Prevention Is Better than Cure—Body Composition and Glycolipid Metabolism after a 24-Week Physical Activity Program without Nutritional Intervention in Healthy Sedentary Women
Abstract
1. Introduction
2. Materials and Methods
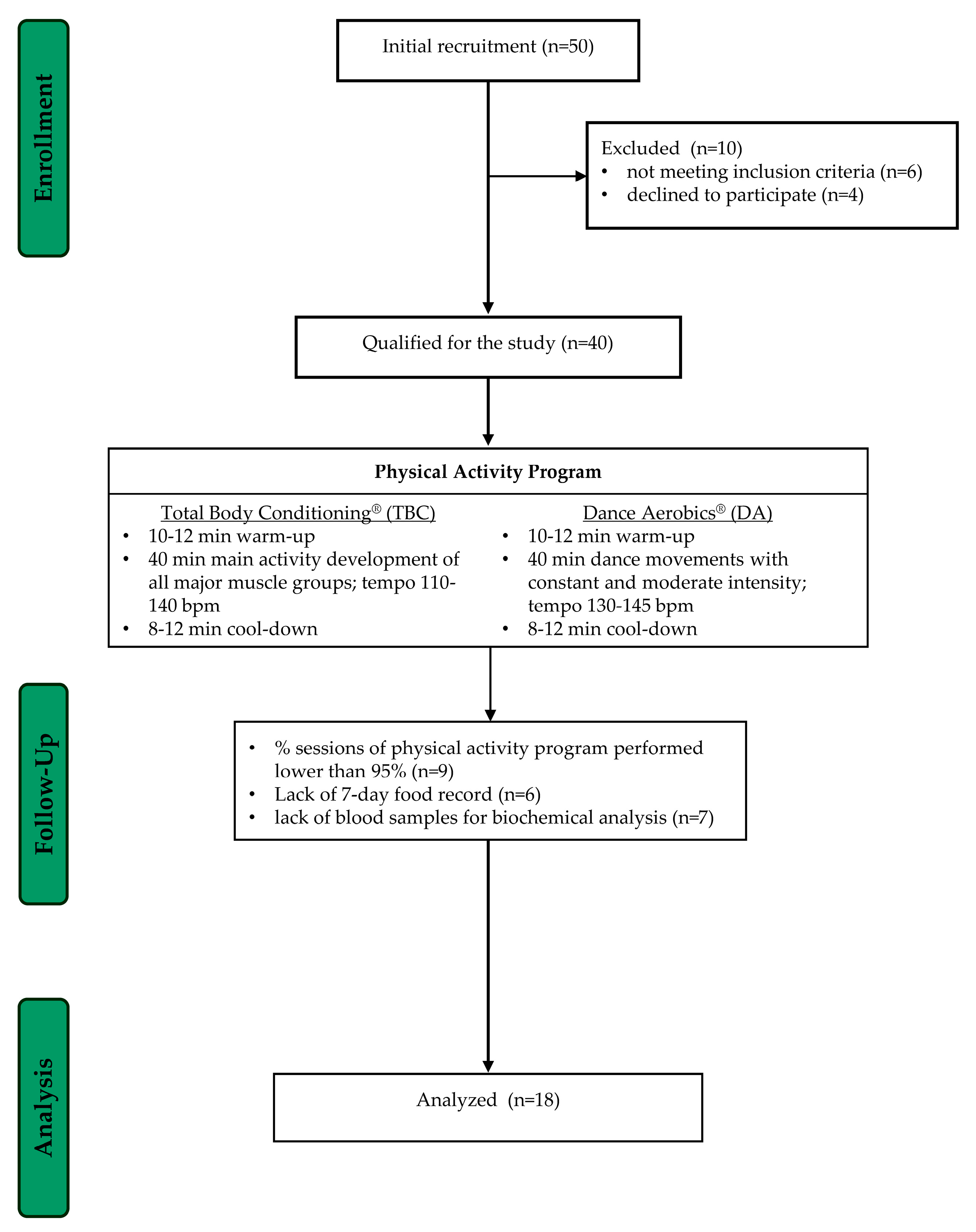
2.1. Participants
2.2. Study Design
2.3. Anthropometric and Body Composition Measurements
2.4. Nutritional Assessment
2.5. Exercise Energy Expenditure
2.6. The Physical Activity Program
2.7. Biochemical Analyses
2.8. Statistical Analyses
3. Results
3.1. Anthropometry and Body Composition
3.2. Biochemical Indices
3.3. Nutritional Evaluation
3.4. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myers, J.; Kokkinos, P.; Nyelin, E. Physical Activity, Cardiorespiratory Fitness, and the Metabolic Syndrome. Nutrients 2019, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
- Posadzki, P.; Pieper, D.; Bajpai, R.; Makaruk, H.; Könsgen, N.; Neuhaus, A.L.; Semwal, M. Exercise/Physical Activity and Health Outcomes: An Overview of Cochrane Systematic Reviews. BMC Public Health 2020, 20, 1724. [Google Scholar] [CrossRef] [PubMed]
- Suárez-López, L.M.; Bru-Luna, L.M.; Martí-Vilar, M. Influence of Nutrition on Mental Health: Scoping Review. Healthcare 2023, 11, 2183. [Google Scholar] [CrossRef] [PubMed]
- Díaz, B.B.; González, D.A.; Gannar, F.; Pérez, M.C.R.; de León, A.C. Myokines, Physical Activity, Insulin Resistance and Autoimmune Diseases. Immunol. Lett. 2018, 203, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.S. Effects of Aerobic Exercise on C-Reactive Protein, Body Composition, and Maximum Oxygen Consumption in Adults: A Meta-Analysis of Randomized Controlled Trials. Metabolism 2006, 55, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.S. Efficacy of Aerobic Exercise on Coronary Heart Disease Risk Factors. Prev. Cardiol. 2008, 11, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Thorogood, A.; Mottillo, S.; Shimony, A.; Filion, K.B.; Joseph, L.; Genest, J.; Pilote, L.; Poirier, P.; Schiffrin, E.L.; Eisenberg, M.J. Isolated Aerobic Exercise and Weight Loss: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Med. 2011, 124, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Missbach, B.; Dias, S.; König, J.; Hoffmann, G. Impact of Different Training Modalities on Glycaemic Control and Blood Lipids in Patients with Type 2 Diabetes: A Systematic Review and Network Meta-Analysis. Diabetologia 2014, 57, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, G.; Blake, C.; Cunningham, C.; Lennon, O.; Perrotta, C. What Exercise Prescription Is Optimal to Improve Body Composition and Cardiorespiratory Fitness in Adults Living with Obesity? A Network Meta-Analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2021, 22, e13137. [Google Scholar] [CrossRef]
- World Health Organization. Global Levels of Physical Inactivity in Adults: Off Track for 2030. Available online: https://iris.who.int/handle/10665/378026 (accessed on 30 June 2024).
- Lumish, H.S.; O’Reilly, M.; Reilly, M.P. Sex Differences in Genomic Drivers of Adipose Distribution and Related Cardiometabolic Disorders: Opportunities for Precision Medicine. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 45–60. [Google Scholar] [CrossRef]
- Barranco-Ruiz, Y.; Ramírez-Vélez, R.; Martínez-Amat, A.; Villa-González, E. Effect of Two Choreographed Fitness Group-Workouts on the Body Composition, Cardiovascular and Metabolic Health of Sedentary Female Workers. Int. J. Environ. Res. Public Health 2019, 16, 4986. [Google Scholar] [CrossRef] [PubMed]
- Johns, D.J.; Hartmann-Boyce, J.; Jebb, S.A.; Aveyard, P. Diet or Exercise Interventions vs Combined Behavioral Weight Management Programs: A Systematic Review and Meta-Analysis of Direct Comparisons. J. Acad. Nutr. Diet. 2014, 114, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Chavarrias, M.; Villafaina, S.; Lavín-Pérez, A.M.; Carlos-Vivas, J.; Merellano-Navarro, E.; Pérez-Gómez, J. Zumba®, Fat Mass and Maximum Oxygen Consumption: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Barranco-Ruiz, Y.; Villa-González, E. Choreographic Group-Based Fitness Classes Improve Cardiometabolic Health-Related Anthropometric Indices and Blood Lipids Profile in Overweight Sedentary Women. Sustainability 2021, 13, 972. [Google Scholar] [CrossRef]
- Rodrigues-Krause, J.; Farinha, J.B.; Krause, M.; Reischak-Oliveira, Á. Effects of Dance Interventions on Cardiovascular Risk with Ageing: Systematic Review and Meta-Analysis. Complement. Ther. Med. 2016, 29, 16–28. [Google Scholar] [CrossRef]
- Vendramin, B.; Bergamin, M.; Gobbo, S.; Cugusi, L.; Duregon, F.; Bullo, V.; Zaccaria, M.; Neunhaeuserer, D.; Ermolao, A. Health Benefits of Zumba Fitness Training: A Systematic Review. PM R 2016, 8, 1181–1200. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical Impedance Analysis—Part I: Review of Principles and Methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Gogojewicz, A.; Śliwicka, E.; Durkalec-Michalski, K. Assessment of Dietary Intake and Nutritional Status in CrossFit-Trained Individuals: A Descriptive Study | EndNote Click. Int. J. Environ. Res. Public Health 2020, 17, 4772. [Google Scholar] [CrossRef]
- Gogojewicz, A.; Straburzyńska-Lupa, A.; Podgórski, T.; Frajtag, P.; Bibrowicz, K.; Śliwicka, E. Assessment of the Dietary Intake and Nutritional Status of Polish Professional Futsal Players: A Descriptive Study-Do Futsal Players Require Nutritional Education? Nutrients 2023, 15, 3720. [Google Scholar] [CrossRef]
- Szponar, L.; Wolnicka, K.; Rychlik, E. Album Fotografii Produktów i Potraw, 1st ed.; Instytut Zywności i Żywienia: Warszawa, Poland, 2008. [Google Scholar]
- Kunachowicz, H.; Przygoda, B.; Nadolna, I.; Iwanow, K. Tabele Składu i Wartości Odżywczej Żywności, 1st ed.; PZWL Wydawnictwo Lekarskie: Warszawa, Poland, 2020. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Matsuhisa, M.; Yamasaki, Y.; Emoto, M.; Shimabukuro, M.; Ueda, S.; Funahashi, T.; Matsuzawa, Y. A Novel Index of Insulin Resistance Determined from the Homeostasis Model Assessment Index and Adiponectin Levels in Japanese Subjects. Diabetes Res. Clin. Pract. 2007, 77, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Endukuru, C.K.; Gaur, G.S.; Yerrabelli, D.; Sahoo, J.; Vairappan, B. Cut-off Values and Clinical Utility of Surrogate Markers for Insulin Resistance and Beta-Cell Function to Identify Metabolic Syndrome and Its Components among Southern Indian Adults. J. Obes. Metab. Syndr. 2020, 29, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Normy Żywieia dla Populacji Polskiej i ich Zastosowanie; Narodowy Instytut Zdrowia Publicznego—Państwowy Zakład Higieny: Warszawa, Poland, 2020. [Google Scholar]
- Pedersen, B.K. Anti-Inflammatory Effects of Exercise: Role in Diabetes and Cardiovascular Disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Gong, L.; Zhang, E.; Wang, X. Exploring Exercise-Driven Exerkines: Unraveling the Regulation of Metabolism and Inflammation. PeerJ 2024, 12, e17267. [Google Scholar] [CrossRef] [PubMed]
- Zorena, K.; Jachimowicz-Duda, O.; Ślęzak, D.; Robakowska, M.; Mrugacz, M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef] [PubMed]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Pilaczynska-Szczesniak, L.; Sliwicka, E.; Deskur-Smielecka, E.; Karolkiewicz, J.; Piechowiak, A. Insulin Resistance and Glucose Tolerance in Obese Women: The Effects of a Recreational Training Program. J. Sports Med. Phys. Fitness 2008, 48, 252–258. [Google Scholar] [PubMed]
- Racil, G.; Ben Ounis, O.; Hammouda, O.; Kallel, A.; Zouhal, H.; Chamari, K.; Amri, M. Effects of High vs. Moderate Exercise Intensity during Interval Training on Lipids and Adiponectin Levels in Obese Young Females. Eur. J. Appl. Physiol. 2013, 113, 2531–2540. [Google Scholar] [CrossRef]
- Lakhdar, N.; Denguezli, M.; Monia, Z.; Zbidi, A.; Tabka, Z.; Bouassida, A. Six Months Training Alone or Combined with Diet Alters HOMA-AD, HOMA-IR and Plasma and Adipose Tissue Adiponectin in Obese Women. Neuroendocrinol. Lett. 2014, 35, 373–379. [Google Scholar]
- Markofski, M.M.; Carrillo, A.E.; Timmerman, K.L.; Jennings, K.; Coen, P.M.; Pence, B.D.; Flynn, M.G. Exercise Training Modifies Ghrelin and Adiponectin Concentrations and Is Related to Inflammation in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Jonas, M.I.; Kurylowicz, A.; Bartoszewicz, Z.; Lisik, W.; Jonas, M.; Domienik-Karlowicz, J.; Puzianowska-Kuznicka, M. Adiponectin/Resistin Interplay in Serum and in Adipose Tissue of Obese and Normal-Weight Individuals. Diabetol. Metab. Syndr. 2017, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Silswal, N.; Singh, A.K.; Aruna, B.; Mukhopadhyay, S.; Ghosh, S.; Ehtesham, N.Z. Human Resistin Stimulates the Pro-Inflammatory Cytokines TNF-Alpha and IL-12 in Macrophages by NF-kappaB-Dependent Pathway. Biochem. Biophys. Res. Commun. 2005, 334, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Würfel, M.; Blüher, M.; Stumvoll, M.; Ebert, T.; Kovacs, P.; Tönjes, A.; Breitfeld, J. Adipokines as Clinically Relevant Therapeutic Targets in Obesity. Biomedicines 2023, 11, 1427. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulou, I.; Fernhall, B.; Carhart, R.; Weinstock, R.S.; Baynard, T.; Figueroa, A.; Kanaley, J.A. Effects of Diet and/or Exercise on the Adipocytokine and Inflammatory Cytokine Levels of Postmenopausal Women with Type 2 Diabetes. Metabolism 2005, 54, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.E.; Basilio, J.L.; Brophy, P.M.; McCammon, M.R.; Hickner, R.C. Long-Term Exercise Training in Overweight Adolescents Improves Plasma Peptide YY and Resistin. Obes. Silver Spring Md. 2009, 17, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Prestes, J.; Shiguemoto, G.; Botero, J.P.; Frollini, A.; Dias, R.; Leite, R.; Pereira, G.; Magosso, R.; Baldissera, V.; Cavaglieri, C.; et al. Effects of Resistance Training on Resistin, Leptin, Cytokines, and Muscle Force in Elderly Post-Menopausal Women. J. Sports Sci. 2009, 27, 1607–1615. [Google Scholar] [CrossRef]
- Jorge, M.L.M.P.; de Oliveira, V.N.; Resende, N.M.; Paraiso, L.F.; Calixto, A.; Diniz, A.L.D.; Resende, E.S.; Ropelle, E.R.; Carvalheira, J.B.; Espindola, F.S.; et al. The Effects of Aerobic, Resistance, and Combined Exercise on Metabolic Control, Inflammatory Markers, Adipocytokines, and Muscle Insulin Signaling in Patients with Type 2 Diabetes Mellitus. Metabolism 2011, 60, 1244–1252. [Google Scholar] [CrossRef]
- Botero, J.P.; Shiguemoto, G.E.; Prestes, J.; Marin, C.T.; Do Prado, W.L.; Pontes, C.S.; Guerra, R.L.F.; Ferreia, F.C.; Baldissera, V.; Perez, S.E.A. Effects of Long-Term Periodized Resistance Training on Body Composition, Leptin, Resistin and Muscle Strength in Elderly Post-Menopausal Women. J. Sports Med. Phys. Fit. 2013, 53, 289–294. [Google Scholar]
- Dakroub, A.; A Nasser, S.; Younis, N.; Bhagani, H.; Al-Dhaheri, Y.; Pintus, G.; Eid, A.A.; El-Yazbi, A.F.; Eid, A.H. Visfatin: A Possible Role in Cardiovasculo-Metabolic Disorders. Cells 2020, 9, 2444. [Google Scholar] [CrossRef]
- Abdalla, M.M.I. Role of Visfatin in Obesity-Induced Insulin Resistance. World J. Clin. Cases 2022, 10, 10840–10851. [Google Scholar] [CrossRef] [PubMed]
- Sethi, J.K.; Vidal-Puig, A. Visfatin: The missing link between intra-abdominal obesity and diabetes? Trends Mol. Med. 2005, 11, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Guerendiain, M.; Villa-González, E.; Barranco-Ruiz, Y. Body Composition and Dairy Intake in Sedentary Employees Who Participated in a Healthy Program Based on Nutrition Education and Zumba. Clin. Nutr. Edinb. Scotl. 2019, 38, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R.; Meijer, G.A.; Janssen, E.M.; Saris, W.H.; Ten Hoor, F. Long-Term Effect of Physical Activity on Energy Balance and Body Composition. Br. J. Nutr. 1992, 68, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Yarizadeh, H.; Eftekhar, R.; Anjom-Shoae, J.; Speakman, J.R.; Djafarian, K. The Effect of Aerobic and Resistance Training and Combined Exercise Modalities on Subcutaneous Abdominal Fat: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Adv. Nutr. Bethesda Md. 2021, 12, 179–196. [Google Scholar] [CrossRef]
- Westerterp, K.R. Exercise, Energy Balance and Body Composition. Eur. J. Clin. Nutr. 2018, 72, 1246–1250. [Google Scholar] [CrossRef]
- Wilk, M.; Zajac, A.; Tufano, J.J. The Influence of Movement Tempo During Resistance Training on Muscular Strength and Hypertrophy Responses: A Review. Sports Med. 2021, 51, 1629–1650. [Google Scholar] [CrossRef] [PubMed]
- Okura, T.; Nakata, Y.; Tanaka, K. Effects of Exercise Intensity on Physical Fitness and Risk Factors for Coronary Heart Disease. Obes. Res. 2003, 11, 1131–1139. [Google Scholar] [CrossRef]
- Westcott, W.L.; Apovian, C.M.; Puhala, K.; Corina, L.; Larosa Loud, R.; Whitehead, S.; Blum, K.; DiNubile, N. Nutrition Programs Enhance Exercise Effects on Body Composition and Resting Blood Pressure. Phys. Sportsmed. 2013, 41, 85–91. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN Exercise & Sports Nutrition Review Update: Research & Recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [CrossRef]
- Poehlman, E.T.; Dvorak, R.V.; DeNino, W.F.; Brochu, M.; Ades, P.A. Effects of Resistance Training and Endurance Training on Insulin Sensitivity in Nonobese, Young Women: A Controlled Randomized Trial. J. Clin. Endocrinol. Metab. 2000, 85, 2463–2468. [Google Scholar] [CrossRef] [PubMed]
- Rodziewicz-Flis, E.A.; Kawa, M.; Kaczor, J.J.; Szaro-Truchan, M.; Flis, D.J.; Lombardi, G.; Ziemann, E. Changes in Selected Exerkines Concentration Post Folk-Dance Training Are Accompanied by Glucose Homeostasis and Physical Performance Improvement in Older Adults. Sci. Rep. 2023, 13, 8596. [Google Scholar] [CrossRef]
- Makni, E.; Moalla, W.; Lac, G.; Aouichaoui, C.; Cannon, D.; Elloumi, M.; Tabka, Z. The Homeostasis Model Assessment-Adiponectin (HOMA-AD) Is the Most Sensitive Predictor of Insulin Resistance in Obese Children. Ann. Endocrinol. 2012, 73, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Vilela, B.S.; Vasques, A.C.J.; Cassani, R.S.L.; Forti, A.C.E.; Pareja, J.C.; Tambascia, M.A.; BRAMS Investigators; Geloneze, B. The HOMA-Adiponectin (HOMA-AD) Closely Mirrors the HOMA-IR Index in the Screening of Insulin Resistance in the Brazilian Metabolic Syndrome Study (BRAMS). PLoS ONE 2016, 11, e0158751. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, N.A.; Irawan, R.; Hanindita, M.H.; Ugrasena, I.; Handajani, R. METS-IR vs. HOMA-AD and Metabolic Syndrome in Obese Adolescents. J. Med. Investig. JMI 2023, 70, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.F.; Klein, S. Lipid Metabolism during Endurance Exercise. Am. J. Clin. Nutr. 2000, 72 (Suppl. S2), 558S–563S. [Google Scholar] [CrossRef] [PubMed]
- Fogelholm, M. Exercise, Substrate Oxidation and Energy Balance. Int. J. Obes. (2005) 2006, 30, 1022. [Google Scholar] [CrossRef]
- Durstine, J.L.; Grandjean, P.W.; Davis, P.G.; Ferguson, M.A.; Alderson, N.L.; DuBose, K.D. Blood Lipid and Lipoprotein Adaptations to Exercise: A Quantitative Analysis. Sports Med. 2001, 31, 1033–1062. [Google Scholar] [CrossRef]
- Tambalis, K.; Panagiotakos, D.B.; Kavouras, S.A.; Sidossis, L.S. Responses of Blood Lipids to Aerobic, Resistance, and Combined Aerobic with Resistance Exercise Training: A Systematic Review of Current Evidence. Angiology 2009, 60, 614–632. [Google Scholar] [CrossRef]
- Greene, N.P.; Martin, S.E.; Crouse, S.F. Acute Exercise and Training Alter Blood Lipid and Lipoprotein Profiles Differently in Overweight and Obese Men and Women. Obes. Silver Spring Md. 2012, 20, 1618–1627. [Google Scholar] [CrossRef]
- Fikenzer, K.; Fikenzer, S.; Laufs, U.; Werner, C. Effects of Endurance Training on Serum Lipids. Vascul. Pharmacol. 2018, 101, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Stasiulis, A.; Mockiene, A.; Vizbaraite, D.; Mockus, P. Aerobic Exercise-Induced Changes in Body Composition and Blood Lipids in Young Women. Med. Kaunas Lith. 2010, 46, 129–134. [Google Scholar] [CrossRef]
- Kostrzewa-Nowak, D.; Nowak, R.; Jastrzębski, Z.; Zarębska, A.; Bichowska, M.; Drobnik-Kozakiewicz, I.; Radzimiński, Ł.; Leońska-Duniec, A.; Ficek, K.; Cięszczyk, P. Effect of 12-Week-Long Aerobic Training Programme on Body Composition, Aerobic Capacity, Complete Blood Count and Blood Lipid Profile among Young Women. Biochem. Medica 2015, 25, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zheng, B. Effect of Yoga Combined with Aerobic Exercise Intervention on Morphological and Blood Lipid Indicators in Female College Students. J. Sports Med. Phys. Fit. 2020, 60, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Poslusna, K.; Ruprich, J.; de Vries, J.H.M.; Jakubikova, M.; van’t Veer, P. Misreporting of Energy and Micronutrient Intake Estimated by Food Records and 24 Hour Recalls, Control and Adjustment Methods in Practice. Br. J. Nutr. 2009, 101 (Suppl. S2), S73–S85. [Google Scholar] [CrossRef]
- Cumberledge, E.A.; Myers, C.; Venditti, J.J.; Dixon, C.B.; Andreacci, J.L. The Effect of the Menstrual Cycle on Body Composition Determined by Contact-Electrode Bioelectrical Impedance Analyzers. Int. J. Exerc. Sci. 2018, 11, 625. [Google Scholar] [PubMed]
| Variables | Before | After | p-Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (years) | 34.7 ± 5.3 | ||
| Body height (cm) | 163.3 ± 5.5 | ||
| Body mass (kg) | 61.7 ± 4.5 | 60.7 ± 4.5 | 0.0000 |
| BMI | 23.2 ± 1.4 | 22.5 ± 1.6 | 0.0044 |
| Waist circumference (cm) | 83.2 ± 5.7 | 80.8 ± 5.9 | 0.0000 |
| Fat (%) | 31.5 ± 4.0 | 30.3 ± 4.4 | 0.0237 |
| FM (kg) | 19.5 ± 3.1 | 18.5 ± 3.4 | 0.0047 |
| FFM (kg) | 42.3 ± 3.3 | 42.3 ± 3.0 | 0.9794 |
| TBW (%) | 49.3 ± 3.1 | 50.1 ± 3.6 | 0.0200 |
| SBP (mmHg) | 117.5 ± 12.6 | 110.0 ± 10.0 | 0.0698 |
| DBP (mmHg) | 73.8 ± 10.8 | 71.7 ± 11.9 | 0.2728 |
| Variables | Before | After | p-Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Glucose (mg·dL−1) | 95.2 ± 8.3 | 87.9 ± 9.6 | 0.0006 |
| Insulin (µIU·mL−1) | 10.4 ± 2.5 | 8.3 ± 2.4 | 0.0000 |
| TG (mg·dL−1) | 98.5 ± 44.5 | 84.6 ± 28.5 | 0.0250 |
| Total cholesterol (mg·dL−1) | 197.0 ± 18.3 | 193.2 ± 15.5 | 0.0641 |
| HDL-CH (mg·dL−1) | 62.0 ± 11.6 | 62.1 ± 12.8 | 0.9034 |
| LDL-CH (mg·dL−1) | 115.4 ± 17.8 | 114.2 ± 19.4 | 0.6231 |
| hsCRP (mg·L−1) | 1.6 ± 2.6 | 1.1 ± 1.4 | 0.0494 |
| Resistin (ng·mL−1) | 5.3 ± 1.8 | 4.3 ± 1.3 | 0.0272 |
| Visfatin (ng·mL−1) | 2.1 ± 1.5 | 2.1 ± 1.3 | 0.9467 |
| Adiponectin (µg·mL−1) | 15.8 ± 2.6 | 18.2 ± 2.5 | 0.0000 |
| HOMA-IR | 2.43 ± 0.67 | 1.87 ± 0.69 | 0.0000 |
| HOMA-AD | 3.57 ± 1.32 | 2.26 ± 0.90 | 0.0001 |
| Nutrient | Mean ± SD | RDA |
|---|---|---|
| Energy intake (kcal) | 1811.8 ± 395.7 | 1800–1900 |
| EEE during DA (kcal) | 331.1 ± 122.6 | |
| EEE during TBC (kcal) | 353.6 ± 61.0 | |
| CHO | ||
| (g) | 230.6 ± 75.8 | |
| (% energy) | 45.6 ± 21.1 | 45–65 |
| (g·kgbm−1) | 3.7 ± 1.1 | |
| Saccharose (g) | 42.8 ± 19.4 | |
| Saccharose (% energy) | 9.2 ± 3.3 | |
| Fiber (g) | 18.3 ± 6.6 | 25 g |
| PRO | ||
| (g) | 76.9 ± 16.2 | |
| (% energy) | 17.4 ± 4.1 | 10–20 |
| (g·kgbm−1) | 1.2 ± 0.2 | 0.9 |
| FAT | ||
| (g) | 66.8 ± 20.5 | |
| (% energy) | 33.8 ± 9.1 | 20–35 |
| (g·kgbm−1) | 1.3 ± 0.3 | 0.5–1.0 |
| Cholesterol (mg) | 252.4 ± 73.2 | <300 |
| SFA (g) SFA (%) | 25.4 ± 6.4 13.0 ± 3.3 | |
| PUFA (g) PUFA (%) | 8.2 ± 2.6 4.1 ± 1.11 | 0.5–1.0 § 6–10 |
| MUFA (g) MUFA (%) | 24.3 ± 6.83 12.3 ± 3.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śliwicka, E.; Popierz-Rydlewska, N.; Straburzyńska-Lupa, A.; Nikolov, J.; Pilaczyńska-Szcześniak, Ł.; Gogojewicz, A. Prevention Is Better than Cure—Body Composition and Glycolipid Metabolism after a 24-Week Physical Activity Program without Nutritional Intervention in Healthy Sedentary Women. Nutrients 2024, 16, 2536. https://doi.org/10.3390/nu16152536
Śliwicka E, Popierz-Rydlewska N, Straburzyńska-Lupa A, Nikolov J, Pilaczyńska-Szcześniak Ł, Gogojewicz A. Prevention Is Better than Cure—Body Composition and Glycolipid Metabolism after a 24-Week Physical Activity Program without Nutritional Intervention in Healthy Sedentary Women. Nutrients. 2024; 16(15):2536. https://doi.org/10.3390/nu16152536
Chicago/Turabian StyleŚliwicka, Ewa, Natalia Popierz-Rydlewska, Anna Straburzyńska-Lupa, Jivko Nikolov, Łucja Pilaczyńska-Szcześniak, and Anna Gogojewicz. 2024. "Prevention Is Better than Cure—Body Composition and Glycolipid Metabolism after a 24-Week Physical Activity Program without Nutritional Intervention in Healthy Sedentary Women" Nutrients 16, no. 15: 2536. https://doi.org/10.3390/nu16152536
APA StyleŚliwicka, E., Popierz-Rydlewska, N., Straburzyńska-Lupa, A., Nikolov, J., Pilaczyńska-Szcześniak, Ł., & Gogojewicz, A. (2024). Prevention Is Better than Cure—Body Composition and Glycolipid Metabolism after a 24-Week Physical Activity Program without Nutritional Intervention in Healthy Sedentary Women. Nutrients, 16(15), 2536. https://doi.org/10.3390/nu16152536







