Nutraceutical Capsules LL1 and Silymarin Supplementation Act on Mood and Sleep Quality Perception by Microbiota–Gut–Brain Axis: A Pilot Clinical Study
Abstract
1. Introduction
2. Materials and Methods
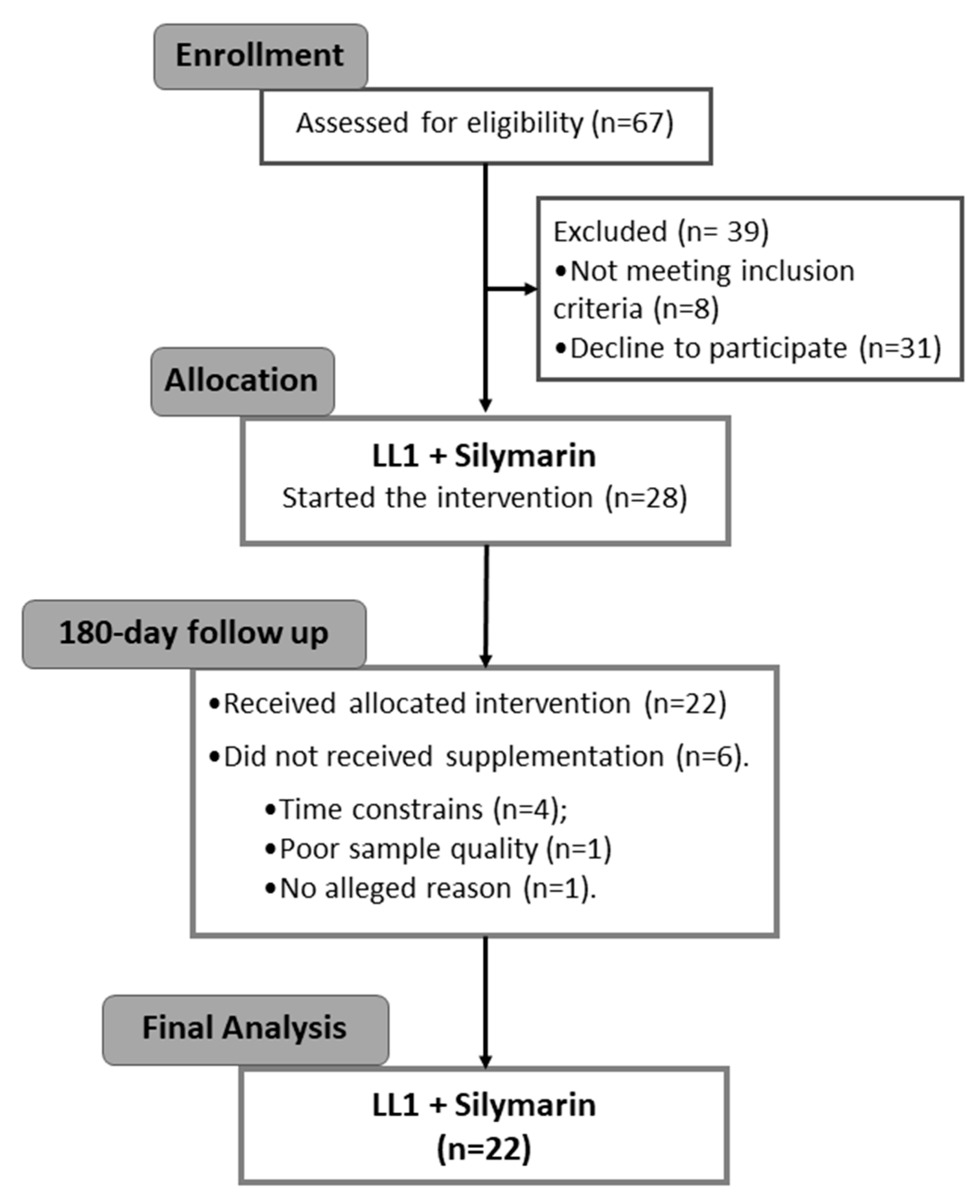
2.1. Ethics, Recruitment, and Experimental Design
2.2. Dietary Intake, Physical Activity, Sleep, Mood, and Quality of Life
2.3. Anthropometrics and Biochemistry Parameter
2.4. Cytokine and Chemokine Levels
2.5. Microbiome Analysis
2.6. Statistical Analysis
3. Results
3.1. Anthropometrics, Dietary Intake, Biochemistry, Cytokines, and Chemokines Modulation
3.2. Sleep, Mood, Physical Activity, and Quality of Life Effects
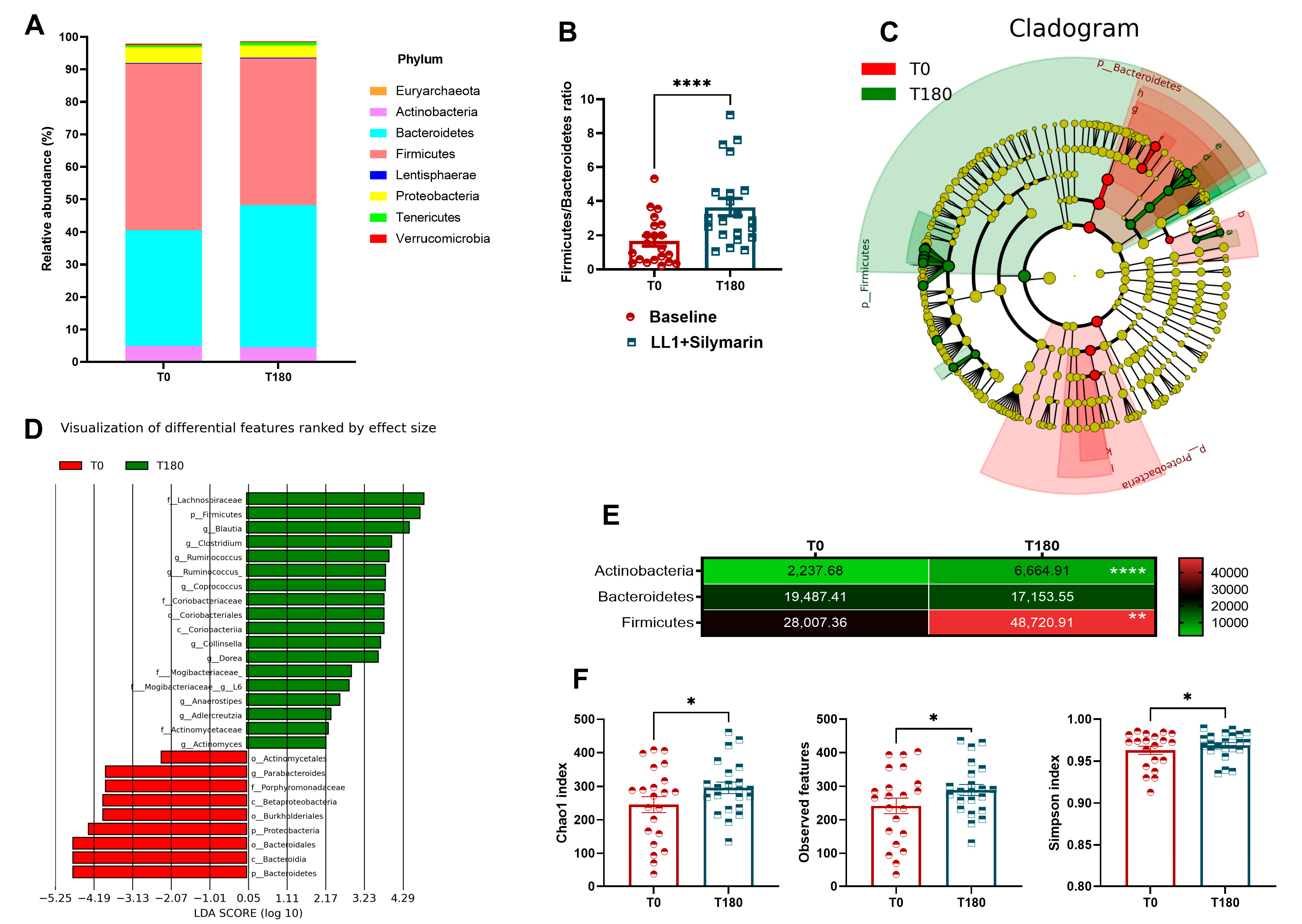
3.3. Gut Microbiota Reshaping
3.4. Predictive Parameters of Microbiota Reshaping
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bremner, J.; Moazzami, K.; Wittbrodt, M.; Nye, J.; Lima, B.; Gillespie, C.; Rapaport, M.; Pearce, B.; Shah, A.; Vaccarino, V. Diet, Stress and Mental Health. Nutrients 2020, 12, 2428. [Google Scholar] [CrossRef] [PubMed]
- Pearson, O.; Uglik-Marucha, N.; Miskowiak, K.W.; Cairney, S.A.; Rosenzweig, I.; Young, A.H.; Stokes, P.R.A. The Relationship between Sleep Disturbance and Cognitive Impairment in Mood Disorders: A Systematic Review. J. Affect. Disord. 2023, 327, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xue, P.; Zhang, H.; Tan, C.; Zhao, S.; Li, X.; Sun, L.; Zheng, H.; Wang, J.; Zhang, B.; et al. Gut Brain Interaction Theory Reveals Gut Microbiota Mediated Neurogenesis and Traditional Chinese Medicine Research Strategies. Front. Cell. Infect. Microbiol. 2022, 12, 1072341. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E.; Teixeira, A.L. Inflammation in Psychiatric Disorders: What Comes First? Ann. N. Y. Acad. Sci. 2019, 1437, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut Microbiota Interact With the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef]
- Chudzik, A.; Orzyłowska, A.; Rola, R.; Stanisz, G.J. Probiotics, Prebiotics and Postbiotics on Mitigation of Depression Symptoms: Modulation of the Brain–Gut–Microbiome Axis. Biomolecules 2021, 11, 1000. [Google Scholar] [CrossRef]
- Cheng, L.-H.; Liu, Y.-W.; Wu, C.-C.; Wang, S.; Tsai, Y.-C. Psychobiotics in Mental Health, Neurodegenerative and Neurodevelopmental Disorders. J. Food Drug Anal. 2019, 27, 632–648. [Google Scholar] [CrossRef]
- Sejbuk, M.; Mirończuk-Chodakowska, I.; Witkowska, A.M. Sleep Quality: A Narrative Review on Nutrition, Stimulants, and Physical Activity as Important Factors. Nutrients 2022, 14, 1912. [Google Scholar] [CrossRef]
- Lu, P.; Mamiya, T.; Lu, L.; Mouri, A.; Niwa, M.; Kim, H.C.; Zou, L.B.; Nagai, T.; Yamada, K.; Ikejima, T.; et al. Silibinin Attenuates Cognitive Deficits and Decreases of Dopamine and Serotonin Induced by Repeated Methamphetamine Treatment. Behav. Brain Res. 2010, 207, 387–393. [Google Scholar] [CrossRef]
- Avery, J.; Hoffmann, P. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef]
- Shrivastava, R.; Upreti, R.K.; Seth, P.K.; Chaturvedi, U.C. Effects of Chromium on the Immune System. FEMS Immunol. Med. Microbiol. 2002, 34, 1–7. [Google Scholar] [CrossRef]
- Siodłak, D.; Nowak, G.; Mlyniec, K. Interaction between Zinc, the GPR39 Zinc Receptor and the Serotonergic System in Depression. Brain Res. Bull. 2021, 170, 146–154. [Google Scholar] [CrossRef]
- Eby, G.A.; Eby, K.L. Magnesium for Treatment-Resistant Depression: A Review and Hypothesis. Med. Hypotheses 2010, 74, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Varlamov, O. Western-Style Diet, Sex Steroids and Metabolism. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 1147–1155. [Google Scholar] [CrossRef]
- Lobato, R.V.; Silva, V.d.O.; Andrade, E.F.; Orlando, D.R.; Zangerônimo, M.G.; de Souza, R.V.; Pereira, L.J. Metabolic Effects of β-Glucans (Saccharomyces Cerevisae) per Os Administration in Rats with Streptozotocin-Induced Diabetes. Nutr. Hosp. 2015, 32, 256–264. [Google Scholar] [CrossRef]
- Torres, D.P.M.; Gonçalves, M.d.P.F.; Teixeira, J.A.; Rodrigues, L.R. Galacto-Oligosaccharides: Production, Properties, Applications, and Significance as Prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Sabater-Molina, M.; Larqué, E.; Torrella, F.; Zamora, S. Dietary Fructooligosaccharides and Potential Benefits on Health. J. Physiol. Biochem. 2009, 65, 315–328. [Google Scholar] [CrossRef]
- Haarhuis, J.E.; Kardinaal, A.; Kortman, G.A.M. Probiotics, Prebiotics and Postbiotics for Better Sleep Quality: A Narrative Review. Benef. Microbes 2022, 13, 169–182. [Google Scholar] [CrossRef]
- Abenavoli, L.; Izzo, A.A.; Milić, N.; Cicala, C.; Santini, A.; Capasso, R. Milk Thistle (Silybum Marianum): A Concise Overview on Its Chemistry, Pharmacological, and Nutraceutical Uses in Liver Diseases. Phyther. Res. 2018, 32, 2202–2213. [Google Scholar] [CrossRef]
- Otzen, T.; Manterola, C. Human Experimentation: Code of Ethics of W.M.A. Br. Med. J. 1964, 2, 177. [Google Scholar] [CrossRef]
- Almutairi, R.; Basson, A.R.; Wearsch, P.A.; Cominelli, F.; Rodriguez-Palacios, A. Validity of Food Additive Maltodextrin as Placebo and Effects on Human Gut Physiology: Systematic Review of Placebo-controlled Clinical Trials. Eur. J. Nutr. 2022, 61, 2853–2871, Erratum in Eur. J. Nutr. 2023, 62, 2345. [Google Scholar] [CrossRef]
- Calgaro, M.; Pandolfo, M.; Salvetti, E.; Marotta, A.; Larini, I.; Pane, M.; Amoruso, A.; Del Casale, A.; Vitulo, N.; Fiorio, M.; et al. Metabarcoding Analysis of Gut Microbiota of Healthy Individuals Reveals Impact of Probiotic and Maltodextrin Consumption. Benef. Microbes 2021, 12, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liang, N.; Zhang, H.; Li, H.; Guo, J.; Zhang, Y.; Chen, Y.; Wang, Y.; Shi, N. Resistant Starch and the Gut Microbiome: Exploring Beneficial Interactions and Dietary Impacts. Food Chem. X 2024, 21, 101118. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.; Romero, R.; Hellhund, A.; Linne, U.; Bertrams, W.; Pinkenburg, O.; Eldin, H.S.; Binder, K.; Jacob, R.; Walker, A.; et al. Dietary Cellulose Induces Anti-Inflammatory Immunity and Transcriptional Programs via Maturation of the Intestinal Microbiota. Gut Microbes 2020, 12, 1829962. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zheng, P.; Qiu, J.; Chen, Q.; Zeng, S.; Zhang, Y.; Lin, S.; Zheng, B. High-Amylose Corn Starch Regulated Gut Microbiota and Serum Bile Acids in High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2022, 23, 5905. [Google Scholar] [CrossRef]
- Kadyan, S.; Park, G.; Wang, B.; Singh, P.; Arjmandi, B.; Nagpal, R. Resistant Starches from Dietary Pulses Modulate the Gut Metabolome in Association with Microbiome in a Humanized Murine Model of Ageing. Sci. Rep. 2023, 13, 10566. [Google Scholar] [CrossRef]
- Moraïs, S.; Winkler, S.; Zorea, A.; Levin, L.; Nagies, F.S.P.; Kapust, N.; Lamed, E.; Artan-Furman, A.; Bolam, D.N.; Yadav, M.P.; et al. Cryptic Diversity of Cellulose-Degrading Gut Bacteria in Industrialized Humans. Science 2024, 383, eadj9223. [Google Scholar] [CrossRef]
- Zaman, S.A.; Sarbini, S.R. The Potential of Resistant Starch as a Prebiotic. Crit. Rev. Biotechnol. 2016, 36, 578–584. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, S.W.; Kim, S.; Lee, Y.S.; Kim, T.Y.; Lee, S.H.; Kim, S.J.; Yoo, H.J.; Kim, E.N.; Kweon, M.N. Dietary Cellulose Prevents Gut Inflammation by Modulating Lipid Metabolism and Gut Microbiota. Gut Microbes 2020, 11, 944–961. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. Br. Med. J. 2010, 340, c332. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Dietary Reference Values for Nutrients Summary Report. EFSA Support. Publ. 2017, 14, e15121. [Google Scholar] [CrossRef]
- Matsudo, S.; Araújo, T.; Matsudo, V.; Andrade, D.; Andrade, E.; Oliveira, L.C.; Braggion, G. Questionário Internacional De Atividade Física (Ipaq): Estupo De Validade E Reprodutibilidade No Brasil. Rev. Bras. Atividade Física Saúde 2012, 6, 5–18. [Google Scholar] [CrossRef]
- Falavigna, A.; De Souza Bezerra, M.L.; Teles, A.R.; Kleber, F.D.; Velho, M.C.; Da Silva, R.C.; Mazzochin, T.; Santin, J.T.; Mosena, G.; De Braga, G.L.; et al. Consistency and Reliability of the Brazilian Portuguese Version of the Mini-Sleep Questionnaire in Undergraduate Students. Sleep Breath. 2011, 15, 351–355. [Google Scholar] [CrossRef]
- Bertolazi, A.N.; Fagondes, S.C.; Hoff, L.S.; Pedro, V.D.; Menna Barreto, S.S.; Johns, M.W. Portuguese-Language Version of the Epworth Sleepiness Scale: Validation for Use in Brazil. J. Bras. Pneumol. 2009, 35, 877–883. [Google Scholar] [CrossRef]
- Bertolazi, A.N.; Fagondes, S.C.; Hoff, L.S.; Dartora, E.G.; da Silva Miozzo, I.C.; de Barba, M.E.F.; Menna Barreto, S.S. Validation of the Brazilian Portuguese Version of the Pittsburgh Sleep Quality Index. Sleep Med. 2011, 12, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Van Esch, L.; Den Oudsten, B.L.; De Vries, J. The World Health Organization Quality of Life Instrument-Short Form (WHOQOL-BREF) in Women with Breast Problems. Int. J. Clin. Heal. Psychol. 2011, 11, 5–22. [Google Scholar]
- Rohlfs, I.C.P.D.M.; Rotta, T.M.; Luft, C.D.B.; Andrade, A.; Krebs, R.J.; De Carvalho, T. A Escala de Humor de Brunel (Brums): Instrumento Para Detecção Precoce Da Síndrome Do Excesso de Treinamento. Rev. Bras. Med. do Esporte 2008, 14, 176–181. [Google Scholar] [CrossRef]
- Nehmi-Filho, V.; de Freitas, J.A.; Franco, L.A.; Martins, R.C.; Turri, J.A.O.; Santamarina, A.B.; Fonseca, J.V.d.S.; Sabino, E.C.; Moraes, B.C.; Souza, E.; et al. Modulation of the Gut Microbiome and Firmicutes Phylum Reduction by a Nutraceutical Blend in the Obesity Mouse Model and Overweight Humans: A Double-Blind Clinical Trial. Food Sci. Nutr. 2024, 12, 2436–2454. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Faith, D.P. Conservation Evaluation and Phylogenetic Diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.; Anheyer, D.; Cramer, H.; Dobos, G. Complementary Therapies for Clinical Depression: An Overview of Systematic Reviews. BMJ Open 2019, 9, e028527. [Google Scholar] [CrossRef]
- Nehmi, V.A.; Murata, G.M.; de Moraes, R.C.M.; Lima, G.C.A.; De Miranda, D.A.; Radloff, K.; Costa, R.G.F.; Jesus, J.C.R.; De Freitas, J.A.; Viana, N.I.; et al. A Novel Supplement with Yeast β-Glucan, Prebiotic, Minerals and Silybum Marianum Synergistically Modulates Metabolic and Inflammatory Pathways and Improves Steatosis in Obese Mice. J. Integr. Med. 2021, 19, 439–450. [Google Scholar] [CrossRef]
- Santamarina, A.B.; Moraes, R.C.M.; Nehmi Filho, V.; Murata, G.M.; de Freitas, J.A.; de Miranda, D.A.; Cerqueira, A.R.A.; Costa, S.K.P.; Ferreira, A.F.F.; Britto, L.R.; et al. The Symbiotic Effect of a New Nutraceutical with Yeast β-Glucan, Prebiotics, Minerals, and Silybum Marianum (Silymarin) for Recovering Metabolic Homeostasis via Pgc-1α, Il-6, and Il-10 Gene Expression in a Type-2 Diabetes Obesity Model. Antioxidants 2022, 11, 447. [Google Scholar] [CrossRef]
- Nehmi-Filho, V.; Alves de Freitas, J.; Augusto Moysés Franco, L.; Vanessa da Silva Fonseca, J.; Cristina Ruedas Martins, R.; Boveto Santamarina, A.; Masahiro Murata, G.; Cerdeira Sabino, E.; Souza, E.; Thomas Ferreira, M.; et al. Novel Nutraceutical (Silymarin, Yeast β-Glucan, Prebiotics, and Minerals) Shifts Gut Microbiota and Restores Large Intestine Histology of Diet-Induced Metabolic Syndrome Mice. J. Funct. Foods 2023, 107, 105671. [Google Scholar] [CrossRef]
- Wilkialis, L.; Rodrigues, N.B.; Cha, D.S.; Siegel, A.; Majeed, A.; Lui, L.M.W.; Tamura, J.K.; Gill, B.; Teopiz, K.; McIntyre, R.S. Social Isolation, Loneliness and Generalized Anxiety: Implications and Associations during the Covid-19 Quarantine. Brain Sci. 2021, 11, 1620. [Google Scholar] [CrossRef]
- da Fonseca, M.; Maffei, G.; Moreno-Bote, R.; Hyafil, A. Mood and Implicit Confidence Independently Fluctuate at Different Time Scales. Cogn. Affect. Behav. Neurosci. 2023, 23, 142–161. [Google Scholar] [CrossRef]
- Galland, L. The Gut Microbiome and the Brain. J. Med. Food 2014, 17, 1261–1272. [Google Scholar] [CrossRef]
- Nehmi-Filho, V.; Santamarina, A.B.; de Freitas, J.A.; Trarbach, E.B.; de Oliveira, D.R.; Palace-Berl, F.; de Souza, E.; de Miranda, D.A.; Escamilla-Garcia, A.; Otoch, J.P.; et al. Novel Nutraceutical Supplements with Yeast β-Glucan, Prebiotics, Minerals, and Silybum Marianum (Silymarin) Ameliorate Obesity-Related Metabolic and Clinical Parameters: A Double-Blind Randomized Trial. Front. Endocrinol. 2023, 13, 1089938. [Google Scholar] [CrossRef]
- Guarino, M.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef]
- Mahindru, A.; Patil, P.; Agrawal, V. Role of Physical Activity on Mental Health and Well-Being: A Review. Cureus 2023, 15, e33475. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.; Naeem, N.; Farooq, Z.; Masood, S.; Iqbal, S.; Naseer, R. Effect of Prebiotic Galacto-Oligosaccharides on Serum Lipid Profile of Hypercholesterolemics. Probiotics Antimicrob. Proteins 2016, 8, 19–30. [Google Scholar] [CrossRef]
- Jung, E.; Kong, S.Y.; Ro, Y.S.; Ryu, H.H.; Shin, S. Do Serum Cholesterol Levels and Risk of Cardiovascular Death: A Systematic Review and a Dose-Response Meta-Analysis of Prospective Cohort Studies. Int. J. Environ. Res. Public Health 2022, 19, 8272. [Google Scholar] [CrossRef]
- Xu, D.; Feng, M.; Chu, Y.F.; Wang, S.; Shete, V.; Tuohy, K.M.; Liu, F.; Zhou, X.; Kamil, A.; Pan, D.; et al. The Prebiotic Effects of Oats on Blood Lipids, Gut Microbiota, and Short-Chain Fatty Acids in Mildly Hypercholesterolemic Subjects Compared With Rice: A Randomized, Controlled Trial. Front. Immunol. 2021, 12, 787797. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Microbiota and Thyroid Interaction in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, J.; Starchl, C.; Tmava Berisha, A.; Amrein, K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769. [Google Scholar] [CrossRef] [PubMed]
- Joffe, R.T.; Sullivan, T.B. The Significance of an Isolated Elevated TSH Level in a Depressed Patient: A Clinical Commentary. Int. J. Psychiatry Med. 2014, 48, 167–173. [Google Scholar] [CrossRef]
- Minuti, A.; Brufani, F.; Menculini, G.; Moretti, P.; Tortorella, A. The Complex Relationship between Gut Microbiota Dysregulation and Mood Disorders: A Narrative Review. Curr. Res. Neurobiol. 2022, 3, 100044. [Google Scholar] [CrossRef]
- Fousek, K.; Horn, L.A.; Palena, C. Interleukin-8: A Chemokine at the Intersection of Cancer Plasticity, Angiogenesis, and Immune Suppression. Pharmacol. Ther. 2021, 219, 107692. [Google Scholar] [CrossRef]
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M. Gut-Brain Axis and Migraine Headache: A Comprehensive Review. J. Headache Pain 2020, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Santamarina, A.B.; de Freitas, J.A.; Franco, L.A.M.; Nehmi-Filho, V.; Fonseca, J.V.; Martins, R.C.; Turri, J.A.; da Silva, B.F.R.B.; Fugi, B.E.I.; da Fonseca, S.S.; et al. Nutraceutical Blends Predict Enhanced Health via Microbiota Reshaping Improving Cytokines and Life Quality: A Brazilian Double-Blind Randomized Trial. Sci. Rep. 2024, 14, 11127. [Google Scholar] [CrossRef] [PubMed]
- Riccio, P.; Rossano, R. Undigested Food and Gut Microbiota May Cooperate in the Pathogenesis of Neuroinflammatory Diseases: A Matter of Barriers and a Proposal on the Origin of Organ Specificity. Nutrients 2019, 11, 2714. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.A.; Sun, E.W.; Martin, A.M.; Keating, D.J. The Ever-Changing Roles of Serotonin. Int. J. Biochem. Cell Biol. 2020, 125, 105776. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Noble, E.E.; Hsu, T.M.; Kanoski, S.E. Gut to Brain Dysbiosis: Mechanisms Linking Western Diet Consumption, the Microbiome, and Cognitive Impairment. Front. Behav. Neurosci. 2017, 11, 9. [Google Scholar] [CrossRef]
- Bruun, C.F.; Hansen, T.H.; Vinberg, M.; Kessing, L.V.; Coello, K. Associations between Short-Chain Fatty Acid Levels and Mood Disorder Symptoms: A Systematic Review. Nutr. Neurosci. 2023, 27, 899–912. [Google Scholar] [CrossRef]
- Dehghani, F.; Abdollahi, S.; Shidfar, F.; Clark, C.C.T.; Soltani, S. Probiotics Supplementation and Brain-Derived Neurotrophic Factor (BDNF): A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Neurosci. 2023, 26, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S.M. The Gut Microbiota in Anxiety and Depression—A Systematic Review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef]
- Chen, M.; Xie, C.R.; Shi, Y.Z.; Tang, T.C.; Zheng, H. Gut Microbiota and Major Depressive Disorder: A Bidirectional Mendelian Randomization. J. Affect. Disord. 2022, 316, 187–193. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, J.; Liang, H.; Ye, L.; Lan, L.; Lu, F.; Wang, Q.; Lei, T.; Yang, X.; Cui, P.; et al. Differences in Alpha Diversity of Gut Microbiota in Neurological Diseases. Front. Neurosci. 2022, 16, 879318. [Google Scholar] [CrossRef]
- Currie, T.L.; Engler, M.M.; Krauthamer, V.; Scott, J.M.; Deuster, P.A.; Flagg, T.P. Considerations for Optimizing Warfighter Psychological Health with a Research-Based Flavonoid Approach: A Review. Nutrients 2023, 15, 1204. [Google Scholar] [CrossRef]
- Hu, M. Commentary: Bioavailability of Flavonoids and Polyphenols: Call to Arms. Mol. Pharm. 2007, 4, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T.; Sharma, V.; Uchitel, S.; Denniston, K.; Chopra, D.; Mills, P.J.; Peterson, S.N. Prebiotic Potential of Herbal Medicines Used in Digestive Health and Disease. J. Altern. Complement. Med. 2018, 24, 656–665. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Wu, S.C. Health Benefits of Silybum Marianum: Phytochemistry, Pharmacology, and Applications. J. Agric. Food Chem. 2020, 68, 11644–11664. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Capasso, R.; Milic, N.; Capasso, F. Milk Thistle in Liver Diseases: Past, Present, Future. Phyther. Res. 2010, 24, 1423–1432. [Google Scholar] [CrossRef]

| LL1 (Long-Life 1) Capsules * | |
|---|---|
| Chromium (Cr) | 75 mcg |
| Zinc (Zn) | 26 mg |
| Magnesium (Mg) | 63.4 mg |
| Fructooligosaccharide (FOS) | 45% |
| Selenium | 140 mcg |
| Galactooligosaccharide (GOS) | 10% |
| 1.3/1.6-(β-glycosidic bonds) yeast β-glucans (Saccharomyces cerevisiae) | 250 mg |
| Silymarin Capsule | |
| Silymarin extract (Silybum marianum (L.) Gaertn.) # | 150 mg |
| LL1 + Silymarin | |||||
|---|---|---|---|---|---|
| Sample size (M/F) | 22 (7/15) | ||||
| Age (years) | 58.54 ± 5.65 | ||||
| Height (m) | 1.62 ± 0.10 | ||||
| Anthropometrics | |||||
| T0 | T180 | ||||
| Mean ± SD | Mean ± SD | p | |||
| BMI (kg/m2) | 28.9 ± 0.67 | 28.3 ± 0.76 | 0.0465 | ||
| Neck (cm) | 36.3 ± 0.70 | 35.7 ± 0.73 | 0.0049 | ||
| Plasmatic profile | |||||
| Total Cholesterol (mg/dL) | 194 ± 7.81 | 208 ± 7.19 | 0.0255 | ||
| HDL-C (mg/dL) | 45.7 ± 2.3 | 51.8 ± 2.61 | 0.0002 | ||
| IgM (mg/dL) | 104 ± 10.4 | 107 ± 10.4 | 0.0106 | ||
| Albumin (g/dL) | 4.65 ± 0.043 | 4.93 ± 0.052 | 0.0001 | ||
| Creatinine (mg/dL) | 0.83 ± 0.36 | 0.90 ± 0.04 | 0.0007 | ||
| TSH (mUI/L) | 2.02 ± 0.243 | 2.72 ± 0.352 | 0.0479 | ||
| Thyroxine (ng/dL) | 0.981 ± 0.045 | 1.36 ± 0.043 | 0.0001 | ||
| IL-8 (pg/mL) | 0.859 ± 0.231 | 0.422 ± 0.156 | 0.0161 | ||
| T0 | T180 | ||||
| Mean ± SD | CI 95% | Mean ± SD | CI 95% | p | |
| Pittsburgh Sleep Quality Index (PSQI) | |||||
| PSQI Global score | 6.39 ± 3.12 | 5.04–7.74 | 5.17 ± 2.77 | 3.97–6.37 | 0.0198 |
| (C1) Sleep Quality | 1.17 ± 0.65 | 0.89–1.46 | 0.83 ± 0.57 | 0.57–1.08 | 0.0078 |
| (C2) Sleep Latency | 1.09 ± 0.85 | 0.72–1.45 | 0.78 ± 0.16 | 0.44–1.13 | |
| (C3) Sleep Duration | 1.17 ± 0.58 | 0.93–1.42 | 1.30 ± 0.87 | 0.93–1.68 | - |
| (C4) Sleep Efficiency | 0.43 ± 0.73 | 0.12–0.75 | 0.43 ± 0.89 | 0.05–0.82 | - |
| (C5) Sleep Disturbance | 1.13 ± 0.55 | 0.89–1.37 | 0.96 ± 0.47 | 0.75–1.16 | - |
| (C6) Sleep Medication | 0.26 ± 0.75 | −0.06–0.59 | 0.13 ± 0.62 | −0.14–0.40 | - |
| (C7) Daytime Dysfunction | 1.35 ± 1.43 | 0.73–1.97 | 0.68 ± 0.65 | 0.39–0.97 | 0.0042 |
| Brunel Mood Scale (BRUMS) | |||||
| BRUMS total score | 20.14 ± 1.31 | 17.40–22.89 | 21.62 ± 1.46 | 18.56–24.68 | - |
| Tension | 3.90 ± 1.09 | 3.41–4.40 | 4.41 ± 1.71 | 3.65–5.17 | - |
| Depression | 2.38 ± 1.66 | 1.63–3.14 | 3.77 ± 3.24 | 2.34–5.21 | - |
| Anger | 3.19 ± 2.27 | 2.16–4.22 | 1.90 ± 1.61 | 1.17–2.64 | 0.0082 |
| Confusion | 3.90 ± 1.04 | 3.43–4.38 | 4.62 ± 1.47 | 3.95–5.29 | 0.0199 |
| Vigor | 3.48 ± 1.66 | 2.72–4.23 | 4.29 ± 1.55 | 3.58–4.99 | 0.0420 |
| Fatigue | 3.10 ± 1.34 | 2.49–3.70 | 3.24 ± 1.30 | 2.65–3.83 | - |
| % | R2 | IC 95% Min–Max | p | ||
|---|---|---|---|---|---|
| Body weight (kg) | Blautia producta | 0.71 | 0.22 | 1.21 | 0.009 |
| WC-mid (cm) | Bacteroidetes | 26.66 | 2.3 | 308.01 | 0.009 |
| Hip (cm) | Roseburia | 23.33 | 1.99 | 273.29 | 0.012 |
| WC-IC (cm) | Parabacteroides | 17.5 | 1.59 | 191.89 | 0.019 |
| Odoribacter | 19.99 | 1.67 | 238.62 | 0.018 | |
| WHR | Actinobacteria | 0.15 | 0.011 | 2.055 | 0.155 |
| Coprococcus | 12.01 | 1.58 | 91.08 | 0.016 | |
| WHtR | Bacteroidetes | 17.5 | 1.59 | 191.89 | 0.019 |
| TNF-α/IL-10 ratio | Anaerostipes | 56.01 | 2.92 | 1071.63 | 0.008 |
| R. Ruminococcus | 15.75 | 1.42 | 174.24 | 0.025 | |
| IL-6 (pg/mL) | Prevotella | 10.5 | 1.11 | 98.91 | 0.04 |
| Oscillospira | 7.11 | 1.08 | 46.44 | 0.04 | |
| Bilophila | 13.5 | 1.19 | 152.21 | 0.035 | |
| RANTES (pg/mL) | α-diversity chao1 index | 12.01 | 1.11 | 128.83 | 0.04 |
| α-diversity Faith’s PD | 12.01 | 1.11 | 128.83 | 0.04 | |
| α-diversity Obs features | 12.01 | 1.11 | 128.83 | 0.04 | |
| CXCL10/IP-10 (pg/mL) | Oscillospira | 7.11 | 1.08 | 46.44 | 0.04 |
| R. Ruminococcus | 7.11 | 1.08 | 46.44 | 0.04 | |
| BRUMS Anger | α-diversity chao1 index | 21.01 | 1.5 | 293.25 | 0.024 |
| α-diversity Obs features | 21.01 | 1.5 | 293.25 | 0.024 | |
| α-diversity Shanon entropy | 21.01 | 1.5 | 293.25 | 0.024 | |
| BRUMS Depression | Bacteroidetes | 32.01 | 2.39 | 427.74 | 0.009 |
| Bacteroides uniformis | 8.166 | 1.027 | 64.93 | 0.047 | |
| Alistipes onderdonkii | 30.01 | 1.471 | 611.79 | 0.027 | |
| Lachnospira | 0.122 | 0.015 | 0.973 | 0.047 | |
| BRUMS Vigor | Ruminococcus bromii | 24.01 | 1.14 | 505.19 | 0.041 |
| BRUMS Fatigue | Streptococcus | 14.01 | 1.13 | 172.64 | 0.039 |
| Variable | (%) | Coef | IC 95% Min–Max | p | |
|---|---|---|---|---|---|
| ↓TNF-α/IL-10 ratio | Bilophila | −4.4251 | −7.7851 | −1.0650 | 0.016 |
| Bifidobacterium | −0.1032 | −0.1740 | −0.0323 | 0.012 | |
| Clostridium spiroforme | −3.2441 | −4.9353 | −1.5529 | 0.009 | |
| Ruminococcus gnavus | −0.3283 | −0.5890 | −0.0677 | 0.022 | |
| Ruminococcus lactaris | 2.6987 | 0.1400 | 5.2573 | 0.042 | |
| ↓IL-6 (pg/mL) | Catenibacterium | −0.1599 | −0.2459 | −0.0739 | 0.003 |
| Ruminococcus callidus | 0.7058 | 0.2054 | 1.2062 | 0.012 | |
| Faecalibacterium prausnitzii | 0.4652 | 0.2876 | 0.6428 | <0.0001 | |
| Parabacteroides distasonis | 0.1206 | 0.0121 | 0.2291 | 0.032 | |
| ↑IL-10 (pg/mL) | Desulfovibrio | 1.3290 | 0.0201 | 2.6379 | 0.047 |
| Prevotella | −0.9742 | −1.6373 | −0.3110 | 0.008 | |
| ↓IL-12p70 (pg/mL) | Holdemania | 0.8647 | 0.0005 | 1.7289 | 0.05 |
| Catenibacterium | −0.1341 | −0.2282 | −0.0401 | 0.01 | |
| Faecalibacterium prausnitzii | 0.4129 | 0.1619 | 0.6639 | 0.003 | |
| Parabacteroides distasonis | 0.1785 | 0.0859 | 0.2711 | 0.001 | |
| ↓IL-8 (pg/mL) | Ruminococcus bromii | 5.4948 | 2.0311 | 8.9585 | 0.012 |
| Bacteroidetes | 1.6105 | 0.0040 | 3.2169 | 0.05 | |
| Alistipes | −0.814 | −1.590 | −0.039 | 0.041 | |
| α-diversity Faith’s PD | 2.021 | 0.258 | 3.784 | 0.027 | |
| Ruminococcus gnavus | 0.280 | 0.028 | 0.532 | 0.033 | |
| ↓RANTES (pg/mL) | Actinomyces | −0.802 | −1.253 | −0.351 | 0.003 |
| α-diversity Faith’s PD | −1.381 | −2.752 | −0.011 | 0.048 | |
| ↓Depression (BRUMS) | Desulfovibrio | −1.693 | −2.670 | −0.715 | 0.005 |
| Paraprevotella | −1.172 | −1.636 | −0.709 | 0.002 | |
| ↓Anger (BRUMS) | Roseburia faecis | 0.302 | 0.085 | 0.519 | 0.010 |
| Blautia producta | −0.303 | −0.597 | −0.009 | 0.045 | |
| ↓Confusion (BRUMS) | Alistipes | 0.182 | 0.011 | 0.353 | 0.038 |
| Firmicutes | −0.220 | −0.388 | −0.053 | 0.013 | |
| ↓PSQI (C2) Sleep Latency | Ruminococcus lactaris | 0.142 | 0.116 | 0.167 | 0.002 |
| ↓PSQI (C3) Sleep Duration | Faecalibacterium | 0.279 | 0.011 | 0.548 | 0.042 |
| ↓PSQI (C4) Sleep Efficiency | Faecalibacterium prausnitzii | −0.608 | −0.785 | −0.431 | 0.002 |
| Alistipes indistinctus | −0.127 | −0.182 | −0.072 | 0.022 | |
| ↓PSQI (C5) Sleep Disturbance | Phascolarctobacterium | 0.136 | 0.044 | 0.229 | 0.009 |
| Faecalibacterium | 0.410 | 0.224 | 0.596 | <0.0001 | |
| Clostridium spiroforme | −0.378 | −0.511 | −0.246 | 0.001 | |
| Bacteroides caccae | −0.102 | −0.191 | −0.013 | 0.030 | |
| ↓PSQI Global score | Eubacterium biforme | 0.507 | 0.128 | 0.886 | 0.014 |
| Ruminococcus callidus | 2.352 | 0.876 | 3.829 | 0.006 | |
| Coprococcus catus | 1.094 | 0.182 | 2.007 | 0.022 | |
| F/B ratio | 0.785 | 0.102 | 1.468 | 0.027 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santamarina, A.B.; Nehmi Filho, V.; Freitas, J.A.d.; Franco, L.A.M.; Fonseca, J.V.; Martins, R.C.; Turri, J.A.O.; Silva, B.F.R.B.d.; Gusmão, A.F.; Olivieri, E.H.R.; et al. Nutraceutical Capsules LL1 and Silymarin Supplementation Act on Mood and Sleep Quality Perception by Microbiota–Gut–Brain Axis: A Pilot Clinical Study. Nutrients 2024, 16, 3049. https://doi.org/10.3390/nu16183049
Santamarina AB, Nehmi Filho V, Freitas JAd, Franco LAM, Fonseca JV, Martins RC, Turri JAO, Silva BFRBd, Gusmão AF, Olivieri EHR, et al. Nutraceutical Capsules LL1 and Silymarin Supplementation Act on Mood and Sleep Quality Perception by Microbiota–Gut–Brain Axis: A Pilot Clinical Study. Nutrients. 2024; 16(18):3049. https://doi.org/10.3390/nu16183049
Chicago/Turabian StyleSantamarina, Aline Boveto, Victor Nehmi Filho, Jéssica Alves de Freitas, Lucas Augusto Moysés Franco, Joyce Vanessa Fonseca, Roberta Cristina Martins, José Antônio Orellana Turri, Bruna Fernanda Rio Branco da Silva, Arianne Fagotti Gusmão, Eloísa Helena Ribeiro Olivieri, and et al. 2024. "Nutraceutical Capsules LL1 and Silymarin Supplementation Act on Mood and Sleep Quality Perception by Microbiota–Gut–Brain Axis: A Pilot Clinical Study" Nutrients 16, no. 18: 3049. https://doi.org/10.3390/nu16183049
APA StyleSantamarina, A. B., Nehmi Filho, V., Freitas, J. A. d., Franco, L. A. M., Fonseca, J. V., Martins, R. C., Turri, J. A. O., Silva, B. F. R. B. d., Gusmão, A. F., Olivieri, E. H. R., Otoch, J. P., & Pessoa, A. F. M. (2024). Nutraceutical Capsules LL1 and Silymarin Supplementation Act on Mood and Sleep Quality Perception by Microbiota–Gut–Brain Axis: A Pilot Clinical Study. Nutrients, 16(18), 3049. https://doi.org/10.3390/nu16183049







