Unraveling the Interaction between Inflammation and the Cardiometabolic Index in Older Men: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Statement
2.3. AI Assistance in Manuscript Preparation
2.4. Participants’ Selection Criteria
2.5. Calculation of the Sample Size
2.6. Clinical and Physical Data
2.7. Blood Sample Collection
2.8. Analysis of the Lipid Profile
2.9. Cardiometabolic Index
2.10. Multiplex Cytokine Analysis
2.11. Statistical Analysis
3. Results
3.1. Characterization of the Sample
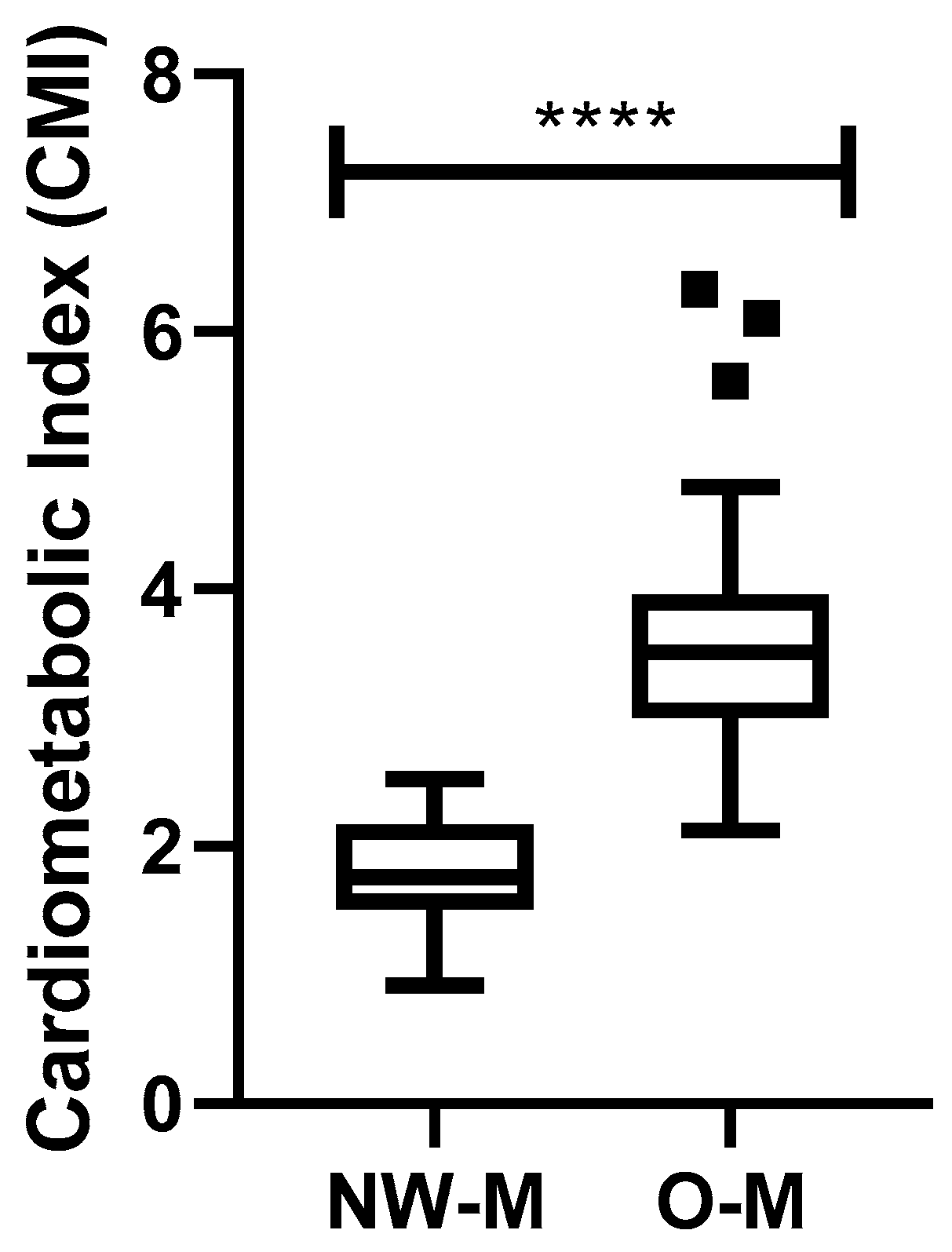
3.2. Analysis of the Cardiometabolic Index
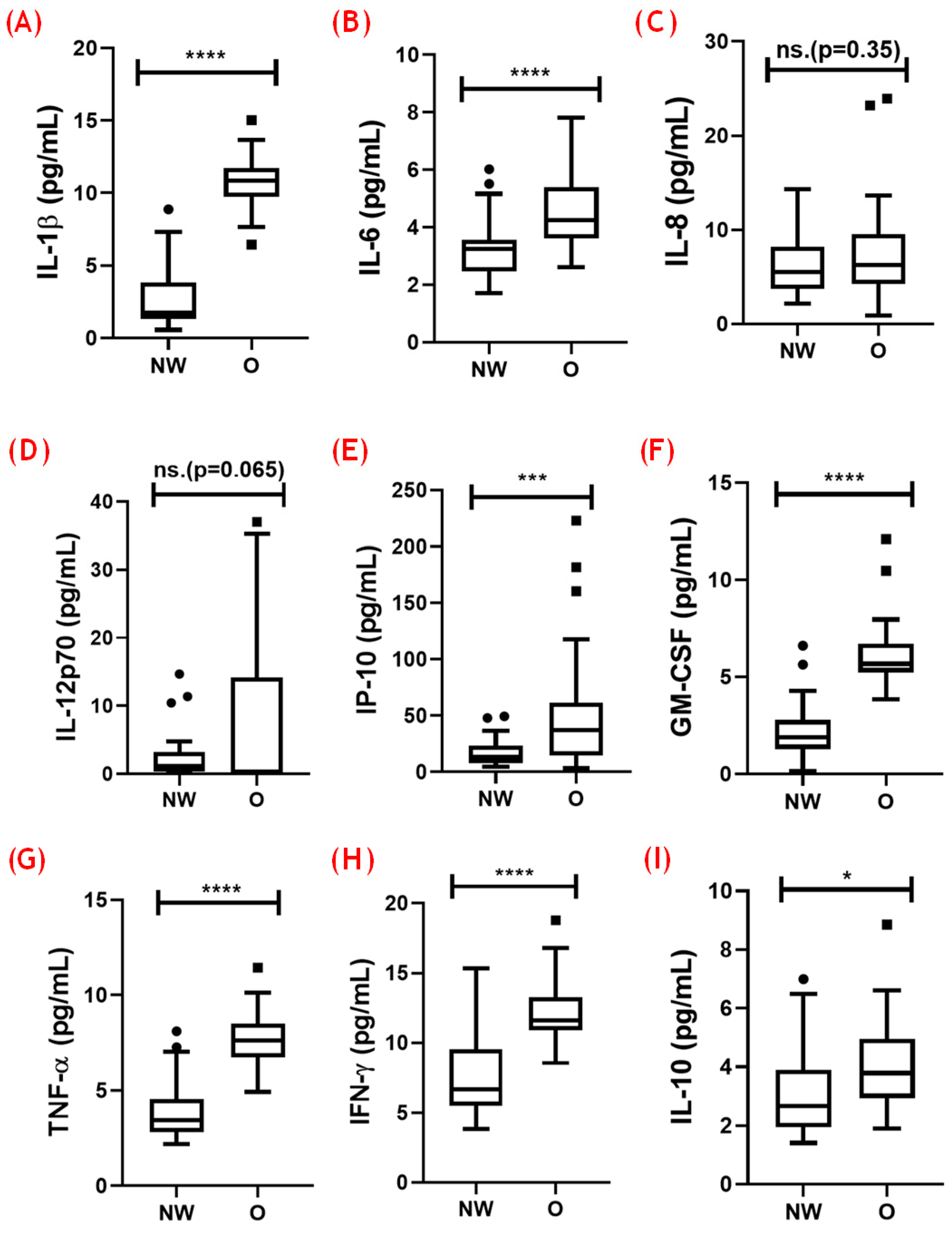
3.3. Analysis of Systemic Cytokines
3.4. Analysis of the Balance between Pro- and Anti-Inflammatory Cytokines
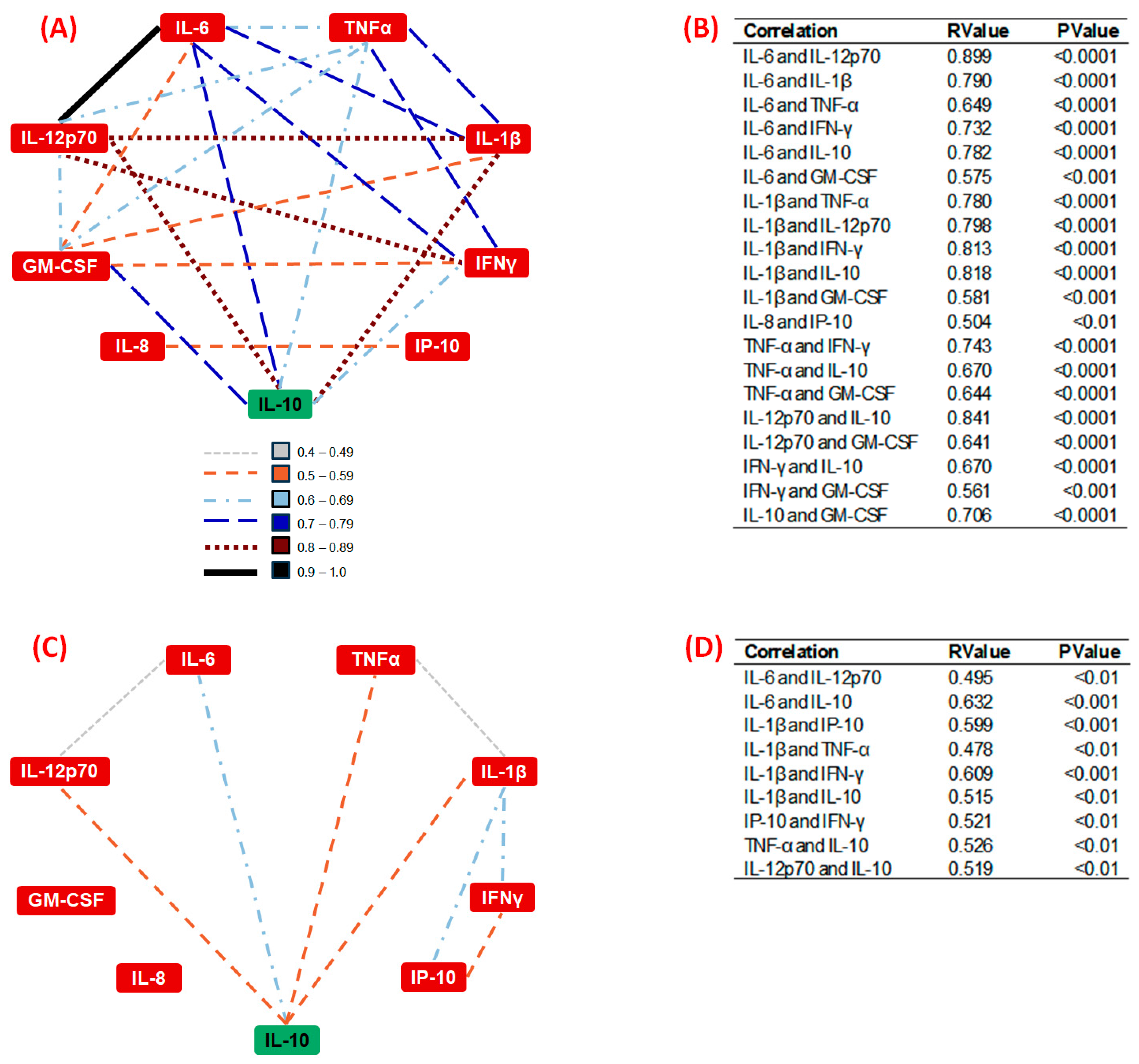
3.5. Correlation Analysis of Systemic Inflammatory Cytokines
3.6. Analysis of the Interplay between Inflammatory Status and the Cardiometabolic Index
3.6.1. Results in the NW Group
3.6.2. Results in the O Group
3.7. Analysis of the Potential Effects of Confounding Factors on CMI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO—World Health Organization Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 17 July 2023).
- Facts & Figures. Available online: https://idf.org/about-diabetes/diabetes-facts-figures/ (accessed on 19 June 2024).
- International Diabetes Federation (IDF). IDF Diabetes Atlas Reports. 2023. Available online: https://diabetesatlas.org/atlas-reports/ (accessed on 1 May 2024).
- Parker, E.D.; Lin, J.; Mahoney, T.; Ume, N.; Yang, G.; Gabbay, R.A.; ElSayed, N.A.; Bannuru, R.R. Economic Costs of Diabetes in the U.S. in 2022. Diabetes Care 2024, 47, 26–43. [Google Scholar] [CrossRef] [PubMed]
- Hypertension. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 21 June 2024).
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. Ser. A 2014, 69, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Meier, H.C.S.; Mitchell, C.; Karadimas, T.; Faul, J.D. Systemic Inflammation and Biological Aging in the Health and Retirement Study. GeroScience 2023, 45, 3257–3265. [Google Scholar] [CrossRef] [PubMed]
- Ruck, L.; Wiegand, S.; Kühnen, P. Relevance and Consequence of Chronic Inflammation for Obesity Development. Mol. Cell. Pediatr. 2023, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H. Obese Visceral Fat. Tissue Inflammation: From Protective to Detrimental? BMC Med. 2022, 20, 494. [Google Scholar] [CrossRef] [PubMed]
- Teissier, T.; Boulanger, E.; Cox, L.S. Interconnections between Inflammageing and Immunosenescence during Ageing. Cells 2022, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory Cytokines, Aging, and Age-Related Diseases. J. Am. Med. Dir. Assoc. 2013, 14, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The Master Regulator of Immunity to Infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef]
- Tang, C.; Pang, T.; Dang, C.; Liang, H.; Wu, J.; Shen, X.; Wang, L.; Luo, R.; Lan, H.; Zhang, P. Correlation between the Cardiometabolic Index and Arteriosclerosis in Patients with Type 2 Diabetes Mellitus. BMC Cardiovasc. Disord. 2024, 24, 186. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Q.; Yan, G.; Duan, J.; Chen, Z.; Yang, P.; Bragazzi, N.L.; Lu, Y.; Yuan, H. Cardiometabolic Index: A New Tool for Screening the Metabolically Obese Normal Weight Phenotype. J. Endocrinol. Invest. 2021, 44, 1253–1261. [Google Scholar] [CrossRef]
- Wakabayashi, I.; Daimon, T. The “Cardiometabolic Index” as a New Marker Determined by Adiposity and Blood Lipids for Discrimination of Diabetes Mellitus. Clin. Chim. Acta 2015, 438, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Yang, D.; Xia, H.; Ren, Z.; Chen, J.; Yao, S. Cardiometabolic Index: A New Predictor for Metabolic Associated Fatty Liver Disease in Chinese Adults. Front. Endocrinol. 2022, 13, 1004855. [Google Scholar] [CrossRef]
- Elm, E.v.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- de Lima, S.G.; de Lima, T.A.G.; de Macedo, L.A.; de Oliveira Sá, M.P.B.; de Lima Vidal, M.; Gomes, A.F.; Oliveira, L.C.; Santos, A.M.A. Ethics in Research with Human Beings: From Knowledge to Practice. Arq. Bras. Cardiol. 2010, 95, 289–294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Picorelli, A.M.A.; Pereira, L.S.M.; Pereira, D.S.; Felício, D.; Sherrington, C. Adherence to Exercise Programs for Older People Is Influenced by Program Characteristics and Personal Factors: A Systematic Review. J. Physiother. 2014, 60, 151–156. [Google Scholar] [CrossRef]
- Draper, N.R.; Smith, H. Applied Regression Analysis; John Wiley & Sons: Hoboken, NJ, USA, 1998; ISBN 978-0-471-17082-2. [Google Scholar]
- Montgomery, D.C.; Peck, E.A.; Vining, G.G. Introduction to Linear Regression Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-0-470-54281-1. [Google Scholar]
- Wenzl, F.A.; Ambrosini, S.; Mohammed, S.A.; Kraler, S.; Lüscher, T.F.; Costantino, S.; Paneni, F. Inflammation in Metabolic Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 742178. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A New Immune–Metabolic Viewpoint for Age-Related Diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Cobos-Palacios, L.; Ruiz-Moreno, M.I.; Vilches-Perez, A.; Vargas-Candela, A.; Muñoz-Úbeda, M.; Porres, J.B.; Navarro-Sanz, A.; Lopez-Carmona, M.D.; Sanz-Canovas, J.; Perez-Belmonte, L.M.; et al. Metabolically Healthy Obesity: Inflammatory Biomarkers and Adipokines in Elderly Population. PLoS ONE 2022, 17, e0265362. [Google Scholar] [CrossRef]
- Guo, W.; Jia, J.; Zhan, M.; Li, X.; Zhu, W.; Lu, J.; Zhao, X.; Xu, N.; Zhang, Q. Association of Metabolically Unhealthy Non-Obese and Metabolically Healthy Obese Individuals with Arterial Stiffness and 10-Year Cardiovascular Disease Risk: A Cross-Sectional Study in Chinese Adults. Nutr. J. 2023, 22, 44. [Google Scholar] [CrossRef]
- Kristiansen, O.P.; Mandrup-Poulsen, T. Interleukin-6 and Diabetes: The Good, the Bad, or the Indifferent? Diabetes 2005, 54, S114–S124. [Google Scholar] [CrossRef]
- Bersch-Ferreira, Â.C.; Sampaio, G.R.; Gehringer, M.O.; Torres, E.A.F.D.S.; Ross-Fernandes, M.B.; da Silva, J.T.; Torreglosa, C.R.; Kovacs, C.; Alves, R.; Magnoni, C.D.; et al. Association between Plasma Fatty Acids and Inflammatory Markers in Patients with and without Insulin Resistance and in Secondary Prevention of Cardiovascular Disease, a Cross-Sectional Study. Nutr. J. 2018, 17, 26. [Google Scholar] [CrossRef] [PubMed]
- Stowe, R.P.; Peek, M.K.; Cutchin, M.P.; Goodwin, J.S. Plasma Cytokine Levels in a Population-Based Study: Relation to Age and Ethnicity. J. Gerontol. Ser. A 2010, 65A, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Fabersani, E.; Marquez, A.; Gauffin-Cano, P. Adipose Tissue Inflammation and Metabolic Syndrome. The Proactive Role of Probiotics. Eur. J. Nutr. 2019, 58, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Shu, W.; Hou, X.; Wang, Y. Innate Immune System Orchestrates Metabolic Homeostasis and Dysfunction in Visceral Adipose Tissue During Obesity. Front. Immunol. 2021, 12, 702835. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.C.H.T.; Rossi, M.; dos Santos, C.A.F.; Oliveira, L.V.F.; Vencio, S.; de Paula Vieira, R.; Juliano, Y.; Armond, J.; Silva, C.H.M.; Fonseca, A.L.; et al. Prevalence of Associations among Sarcopenia, Obesity, and Metabolic Syndrome in Brazilian Older Adults. Front. Med. 2023, 10, 1206545. [Google Scholar] [CrossRef]
- Rangel, S.C.; da Silva, M.D.; Natrielli Filho, D.G.; Santos, S.N.; do Amaral, J.B.; Victor, J.R.; Silva, K.C.N.; Tuleta, I.D.; França, C.N.; Shio, M.T.; et al. HERV-W Upregulation Expression in Bipolar Disorder and Schizophrenia: Unraveling Potential Links to Systemic Immune/Inflammation Status. Retrovirology 2024, 21, 7. [Google Scholar] [CrossRef]
- Fico, B.G.; Maharaj, A.; Pena, G.S.; Huang, C.-J. The Effects of Obesity on the Inflammatory, Cardiovascular, and Neurobiological Responses to Exercise in Older Adults. Biology 2023, 12, 865. [Google Scholar] [CrossRef]
- Dragon-Durey, M.-A.; Chen, X.; Kirilovsky, A.; Hamouda, N.B.; Sissy, C.E.; Russick, J.; Charpentier, E.; Binois, Y.; Marliot, F.; Meylan, M.; et al. Differential Association between Inflammatory Cytokines and Multiorgan Dysfunction in COVID-19 Patients with Obesity. PLoS ONE 2021, 16, e0252026. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B.; Paganelli, R. Aging, Obesity, and Inflammatory Age-Related Diseases. Front. Immunol. 2017, 8, 1745. [Google Scholar] [CrossRef]
- Shimi, G.; Sohouli, M.H.; Ghorbani, A.; Shakery, A.; Zand, H. The Interplay between Obesity, Immunosenescence, and Insulin Resistance. Immun. Ageing 2024, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.D.S.; de Souza Silva, C.M.; Ferreira Fratelli, C.; Ramos de Lima, L.; Morato Stival, M.; Schwerz Funghetto, S.; Rodrigues da Silva, I.C.; Vieira de Andrade, R. IL-10 and IL-1β Serum Levels, Genetic Variants, and Metabolic Syndrome: Insights into Older Adults’ Clinical Characteristics. Nutrients 2024, 16, 1241. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.M.; Bergamini, E.; Brunk, U.T.; Dröge, W.; Ffrench, M.; Terman, A. Autophagy and Aging: The Importance of Maintaining “Clean” Cells. Autophagy 2005, 1, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Vitale, G.; Capri, M.; Salvioli, S. Inflammaging and ‘Garb-Aging’. Trends Endocrinol. Metab. 2017, 28, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Pinti, M.; Gibellini, L.; Lo Tartaro, D.; De Biasi, S.; Nasi, M.; Borella, R.; Fidanza, L.; Neroni, A.; Troiano, L.; Franceschi, C.; et al. A Comprehensive Analysis of Cytokine Network in Centenarians. Int. J. Mol. Sci. 2023, 24, 2719. [Google Scholar] [CrossRef] [PubMed]
- Silveira-Nunes, G.; Speziali, E.; Teixeira-Carvalho, A.; Vitelli-Avelar, D.M.; Sathler-Avelar, R.; Figueiredo-Soares, T.; Silva, M.L.; Peruhype-Magalhães, V.; Chaves, D.G.; Brito-Melo, G.E.; et al. Lifewide Profile of Cytokine Production by Innate and Adaptive Immune Cells from Brazilian Individuals. Immun. Ageing 2017, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergl, R.; Kirkby, K.C.; Faßhauer, M.; Stumvoll, M.; et al. Inflammatory Cytokines in General and Central Obesity and Modulating Effects of Physical Activity. PLoS ONE 2015, 10, e0121971. [Google Scholar] [CrossRef] [PubMed]
- Neves, C.V.B.; Mambrini, J.V.d.M.; Torres, K.C.L.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Lima-Costa, M.F.; Peixoto, S.V. Association of Metabolic Syndrome with Inflammatory Markers in a Sample of Community-Dwelling Older Adults. Cad. Saude Publica 2019, 35, e00129918. [Google Scholar] [CrossRef] [PubMed]
- Kaptoge, S.; Seshasai, S.R.K.; Gao, P.; Freitag, D.F.; Butterworth, A.S.; Borglykke, A.; Di Angelantonio, E.; Gudnason, V.; Rumley, A.; Lowe, G.D.O.; et al. Inflammatory Cytokines and Risk of Coronary Heart Disease: New Prospective Study and Updated Meta-Analysis. Eur. Heart J. 2014, 35, 578–589. [Google Scholar] [CrossRef]
- Jung, H.N.; Jung, C.H. The Role of Anti-Inflammatory Adipokines in Cardiometabolic Disorders: Moving beyond Adiponectin. Int. J. Mol. Sci. 2021, 22, 13529. [Google Scholar] [CrossRef]
- Kim, S.J.; Lim, J.; Nam, G.E.; Park, H.S. Correlation between Serum Lipid Parameters and Interleukin-10 Concentration in Obese Individuals. J. Obes. Metab. Syndr. 2021, 30, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Torres-Orozco, A.K.; De León, L.G.; Ortiz-Rodríguez, B.; Candia-Luján, R. Wakabayashi & Daimon Cardiometabolic Index as an Indicator to Assess Risk in Adults. A Syst. Review. Aten. Primaria 2024, 56, 102846. [Google Scholar] [CrossRef]
- He, L.; Lin, C.; Tu, Y.; Yang, Y.; Lin, M.; Tu, H.; Li, J. Correlation of Cardiometabolic Index and Sarcopenia with Cardiometabolic Multimorbidity in Middle-Aged and Older Adult: A Prospective Study. Front. Endocrinol. 2024, 15, 1387374. [Google Scholar] [CrossRef]
- Yan, L.; Hu, X.; Wu, S.; Cui, C.; Zhao, S. Association between the Cardiometabolic Index and NAFLD and Fibrosis. Sci. Rep. 2024, 14, 13194. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Lin, W.; Zhou, Q.; Yang, Y.; Liu, X.; Chen, J.; Liu, H.; Zhang, C. Association of Adiposity Indicators with Cardiometabolic Multimorbidity Risk in Hypertensive Patients: A Large Cross-Sectional Study. Front. Endocrinol. 2024, 15, 1302296. [Google Scholar] [CrossRef]
- Xu, B.; Wu, Q.; La, R.; Lu, L.; Abdu, F.A.; Yin, G.; Zhang, W.; Ding, W.; Ling, Y.; He, Z.; et al. Is Systemic Inflammation a Missing Link between Cardiometabolic Index with Mortality? Evidence from a Large Population-Based Study. Cardiovasc. Diabetol. 2024, 23, 212. [Google Scholar] [CrossRef]


| NW | O | p-Value | |
|---|---|---|---|
| Demographic data | |||
| n | 34 | 32 | |
| Age | 71.79 ± 6.85 | 71.78 ± 7.95 | >0.05 |
| Anthropometric data | |||
| Height (cm) | 168.80 ± 6.37 | 167.00 ± 7.54 | >0.05 |
| Weight (kg) | 64.40 ± 6.50 | 93.35 ± 3.88 | <0.0001 |
| Waist circ. (cm) | 87.72 ± 9.96 | 113.30 ± 22.99 | <0.0001 |
| BMI (kg/m²) | 22.58 ± 1.53 | 33.31 ± 3.02 | <0.0001 |
| Clinical data | |||
| HDL-c (mg/dL) | 48.52 ± 9.70 | 37.15 ± 7.31 | <0.0001 |
| LDL-c (mg/dL) | 117.4 ± 50.63 | 97.20 ± 30.74 | >0.05 |
| Triglycerides (mg/dL) | 164.60 ± 48.84 | 200.80 ± 51.18 | <0.001 |
| Total cholesterol (mg/dL) | 198.9 ± 59.78 | 164.2 ± 46.66 | >0.05 |
| Glucose (mg/dL) | 104.7 ± 36.62 | 101.9 ± 36.63 | >0.05 |
| Hypertension | 18 (52.9%) | 30 (93.7%) | <0.001 |
| DM-2 | 11 (32.3%) | 17 (53.1%) | >0.05 |
| CVD | 8 (23.5%) | 9 (28.1%) | >0.05 |
| Stroke | 2 (5.8%) | 5 (15.6%) | >0.05 |
| Cancer | 4 (11.7%) | 5 (15.6%) | >0.05 |
| Physical activity | |||
| Walking | 13 (38.2%) | 6 (18.7%) | >0.05 |
| Light jogging | 0 | 0 | >0.05 |
| Gymnastics | 1 (2.9%) | 0 | >0.05 |
| Weight training | 0 | 1 (3.1%) | >0.05 |
| Swimming | 1 (2.9%) | 1 (3.1%) | >0.05 |
| Physically active | 3 (8.8%) | 1 (3.1%) | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, R.L.; Brito, T.R.P.; Amaral, J.B.; Monteiro, F.R.; Lima, D.B.; Pereira, T.A.M.; da Costa, B.F.; Furtado, G.E.; Rodrigues, P.M.M.; dos Santos, C.A.F.; et al. Unraveling the Interaction between Inflammation and the Cardiometabolic Index in Older Men: A Pilot Study. Nutrients 2024, 16, 2529. https://doi.org/10.3390/nu16152529
Carvalho RL, Brito TRP, Amaral JB, Monteiro FR, Lima DB, Pereira TAM, da Costa BF, Furtado GE, Rodrigues PMM, dos Santos CAF, et al. Unraveling the Interaction between Inflammation and the Cardiometabolic Index in Older Men: A Pilot Study. Nutrients. 2024; 16(15):2529. https://doi.org/10.3390/nu16152529
Chicago/Turabian StyleCarvalho, Rafael L., Tábatta R. P. Brito, Jônatas B. Amaral, Fernanda R. Monteiro, Daniela B. Lima, Thalles A. M. Pereira, Beatriz F. da Costa, Guilherme E. Furtado, Pamella M. M. Rodrigues, Carlos A. F. dos Santos, and et al. 2024. "Unraveling the Interaction between Inflammation and the Cardiometabolic Index in Older Men: A Pilot Study" Nutrients 16, no. 15: 2529. https://doi.org/10.3390/nu16152529
APA StyleCarvalho, R. L., Brito, T. R. P., Amaral, J. B., Monteiro, F. R., Lima, D. B., Pereira, T. A. M., da Costa, B. F., Furtado, G. E., Rodrigues, P. M. M., dos Santos, C. A. F., Bachi, A. L. L., & Sarmento, A. d. O. (2024). Unraveling the Interaction between Inflammation and the Cardiometabolic Index in Older Men: A Pilot Study. Nutrients, 16(15), 2529. https://doi.org/10.3390/nu16152529







