Eritropatología de la Sociedad Española de Hematología y Hemoterapia; Otros componentes del Grupo Español de Eritropatología de la Sociedad Española de Hematología y Hemoterapia. Management of iron deficiency in various clinical conditions and the role of intravenous iron: Recommendations of the Spanish Erythropathology Group of the Spanish Society of Haematology and Haemotherapy. Rev. Clin. Esp. 2020, 220, 31–42. [Google Scholar] [CrossRef]
- Iron Deficiency. Starship NZ. Available online: https://www.starship.org.nz/guidelines/iron-deficiency/ (accessed on 28 May 2021).
- Toward Optimized Practice. Iron Deficiency Anemia (IDA) Clinical Practice Guideline. Available online: https://actt.albertadoctors.org/media/tc4lq52r/ida-cpg.pdf (accessed on 7 March 2021).
- World Health Organization. Nutritional Anaemias: Tools for Effective Prevention and Control. Available online: https://www.who.int/publications/i/item/9789241513067 (accessed on 7 March 2021).
- O’Connor, D.L.; Blake, J.; Bell, R.; Bowen, A.; Callum, J.; Fenton, S.; Gray-Donald, K.; Rossiter, M.; Adamo, K.; Brett, K.; et al. Canadian consensus on female nutrition: Adolescence, reproduction, menopause, and beyond. J. Obstet. Gynaecol. Can. 2016, 38, 508–554. [Google Scholar] [CrossRef]
- Pavord, S.; Myers, B.; Robinson, S.; Allard, S.; Strong, J.; Oppenheimer, C.; British Committee for Standards in Haematology. UK guidelines on the management of iron deficiency in pregnancy. Br. J. Haematol. 2012, 156, 588–600. [Google Scholar] [CrossRef]
- Aleem, A.; Alsayegh, F.; Keshav, S.; Alfadda, A.; Alfadhli, A.A.; Al-Jebreen, A.; Al-Kasim, F.; Almuhaini, A.; Al-Zahrani, H.; Batwa, F.; et al. Consensus statement by an expert panel on the diagnosis and management of iron deficiency anemia in the gulf cooperation council countries. Med. Princ. Pract. 2020, 29, 371–381. [Google Scholar] [CrossRef]
- Muñoz, M.; Acheson, A.G.; Bisbe, E.; Butcher, A.; Gómez-Ramírez, S.; Khalafallah, A.A.; Kehlet, H.; Kietaibl, S.; Liumbruno, G.M.; Meybohm, P.; et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 2018, 73, 1418–1431. [Google Scholar] [CrossRef]
- Abdullah, K.; Zlotkin, S.; Parkin, P.; Grenier, D.; Canadian Pediatric Surveillance Program. Iron Deficiency Anemia in Children. Available online: https://cpsp.cps.ca/uploads/publications/RA-iron-deficiency-anemia.pdf (accessed on 28 May 2021).
- Aapro, M.; Beguin, Y.; Bokemeyer, C.; Dicato, M.; Gascón, P.; Glaspy, J.; Hofmann, A.; Link, H.; Littlewood, T.; Ludwig, H.; et al. Management of anaemia and iron deficiency in patients with cancer: ESMO clinical practice guidelines. Ann. Oncol. 2018, 29, 271. [Google Scholar] [CrossRef]
- Kidney Disease Improving Global Outcomes (KDIGO). KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Nature Publishing Group. Available online: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-Anemia-Guideline-English.pdf (accessed on 25 May 2021).
- King Edward Memorial Hospital. Anaemia and Iron Deficiency: Management in Pregnancy and Postpartum; King Edward Memorial Hospital: Subiaco, Australia, 2013. Available online: https://www.kemh.health.wa.gov.au/~/media/HSPs/NMHS/Hospitals/WNHS/Documents/Clinical-guidelines/Obs-Gyn-Guidelines/Anaemia-Management-during-Pregnancy-and-the-Postnatal-Period.pdf?thn=0 (accessed on 25 May 2021).
- Randall Children’s Hospital. Evaluation and Treatment of Iron Deficiency Anemia (IDA). Available online: https://www.legacyhealth.org/Children/provider-resources/rch-referral-guidelines (accessed on 25 May 2021).
- The Federation of Obstetric and Gynaecological Societies of India. FOGSI General Clinical Practice Recommendations: Management of Iron Deficiency Anemia in Pregnancy; The Federation of Obstetric and Gynaecological Societies of India: Mumbai, India, 2016. [Google Scholar]
- Central Manchester Hospitals Anaemia Guide (Adults Excluding Pregnant Women). Available online: https://mft.nhs.uk/app/uploads/2022/11/Manchester-Adult-Anaemia-Guide-excl.-pregnancy-v5-Aug-2021.pdf (accessed on 25 May 2021).
- Guidelines and Audit Implementation Network (GAIN). Investigation and Management of the Adult Patient with Anaemia. Available online: https://www.rqia.org.uk/RQIA/files/1e/1e2a9adc-7517-4a47-858a-5192b0746456.pdf (accessed on 25 May 2021).
- Cohen-Solal, A.; Leclercq, C.; Mebazaa, A.; De Groote, P.; Damy, T.; Isnard, R.; Galinier, M. Diagnosis and treatment of iron deficiency in patients with heart failure: Expert position paper from French cardiologists. Arch. Cardiovasc. Dis. 2014, 107, 563–571. [Google Scholar] [CrossRef]
- Buckinghamshire Clinical Commissioning Group. Iron Deficiency Anaemia in Adults; Buckinghamshire Clinical Commissioning Group: Aylesbury, England, 2018. [Google Scholar]
- University of Washington Pediatrics Clinical Guidelines. Iron Deficiency Anemia. 2018.
- Yamamoto, H.; Nishi, S.; Tomo, T.; Masakane, I.; Saito, K.; Nangaku, M.; Hattori, M.; Suzuki, T.; Morita, S.; Ashida, A.; et al. 2015 Japanese Society for Dialysis Therapy: Guidelines for renal anemia in chronic kidney disease. Ren. Replace. Ther. 2017, 3, 36. [Google Scholar] [CrossRef]
- Thomas, D.W.; Hinchliffe, R.F.; Briggs, C.; Macdougall, I.C.; Littlewood, T.; Cavill, I.; British Committee for Standards in Haematology. Guideline for the laboratory diagnosis of functional iron deficiency. Br. J. Haematol. 2013, 161, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Comin-Colet, J.; de Francisco, A.; Dignass, A.; Doehner, W.; Lam, C.S.; Macdougall, I.C.; Rogler, G.; Camaschella, C.; Kadir, R.; et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am. J. Hematol. 2017, 92, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Bohlius, J.; Bohlke, K.; Castelli, R.; Djulbegovic, B.; Lustberg, M.B.; Martino, M.; Mountzios, G.; Peswani, N.; Porter, L.; Tanaka, T.N.; et al. Management of cancer-associated anemia with erythropoiesis-stimulating agents: ASCO/ASH clinical practice guideline update. Blood Adv. 2019, 3, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.U.; Gasche, C.; Bettenworth, D.; Birgegård, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, K.; Koutroubakis, I.; et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J. Croh. Colitis 2015, 9, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.D.; Greer, F.R.; The Committee on Nutrition. Clinical report—Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics 2010, 126, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Clinical Practice Guidelines: Iron Deficiency. The Royal Children’s Hospital Melbourne. Available online: https://www.rch.org.au/clinicalguide/guideline_index/iron_deficiency/ (accessed on 9 May 2023).
- NCCN Clinical Practice Guidelines in Oncology. Cancer- and Chemotherapy- Induced Anemia. 2010. Available online: https://www.sabm.org/assets/pdfs/nccn_anemia_guidelines.pdf (accessed on 25 May 2021).
- The Royal Women’s Hospital. Management of Iron Deficiency in Maternity and Gynaecology Patients. Available online: https://thewomens.r.worldssl.net/images/uploads/downloadable-records/clinical-guidelines/iron-deficiency-management-inmaternity-and-gynaecology-patients_280720.pdf (accessed on 25 May 2021).
- Pavord, S.; Daru, J.; Prasannan, N.; Robinson, S.; Stanworth, S.; Girling, J.; on behalf of the BSH Committee. UK guidelines on the management of iron deficiency in pregnancy. Br. J. Haematol. 2020, 188, 819–830. [Google Scholar] [CrossRef]
- Escobar Álvarez, Y.; de las Peñas Bataller, R.; Perez Altozano, J.; Ros Martínez, S.; Sabino Álvarez, A.; Blasco Cordellat, A.; Brozos Vázquez, E.; Corral Jaime, J.; García Escobar, I.; Beato Zambrano, C. SEOM clinical guidelines for anaemia treatment in cancer patients (2020). Clin. Transl. Oncol. 2021, 23, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, K. Iron deficiency anemia: Guidelines from the American Gastroenterological Association. Am. Fam. Physician 2021, 104, 211–212. [Google Scholar] [PubMed]
- Snook, J.; Bhala, N.; Beales, I.L.P.; Cannings, D.; Kightley, C.; Logan, R.P.; Pritchard, D.M.; Sidhu, R.; Surgenor, S.; Thomas, W.; et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut 2021, 70, 2030–2051. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, A.; Forbes, A.; Svenson, N.; Wayne Thomas, D. A British Society for Haematology Good Practice Paper. Guideline for the laboratory diagnosis of iron deficiency in adults (excluding pregnancy) and children. Br. J. Haematol. 2022, 196, 523–529. [Google Scholar] [CrossRef]
- Bloomquist; Maccabe-Ryaboy; Kuldanek. Outpatient evaluation and management of iron deficiency anemia (IDA) in Children ≤5 Years Old. Children’s Minnesota. Available online: https://www.childrensmn.org/references/cds/iron-deficiency-anemia-guideline.pdf (accessed on 9 May 2023).
- World Health Organization. WHO Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations. Available online: https://www.who.int/publications-detail-redirect/9789240000124 (accessed on 10 May 2023).
- Caljé, E. Anaemia in pregnancy: A practice update for midwives in Aotearoa. Aotearoa N. Z. Midwife 2021, 101, 20–24. [Google Scholar]
- Chandra, J.; Dewan, P.; Kumar, P.; Mahajan, A.; Singh, P.; Dhingra, B.; Radhakrishnan, N.; Sharma, R.; Manglani, M.; Rawat, A.K.; et al. Diagnosis, treatment and prevention of nutritional anemia in children: Recommendations of the joint committee of Pediatric Hematology-Oncology Chapter and Pediatric and Adolescent Nutrition Society of the Indian Academy of Pediatrics. Indian. Pediatr. 2022, 59, 782–801. [Google Scholar] [CrossRef]
- Royal Berkshire NHS. Iron Deficiency Anaemia in Maternity—Guideline for the Management of; Royal Berkshire NHS: Reading, UK, 2020. [Google Scholar]
- Sieroszewski, P.; Bomba-Opon, D.; Cnota, W.; Drosdzol-Cop, A.; Gogacz, M.; Grzesiak, M.; Huras, H.; Jakimiuk, A.; Kaczmarek, P.; Kwiatkowski, S.; et al. Guidelines of the Polish Society of Gynecologists and Obstetricians on the diagnosis and treatment of iron deficiency and iron deficiency with anemia. Ginekol. Pol. 2023, 94, 415–422. [Google Scholar] [CrossRef]
- Talathi, S.; Namjoshi, S.; Raghu, V.; Wendel, D.; Oliveira, S.B.; Reed, K.; Yanchis, D.; Mezoff, E.A. Evaluation and management of iron deficiency in children undergoing intestinal rehabilitation—A position paper from the NASPGHAN Intestinal Rehabilitation Special Interest Group. JPGN 2023, 76, 672–683. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 95, anemia in pregnancy. Obstet. Gynecol. 2008, 112, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.F.; McIntyre, A.S.; Scott, B.B. Guidelines for the management of iron deficiency anaemia. Gut 2000, 46, iv1–iv5. [Google Scholar] [CrossRef] [PubMed]
- Madore, F.; White, C.T.; Foley, R.N.; Barrett, B.J.; Moist, L.M.; Klarenbach, S.W.; Culleton, B.F.; Tonelli, M.; Manns, B.J. Clinical practice guidelines for assessment and management of iron deficiency. Kidney Int. Suppl. 2008, 110, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Roger, S. The CARI Guidelines. Haematol. Targets 2006, 11, 217–229. [Google Scholar]
- Kidney Disease Outcomes Quality Initiative (KDOQI); National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am. J. Kidney Dis. 2006, 47, 11–145. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR 1998, 47, 1–36. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00051880.htm (accessed on 7 March 2021).
- Centers for Disease Control and Prevention. CDC criteria for anemia in children and childbearing-aged women. MMWR 1989, 38, 400–404. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00001405.htm (accessed on 7 March 2021).
- Association of Ontario Midwives. Iron Deficiency Anemia in the Childbearing Year. No Date. Available online: https://www.ontariomidwives.ca/sites/default/files/Iron-deficiency-anemia-in-the-childbearing-year.pdf (accessed on 7 March 2021).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Sanders, S.; Doust, J.; Beller, E.; Glasziou, P. Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics 2015, 135, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Fereshtehnejad, S.M.; Rahmani, A.; Shafieesabet, M.; Soori, M.; Delbari, A.; Motamed, M.R.; Lökk, J. Prevalence and associated comorbidities of restless legs syndrome (RLS): Data from a large population-based door-to-door survey on 19176 adults in Tehran, Iran. PLoS ONE 2017, 12, e0172593. [Google Scholar] [CrossRef] [PubMed]
- Balendran, S.; Forsyth, C. Non-anaemic iron deficiency. Aust. Prescr. 2021, 44, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Addo, O.Y.; Jefferds, M.E.; Sharma, A.J.; Flores-Ayala, R.C.; Brittenham, G.M. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: A US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021, 8, e572–e582. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, E.; Duque, X.; Moran, S.; Martínez-Andrade, G.; Reyes-Maldonado, E.; Flores-Huerta, S.; Martinez, H. Hepcidin and other indicators of iron status, by alpha-1 acid glycoprotein levels, in a cohort of Mexican infants. Ann. Hematol. 2021, 100, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Konofal, E.; Lecendreux, M.; Deron, J.; Marchand, M.; Cortese, S.; Zaïm, M.; Mouren, M.C.; Arnulf, I. Effects of iron supplementation on attention deficit hyperactivity disorder in children. Pediatr. Neurol. 2008, 38, 20–26. [Google Scholar] [CrossRef]
- Panahandeh, G.; Vatani, B.; Safavi, P.; Khoshdel, A. The effect of adding ferrous sulfate to methylphenidate on attention-deficit/hyperactivity disorder in children. J. Adv. Pharm. Technol. Res. 2017, 8, 138–142. [Google Scholar] [CrossRef]
- De Barros, A.; Arribarat, G.; Combis, J.; Chaynes, P.; Péran, P. Matching ex vivo MRI With Iron Histology: Pearls and Pitfalls. Front. Neuroanat. 2019, 13, 68. [Google Scholar] [CrossRef]
- Péran, P.; Hagberg, G.; Luccichenti, G.; Cherubini, A.; Brainovich, V.; Celsis, P.; Caltagirone, C.; Sabatini, U. Voxel-based analysis of R2* maps in the healthy human brain. J. Magn. Reson. Imaging 2007, 26, 1413–1420. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Tong, K.A.; Yeom, K.W.; Kuzminski, S. Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J. Magn. Reson. Imaging 2015, 42, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Sato, N.; Ishiyama, A.; Shigemoto, Y.; Suzuki, F.; Fujii, H.; Maikusa, N.; Matsuda, H.; Nishioka, K.; Hattori, N.; et al. Serial MRI alterations of pediatric patients with beta-propeller protein associated neurodegeneration (BPAN). J. Neuroradiol. 2021, 48, 88–93. [Google Scholar] [CrossRef]
- Prayer, D.; Brugger, P.C.; Prayer, L. Fetal MRI: Techniques and protocols. Pediatr. Radiol. 2004, 34, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Deistung, A.; Schweser, F.; Reichenbach, J.R. Overview of quantitative susceptibility mapping. NMR Biomed. 2017, 30, e3569. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; He, N.; Lin, H.; Li, R. Iron deposition quantification: Applications in the brain and liver. J. Magn. Reson. Imaging 2018, 48, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Degremont, A.; Jain, R.; Philippou, E.; Latunde-Dada, G.O. Brain iron concentrations in the pathophysiology of children with attention deficit/hyperactivity disorder: A systematic review. Nutr. Rev. 2021, 79, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Möller, H.E.; Bossoni, L.; Connor, J.R.; Crichton, R.R.; Does, M.D.; Ward, R.J.; Zecca, L.; Zucca, F.A.; Ronen, I. Iron, myelin, and the brain: Neuroimaging meets neurobiology. Trends Neurosci. 2019, 42, 384–401. [Google Scholar] [CrossRef] [PubMed]
- Birkl, C.; Birkl-Toeglhofer, A.M.; Kames, C.; Goessler, W.; Haybaeck, J.; Fazekas, F.; Ropele, S.; Rauscher, A. The influence of iron oxidation state on quantitative MRI parameters in post mortem human brain. Neuroimage 2020, 220, 117080. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.R.; Wang, X.S.; Patton, S.M.; Menzies, S.L.; Troncoso, J.C.; Earley, C.J.; Allen, R.P. Decreased transferrin receptor expression by neuromelanin cells in restless legs syndrome. Neurology 2004, 62, 1563–1567. [Google Scholar] [CrossRef]
- Chenini, S.; Delaby, C.; Rassu, A.L.; Barateau, L.; Vialaret, J.; Hirtz, C.; Dupuy, A.M.; Lehmann, S.; Jaussent, I.; Dauvilliers, Y. Hepcidin and ferritin levels in restless legs syndrome: A case–control study. Sci. Rep. 2020, 10, 11914. [Google Scholar] [CrossRef]
- Alaçam Köksal, S.; Boncuk Ulaş, S.; Acar, B.A.; Acar, T.; Güzey Aras, Y.; Köroğlu, M. Evaluation of the relationship between idiopathic restless legs syndrome and serum hepcidin levels. Brain Behav. 2023, 13, e3259. [Google Scholar] [CrossRef] [PubMed]
- Yazici, K.U.; Yazici, I.P.; Ustundag, B. Increased Serum Hepcidin Levels in Children and Adolescents with Attention Deficit Hyperactivity Disorder. Clin. Psychopharmacol. Neurosci. 2019, 17, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Girelli, D.; Nemeth, E.; Swinkels, D.W. Hepcidin in the diagnosis of iron disorders. Blood 2016, 127, 2809–2813. [Google Scholar] [CrossRef]
- Vela, D. Hepcidin, an emerging and important player in brain iron homeostasis. J. Transl. Med. 2018, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Miano, S.; Amato, N.; Foderaro, G.; Pezzoli, V.; Ramelli, G.P.; Toffolet, L.; Manconi, M. Sleep phenotypes in attention deficit hyperactivity disorder. Sleep Med. 2019, 60, 123–131. [Google Scholar] [CrossRef]
- Bruni, O.; Angriman, M.; Luchetti, A.; Ferri, R. Leg kicking and rubbing as a highly suggestive sign of pediatric restless legs syndrome. Sleep Med. 2015, 16, 1576–1577. [Google Scholar] [CrossRef]
- Ipsiroglu, O.S.; Beyzaei, N.; Berger, M.; Wagner, A.L.; Dhalla, S.; Garden, J.; Stockler, S. “Emplotted Narratives” and structured “Behavioral Observations” Supporting the diagnosis of Willis-Ekbom Disease/restless legs syndrome in children with neurodevelopmental conditions. CNS Neurosci. Ther. 2016, 22, 894–905. [Google Scholar] [CrossRef]
- Picchietti, D.L.; Bruni, O.; de Weerd, A.; Durmer, J.S.; Kotagal, S.; Owens, J.A.; Simakajornboon, N.; International Restless Legs Syndrome Study Group (IRLSSG). Pediatric restless legs syndrome diagnostic criteria: An update by the International Restless Legs Syndrome Study Group. Sleep Med. 2013, 14, 1253–1259. [Google Scholar] [CrossRef]
- Allen, R.P.; Picchietti, D.L.; Auerbach, M.; Cho, Y.W.; Connor, J.R.; Earley, C.J.; Garcia-Borreguero, D.; Kotagal, S.; Manconi, M.; Ondo, W.; et al. Evidence-based and consensus clinical practice guidelines for the iron treatment of restless legs syndrome/Willis-Ekbom disease in adults and children: An IRLSSG task force report. Sleep Med. 2018, 41, 27. [Google Scholar] [CrossRef]
- Picchietti, D.L.; Hesley, J.G.; Bainbridge, J.L.; Lee, K.A.; Manconi, M.; McGregor, J.A.; Silver, R.M.; Trenkwalder, C.; Walters, A.S.; International Restless Legs Syndrome Study Group (IRLSSG). Consensus clinical practice guidelines for the diagnosis and treatment of restless legs syndrome/Willis-Ekbom disease during pregnancy and lactation. Sleep Med. Rev. 2015, 22, 64–77. [Google Scholar] [CrossRef]
- Siu, A.L.; US Preventive Services Task Force. Screening for iron deficiency anemia in young children: USPSTF recommendation statement. Pediatrics 2015, 136, 746–752. [Google Scholar] [CrossRef]
- Carsley, S.; Fu, R.; Borkhoff, C.M.; Reid, N.; Baginska, E.; Birken, C.S.; Maguire, J.L.; Hancock-Howard, R.; Parkin, P.C.; Coyte, P.C. Iron deficiency screening for children at 18 months: A cost-utility analysis. CMAJ Open 2019, 7, E689–E698. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, R. The importance of diagnosing and treating iron deficiency in sleep disorders. Sleep Med. Rev. 2020, 51, 101314. [Google Scholar] [CrossRef] [PubMed]
- Kanney, M.L.; Durmer, J.S.; Trotti, L.M.; Leu, R. Rethinking bedtime resistance in children with autism: Is restless legs syndrome to blame? J. Clin. Sleep Med. 2020, 16, 2029–2035. [Google Scholar] [CrossRef]
- Chen, J.; Gong, N.J.; Chaim, K.T.; Otaduy, M.C.G.; Liu, C. Decompose quantitative susceptibility mapping (QSM) to sub-voxel diamagnetic and paramagnetic components based on gradient-echo MRI data. Neuroimage 2021, 242, 118477. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Feng, R.; Liu, Q.; Feng, J.; Lao, G.; Zhang, M.; Li, J.; Zhang, Y.; Wei, H. APART-QSM: An improved sub-voxel quantitative susceptibility mapping for susceptibility source separation using an iterative data fitting method. Neuroimage 2023, 274, 120148. [Google Scholar] [CrossRef] [PubMed]
- Kames, C.; Doucette, J.; Birkl, C.; Rauscher, A. Recovering SWI-filtered phase data using deep learning. Magn. Reson. Med. 2022, 87, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Walters, A.S.; Silvestri, R.; Zucconi, M.; Chandrashekariah, R.; Konofal, E. Review of the possible relationship and hypothetical links between attention deficit hyperactivity disorder (ADHD) and the simple sleep related movement disorders, parasomnias, hypersomnias, and circadian rhythm disorders. J. Clin. Sleep Med. 2008, 4, 591–600. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Comparan-Meza, M.; Vargas de la Cruz, I.; Jauregui-Huerta, F.; Gonzalez-Castañeda, R.E.; Gonzalez-Perez, O.; Galvez-Contreras, A.Y. Biopsychological correlates of repetitive and restricted behaviors in autism spectrum disorders. Brain Behav. 2021, 11, e2341. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMAScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
| Database | ||
|---|---|---|
| CINAHL | (MH “Anemia, Iron Deficiency”) OR (TI “iron deficiency anemia” OR AB “iron deficiency anemia”) OR (TI “iron deficiency” OR AB “iron deficiency”) | (MH “Practice Guidelines”) OR (TI guideline or AB guideline) |
| Embase | (iron deficiency/) OR (iron deficiency anemia/) OR (iron deficiency anemia. tw, kw.) OR (iron deficiency. tw, kw.) | (practice guideline/) OR (guideline. tw, kw.) |
| Medline | (Anemia, Iron Deficiency/) OR (iron deficiency. tw, kf.) OR (iron deficiency anemia. tw, kf.) | (Practice Guideline/OR Guideline/) OR (Guideline. tw, kf.) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McWilliams, S.; Hill, O.; Ipsiroglu, O.S.; Clemens, S.; Weber, A.M.; Chen, M.; Connor, J.; Felt, B.T.; Manconi, M.; Mattman, A.; et al. Iron Deficiency and Sleep/Wake Behaviors: A Scoping Review of Clinical Practice Guidelines—How to Overcome the Current Conundrum? Nutrients 2024, 16, 2559. https://doi.org/10.3390/nu16152559
McWilliams S, Hill O, Ipsiroglu OS, Clemens S, Weber AM, Chen M, Connor J, Felt BT, Manconi M, Mattman A, et al. Iron Deficiency and Sleep/Wake Behaviors: A Scoping Review of Clinical Practice Guidelines—How to Overcome the Current Conundrum? Nutrients. 2024; 16(15):2559. https://doi.org/10.3390/nu16152559
Chicago/Turabian StyleMcWilliams, Scout, Olivia Hill, Osman S. Ipsiroglu, Stefan Clemens, Alexander Mark Weber, Michael Chen, James Connor, Barbara T. Felt, Mauro Manconi, Andre Mattman, and et al. 2024. "Iron Deficiency and Sleep/Wake Behaviors: A Scoping Review of Clinical Practice Guidelines—How to Overcome the Current Conundrum?" Nutrients 16, no. 15: 2559. https://doi.org/10.3390/nu16152559
APA StyleMcWilliams, S., Hill, O., Ipsiroglu, O. S., Clemens, S., Weber, A. M., Chen, M., Connor, J., Felt, B. T., Manconi, M., Mattman, A., Silvestri, R., Simakajornboon, N., Smith, S. M., & Stockler, S. (2024). Iron Deficiency and Sleep/Wake Behaviors: A Scoping Review of Clinical Practice Guidelines—How to Overcome the Current Conundrum? Nutrients, 16(15), 2559. https://doi.org/10.3390/nu16152559








