Abstract
Background: Excessive fluoride exposure induces skeletal fluorosis, but the specific mechanism responsible is still unclear. Therefore, this study aimed to identify the pathogenesis of fluoride-induced bone injuries. Methods: We systematically searched fluoride-induced bone injury-related genes from five databases. Then, these genes were subjected to enrichment analyses. A TF (transcription factor)–mRNA–miRNA network and protein–protein interaction (PPI) network were constructed using Cytoscape, and the Human Protein Atlas (HPA) database was used to screen the expression of key proteins. The candidate pharmacological targets were predicted using the Drug Signature Database. Results: A total of 85 studies were included in this study, and 112 osteoblast-, 35 osteoclast-, and 41 chondrocyte-related differential expression genes (DEGs) were identified. Functional enrichment analyses showed that the Atf4, Bcl2, Col1a1, Fgf21, Fgfr1 and Il6 genes were significantly enriched in the PI3K-Akt signaling pathway of osteoblasts, Mmp9 and Mmp13 genes were enriched in the IL-17 signaling pathway of osteoclasts, and Bmp2 and Bmp7 genes were enriched in the TGF-beta signaling pathway of chondrocytes. With the use of the TF–mRNA–miRNA network, the Col1a1, Bcl2, Fgfr1, Mmp9, Mmp13, Bmp2, and Bmp7 genes were identified as the key regulatory factors. Selenium methyl cysteine, CGS-27023A, and calcium phosphate were predicted to be the potential drugs for skeletal fluorosis. Conclusions: These results suggested that the PI3K-Akt signaling pathway being involved in the apoptosis of osteoblasts, with the IL-17 and the TGF-beta signaling pathways being involved in the inflammation of osteoclasts and chondrocytes in fluoride-induced bone injuries.
1. Introduction
Fluorine, an essential trace element for human beings, exists in the environment in the form of fluoride []. Fluoride is widely distributed in the soil, rocks, and water throughout the world []. Due to volcanic eruptions, mineral dissolution, and human activities, fluoride accumulates in the natural environment []. Fluoride has a strong affinity for bones, and a large amount of absorbed fluoride tends to accumulate in bones []. Therefore, the concentration of fluoride is closely related to bone development. The World Health Organization (WHO) has established a maximum limit of 1.5 mg/L for fluorine in drinking water []. An adequate intake of fluoride promotes the formation of the skeleton [,]. On the contrary, excessive accumulation of fluoride can lead to skeletal fluorosis [,].
Skeletal fluorosis is a metabolic bone disease, and it mostly involves bone joints. The manifestations of skeletal fluorosis include diffuse osteosclerosis, skeletal pain, connective tissue calcification, and stiffening of bone joints [,]. Recent studies have indicated that skeletal fluorosis is associated with the imbalance of bone metabolism, mainly referring to the imbalance between bone formation by osteoblasts and bone resorption by osteoclasts [,]. A moderate amount of fluoride promotes the proliferation of osteoblasts and increases bone mass, alkaline phosphatase, bone morphogenetic protein (BMP), and bone gla protein levels []. Excessive fluoride leads to the apoptosis of osteoblasts through the activation of caspase-3, resulting in an imbalance of bone remodeling and triggering various bone diseases []. Bone resorption in osteoclasts is also essential for normal bone remodeling []. Excessive osteoclastic bone resorption can induce osteoporosis and autoimmune arthritis, whereas defective osteoclastic bone resorption can cause osteopetrosis []. Moreover, damaged cartilage is common among fluoride-induced bone injuries, primarily including chondrocytes necrosis, proteoglycan changes, a decreased ability of collagen synthesis, and an imbalance of enzyme activity in cartilage tissues [,]. Although many more studies have been conducted on the effects of fluoride on osteoblasts, osteoclasts, and chondrocytes, it is still unclear whether fluoride affects bone development via these cells.
Therefore, this study aimed to review studies focusing on fluoride-induced bone injuries and identify fluoride-induced bone injuries-related genes, exploring the pathogenesis of bone disorders’ exposure to fluoride.
2. Materials and Methods
2.1. Search Strategy
Studies were identified through searching the PubMed, Web of Science, EMBASE, Cochrane Library, and Chinese National Knowledge Infrastructure (CNKI) databases with the keywords (“fluorine” or “fluoride”) and (“skeletal fluorosis” or “SF” or “bone injury”) up to October 2023. The search strategy followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
2.2. Inclusion and Exclusion Criteria
We focused on studies that related to fluoride-induced bone injuries. The included studies were selected based on the following criteria: (1) Study design: the rats/mice of fluoride exposure group and control group were included in the study. (2) Study samples: the rats/mice in the fluoride exposure group were exposed to fluoride, while the control group was not exposed to fluoride. (3) Study outcome was associated with fluoride-induced bone injuries.
Studies were excluded based on the following criteria: (1) The full text of the article could not be obtained, contained incomplete data, and/or was unextractable. (2) Was a review, report, and letter. (3) There were duplicate studies from different databases. (4) The outcomes were not associated with fluoride-induced bone injuries.
2.3. Study Selection and Data Extraction
The titles and abstracts were reviewed, and the eligibility of selected studies was determined by two investigators (M Wang and FF Yu) independently. After removing studies that obviously violated the inclusion criteria, the remaining articles were reviewed. Any disagreements about study eligibility between the two investigators were resolved through discussion. Subsequently, we systematically searched and identified fluoride-induced bone injury-related genes from the identified studies. These genes were classified into different sets according to the different cell types: osteoblasts, osteoclasts, and chondrocytes.
2.4. Gene Ontology and Kyoto Encyclopedia of Genes Genomes Pathway Enrichment Analyses
The Gene Ontology (GO) database (https://www.geneontology.org/, accessed on 15 January 2024) contains information of gene functions. GO analysis annotates genes into biological process (BP), cellular component (CC), and molecular function (MF) terms. The Kyoto Encyclopedia of Genes Genomes (KEGG) database (https://www.genome.jp/kegg/, accessed on 20 January 2024) includes the functional subcategory, route, and annotation of the biological system. In this study, GO and KEGG enrichment analyses were conducted to evaluate the molecular mechanism of fluoride-induced bone injuries, and analysis of the functions of differentially expressed genes (DEGs) was carried out in various cell types using R software v4.3.1 (https://mirrors.tuna.tsinghua.edu.cn/CRAN/, accessed on 1 February 2024).
2.5. Protein–Protein Interaction Network Construction
The STRING database (https://cn.string-db.org/, accessed on 6 March 2024) was used to construct the interactive network among the DEGs related to fluoride-induced bone injuries. The interactions were reflected by the comprehensive score, and an interaction score of >0.4 was considered the reliability threshold value []. Based on the inter-relationships, the PPI network was established. Then, they were visualized using Cytoscape v3.9.0 (https://cytoscape.org/, accessed on 15 March 2024) []. According to Cyto-Hubba, the top five hub genes were screened [].
2.6. Transcription Factor-mRNA-miRNA Regulatory Network Construction and Key Gene Identification
The transcription factors (TFs) and miRNAs of the DEGs that were in the primary pathway were predicted. In this analysis, the TFs were decoded using the GTRD database (https://gtrd20-06.biouml.org, accessed on 20 March 2024), and the miRNAs were identified using the TargetScanMouse database (https://www.targetscan.org/mmu_80/, accessed on 21 March 2024). Furthermore, the interactive network of TF–mRNA–miRNA was constructed via Cytoscape v3.9.0 (https://cytoscape.org/, accessed on 21 March 2024).
The HPA database (https://www.proteinatlas.org/, accessed on 26 March 2024) provides information regarding transcriptome and protein mapping for specific human tissues. In this study, the HPA database was used to obtain the key protein expression levels in skeletal muscle and bone marrow tissues.
2.7. Candidate Pharmacological Target Prediction
To identify the candidate drugs that target fluoride-induced bone injuries, we leveraged the Drug Signature Database (DSigDB) via Enrichr v3.2.0 (https://maayanlab.cloud/Enrichr/, accessed on 3 April 2024). The candidate targeted drugs of the key genes were sorted by p value, and a p value of < 0.05 was considered statistically significant [].
3. Results
3.1. General Characteristics
A total of 7224 relevant articles were identified among the five databases. The selection process of studies is shown in Figure 1. Initially, 927 duplicate records were excluded. Subsequently, the titles and abstracts were reviewed, and 5998 records were excluded after the preliminary screening. Then, full-text articles were evaluated, and 214 records were excluded. Finally, 85 studies were included in this study, with 63 studies focusing on osteoblasts, 16 studies on osteoclasts, and 14 studies on chondrocytes.
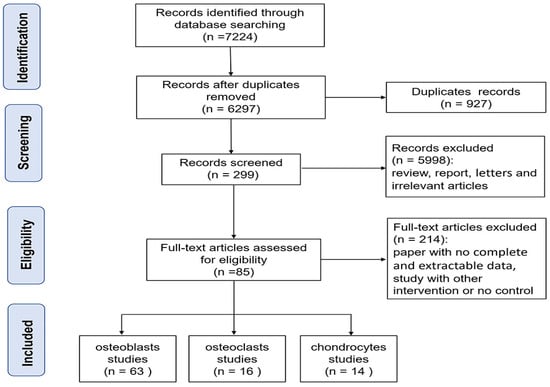
Figure 1.
Flow diagram for the study selection procedure.
3.2. Identification and Classification of Differentially Expressed Genes
In accordance with the different cell types (osteoblasts, osteoclasts, and chondrocytes), studies were classified into different groups. The baseline data including 85 studies are presented in Table 1. Among the 63 osteoblast studies, 112 osteoblast-related DEGs were identified between the fluoride exposure group and control group. Among the 16 osteoclast studies, 35 osteoclast-related DEGs were identified between the fluoride exposure group and control group. Among the 14 chondrocyte studies, 41 chondrocyte-related DEGs were identified between the above-mentioned two groups.

Table 1.
Basic information of included studies.
3.3. Functional Enrichment Analyses of Differentially Expressed Genes
GO and KEGG enrichment analyses were performed on fluoride-induced bone injuries related to DEGs. As shown in Figure 2A, the top 10 BP, CC, and MF terms were presented according to their p value. In osteoblasts, the BP term was significantly enriched in the cellular response to chemical stimulus. The MF term was related to protein heterodimerization activity. In osteoclasts, GO analysis indicated that skeletal system development, ossification, and bone morphogenesis terms were significantly enriched (Figure 2B). In chondrocytes, the BP term was mainly enriched in cartilage development, skeletal system development, and skeletal system morphogenesis. The CC term was enriched in the extracellular matrix, and the MF term was related to signaling receptor binding (Figure 2C).
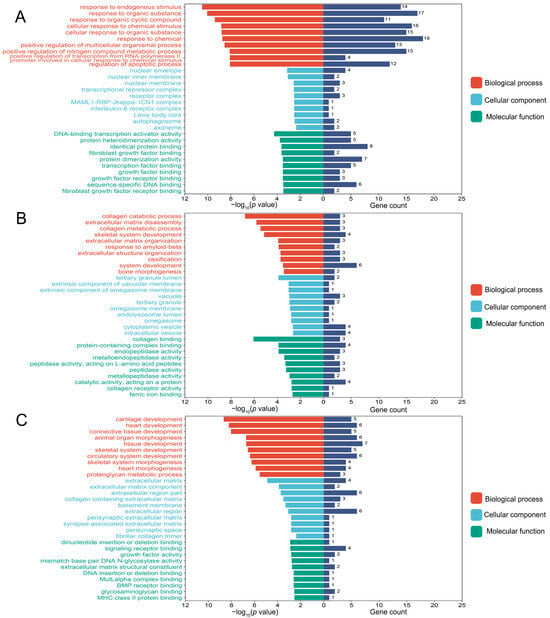
Figure 2.
The top 10 biological process (BP), cellular component (CC), and molecular function (MF) terms of the osteoblast-related differentially expressed genes (DEGs) (A), osteoclast-related DEGs (B), and chondrocyte-related DEGs (C) in the fluoride exposure group compared with the control group. p value < 0.05 was considered significant.
Moreover, KEGG pathway enrichment analysis revealed that Atf4, Bcl2, Col1a1, Fgf21, Fgfr1, and Il6 genes were significantly enriched in the PI3K-Akt signaling pathway of osteoblasts (Figure 3A). Similarly, the Mmp13 and Mmp9 genes were significantly enriched in the IL-17 signaling pathway of osteoclasts (Figure 3B). The Bmp2 and Bmp7 genes were enriched in the TGF-beta signaling pathway of chondrocytes (Figure 3C).
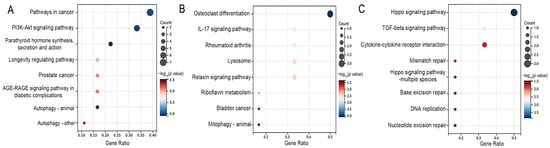
Figure 3.
The top 8 enriched signaling pathways of the osteoblast-related DEGs (A), osteoclast-related DEGs (B), and chondrocyte-related DEGs (C) in the fluoride exposure group compared with the control group. Pathways with p value < 0.05 were considered significant.
3.4. Protein–Protein Interaction Network Analysis
The identified DEGs were used to construct the interaction network with STRING, and the results were visualized using Cytoscape. As shown in Figure 4A, the network consisted of 81 nodes and 870 edges, and the top 5 hub genes were Akt, Il6, P53, Bcl2, and Jag1 in the osteoblasts. A total of 21 nodes and 86 edges were shown in the interaction network of osteoclasts, and the Rankl, Mtor, Acp5, Cathepsin K, and Akt genes were identified as the hub genes (Figure 4B). Similarly, 23 nodes and 111 edges were identified in the interaction network of chondrocytes, and the Akt, Bcl2, Il6, Runx2, and Beclin1 genes were ranked as the top 5 hub genes according to the Maximal Clique Centrality (MCC) algorithm (Figure 4C).
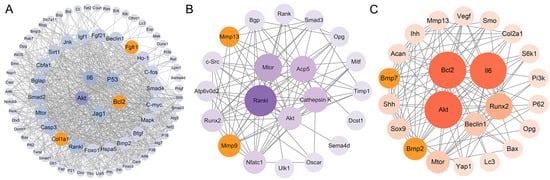
Figure 4.
Protein–protein interaction (PPI) network among the osteoblast-related DEGs (A), osteoclast-related DEGs (B), and chondrocyte-related DEGs (C) was constructed by STRING database and visualized by Cytoscape. The top 5 hub genes were identified via the MCC algorithm of the cyto-hubba plug-in. Orange represents the key genes.
3.5. Transcription Factor–mRNA–miRNA Regulatory Network Construction and Key Gene Identification
According to the TargetScanMouse database, the Atf4, Fgf21, and Il6 genes were not predicted to target any miRNAs, so they were excluded from the key genes. Combined with the GTRD database, we found that a total of 35 TFs and 22 miRNAs regulated the expression of key genes. Eventually, 131 TF–mRNA pairs and 22 miRNA–mRNA pairs were integrated to construct a TF–miRNA–mRNA regulatory network (Figure 5A).
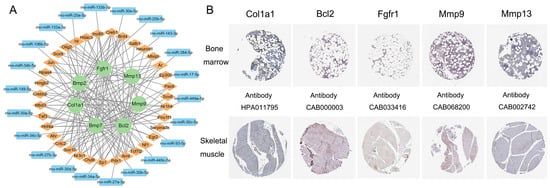
Figure 5.
The transcription factor (TF)–mRNA–miRNA regulatory network (A) and immunohistochemistry images of the key genes from the HPA database (B). Green represents the key genes. Yellow represents the target TFs. Blue represents the target miRNAs. The scale bar is 200 µm.
As shown in Figure 5B, the key genes of Col1a1, Bcl2, Fgfr1, Mmp9, and Mmp13 were screened using the HPA database. These genes were expressed both in bone marrow and skeletal muscle tissues. Unfortunately, the normal tissue analysis of Bmp2 and Bmp7 required more information.
3.6. Prediction of Candidate Drugs
The pharmacological targets of fluoride-induced bone injuries were predicted via the Enrichr platform. The top 5 candidate drugs were listed according to their p value (Table 2). These results showed that selenium methyl cysteine (CTD 00000103), CGS-27023A (TTD 00002801) and calcium phosphate (BOSS) were possibly the key drugs responsible for skeletal fluorosis.

Table 2.
Prediction of the top 5 candidate drugs in three cell types.
4. Discussion
Fluoride affects bone metabolism by intervening with osteoblast and osteoclast activity, and fluoride exposure also triggers chondrocyte degradation [,]. To shed light on the specific mechanisms of fluoride-induced bone injuries, we discovered that the apoptosis of osteoblasts and the inflammation of osteoclasts and chondrocytes were involved in fluoride-induced bone injuries. Additionally, the Col1a1, Bcl2, Fgfr1, Mmp9, Mmp13, Bmp2, and Bmp7 genes were identified as the key regulatory factors of fluoride-induced bone injuries (Figure 6).
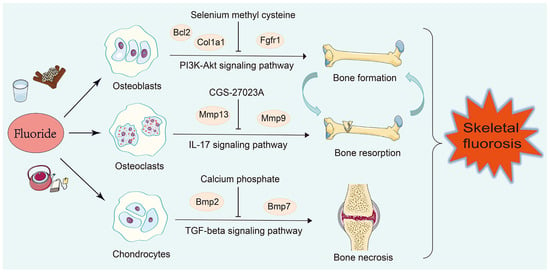
Figure 6.
The regulatory mechanism of bone injuries induced by fluoride. The Bcl2, Col1a1, and Fgfr1 genes were involved in the apoptosis of osteoblasts via the PI3K-Akt signaling pathway, inhibiting bone formation. The Mmp13 and Mmp9 genes caused the inflammation of osteoclasts by activating the IL-17 signaling pathway, promoting bone resorption. The Bmp2 and Bmp7 genes caused the inflammation of chondrocytes by activating the TGF-beta signaling pathway, resulting in bone necrosis.
In this study, a total of 112, 35, and 41 DEGs were obtained in the identified 63 osteoblast studies, 16 osteoclast studies, and 14 chondrocyte studies, respectively. According to the KEGG enrichment analysis in osteoblasts, the PI3K-Akt signaling pathway was significantly enriched. The PI3K-Akt signaling pathway was closely associated with bone metabolism [,,]. Studies demonstrated that the PI3K-Akt signaling pathway participated in the excessive proliferation and differentiation of osteoblasts in rats []. Combined with the TF–mRNA–miRNA regulatory network, the Col1a1, Bcl2, and Fgfr1 genes were identified as the key genes for bone formation, which was regulated by the PI3K-Akt signaling pathway (Figure S1). Col1a1 is a hydrophilic protein that belongs to the collagen family and is closely associated with osteogenesis imperfecta, osteoporosis, and other skeletal injuries [,,]. The LINC00313/miR-218-5p/COL1A1 axis contributed to osimertinib resistance through the PI3K-Akt signaling pathway, confirming that Col1a1 is likely to be the upstream gene for the PI3K-Akt signaling pathway []. Additionally, the overexpression of Fgfr1 promoted the activation of the PI3K-Akt pathway []. Fgfr1 plays essential roles in osteocytes during bone remodeling, and this gene is suggested to be a potential therapeutic target for the prevention of bone loss []. Moreover, the low expression of Akt increased the level of the pro-apoptotic protein Bax, which failed to form heterodimers with the anti-apoptotic protein Bcl2, resulting in osteoblast apoptosis []. Based on these studies, fluoride may activate Col1a1, Bcl2, and Fgfr1 and regulate osteoblast apoptosis through the PI3K-Akt signaling pathway. Furthermore, selenium methyl cysteine (CTD 00000103) was predicted to be a potential drug according to the DSigDB database. Supplementation with selenium methyl cysteine increased bone mineral density []. Studies have demonstrated that selenium methyl cysteine protects against liver injuries by inhibiting apoptosis []. In the future, it could be applied for treating fluoride-induced bone injuries based on these findings.
Osteoclasts are a type of multinucleated bone-resorbing cells that originate from the myeloid lineage of hematopoietic stem cells in bone marrow []. In our study, GO analysis indicated that osteoclasts were responsible for bone resorption. Moreover, the Mmp13 and Mmp9 genes were mainly enriched in the IL17 signaling pathway (Figure S2). IL-17 is a proinflammatory cytokine. Recent studies have shown that IL-17 promotes osteoclast-induced bone loss by regulating glutamine-dependent energy metabolism. Moreover, IL-17 treatment increases the expression of osteoclast marker genes Mmp9 and Mmp13 []. The Mmp9 and Mmp13 genes are involved in the process of bone resorption through the activation and differentiation of osteoclasts [,]. In this study, we found that fluoride exposure activated the Mmp9 and Mmp13 genes and regulated bone resorption through the IL-17 signaling pathway. Among the top 5 candidate drugs in osteoclasts, CGS-27023A was identified as a potent matrix metalloproteinase inhibitor. It can be utilized to regulate the process of bone resorption through Mmp13 and Mmp9 [].
Furthermore, we focused on the enrichment analysis of chondrocyte-related DEGs. GO analysis suggested that chondrocytes were significantly important for the development of the skeletal system. KEGG enrichment analysis showed that the Bmp2 and Bmp7 genes were mainly enriched in the TGF-beta signaling pathway (Figure S3). BMPs are a type of extracellular multifunctional signaling cytokine that belongs to the TGF-beta family []. Bmp2 and Bmp7 have been shown to induce endochondral bone formation and subsequently form long bones [,]. The high expression of Bmp2, along with TGF-beta1, promotes the expansion of Treg cells, leading to significant inflammation [,]. A large number of studies has also confirmed that fluoride exposure leads to an inflammatory response [,,]. Moreover, the PPI analysis identified several hub proteins related to inflammation, such as Akt, Il6, Runx2, Bcl2, and Beclin1 [,,,]. Studies have shown that TGF-beta degrades the extracellular matrix, induces chondrocyte differentiation, and even leads to bone necrosis [,]. According to the analysis of candidate drugs, calcium phosphate is supposed to be used for inhibiting bone necrosis. Studies have indicated that calcium phosphate could be used in bone grafts due to its composition, supporting our findings [].
Overall, the above findings provide promising insights for skeletal fluorosis. However, our study still has certain limitations. The specific regulatory mechanisms of the PI3K-Akt signaling pathway, IL-17 signaling pathway, and TGF-beta signaling pathway in bone disorders’ exposure to fluoride are needed to be further verified via in vivo and in vitro experiments.
5. Conclusions
In conclusion, the PI3K-Akt signaling pathway is involved in the apoptosis of osteoblasts and the IL-17 and TGF-beta signaling pathways are involved in the inflammation of osteoclasts and chondrocytes, playing a part in the process of fluoride-induced bone injuries. The Col1a1, Bcl2, Fgfr1, Mmp9, Mmp13, Bmp2, and Bmp7 genes are the key regulatory factors in fluorosis bone metabolism.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16152500/s1, Figure S1: The Fgfr1, Bcl2, and Col1a1 genes involved in the PI3K-Akt signaling pathway; Figure S2: The Mmp9 and Mmp13 genes involved in the IL-17 signaling pathway; Figure S3: The Bmp2 and Bmp7 genes involved in the TGF-beta signaling pathway. The positions of key genes are highlighted.
Author Contributions
Conceptualization, M.W. and F.Y.; methodology, M.W. and F.Y.; software, M.W., G.Z., Y.B. and F.Y.; validation, M.W. and F.Y.; formal analysis, M.W., K.L., T.S., Z.D., Q.L., Y.D., H.Z., G.Z. and Y.B.; investigation, F.Y., M.W., K.L., T.S., Z.D. and Q.L.; resources, F.Y.; data curation, F.Y., M.W., K.L., T.S., Z.D., Q.L., Y.D., H.Z., G.Z. and Y.B.; writing—original draft preparation, M.W.; writing—review and editing, M.W. and F.Y.; visualization, M.W.; supervision, F.Y.; project administration, F.Y.; funding acquisition, F.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Scientific Foundation of China (82003400), Science and Technology Program of Henan Province (232102311079).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Srivastava, S.; Flora, S.J.S. Fluoride in drinking water and skeletal fluorosis: A review of the global impact. Curr. Environ. Health Rep. 2020, 7, 140–146. [Google Scholar] [CrossRef]
- Qin, M.; Gao, Y.; Zhang, M.; Wu, J.; Liu, Y.; Jiang, Y.; Zhang, X.; Wang, X.; Yang, Y.; Gao, Y. Association between ADAMTS14_rs4747096 gene polymorphism and bone mineral density of Chinese Han population residing in fluorine exposed areas in ShanXi province, China. Environ. Sci. Pollut. Res. Int. 2023, 30, 106059–106067. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Y.; Li, M.; Zhao, T.; Zhang, W.; Wang, Y.; He, Y.; Zhao, H.; Li, H.; Wang, T.; et al. Calcium supplementation attenuates fluoride-induced bone injury via pink1/parkin-mediated mitophagy and mitochondrial apoptosis in mice. J. Hazard. Mater. 2024, 465, 133411. [Google Scholar] [CrossRef] [PubMed]
- Veneri, F.; Iamandii, I.; Vinceti, M.; Birnbaum, L.S.; Generali, L.; Consolo, U.; Filippini, T. Fluoride exposure and skeletal fluorosis: A systematic review and dose-response Meta-analysis. Curr. Environ. Health Rep. 2023, 10, 417–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lv, M.; Zhang, Y.; Gao, Y.; Cai, Z.; Zhang, Y.; Song, J.; Liu, J.; Yin, H.; Shang, F. TDDFT study on the ESIPT properties of 2-(2′-hydroxyphenyl)-benzothiazole and sensing mechanism of a derived fluorescent probe for fluoride ion. Molecules 2024, 29, 1541. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.Y.; Parr, C.; Pesti, G.M. The effects of dietary fluoride on growth and bone mineralization in broiler chicks. Poult. Sci. 2011, 90, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yu, S.; Duan, L.; Yang, S.; Hou, X.; Du, Y.; Gao, M.; Zuo, J.; Sun, L.; Fu, X.; et al. Proteomics sequencing reveals the role of TGF-β signaling pathway in the peripheral blood of offspring rats exposed to fluoride. Biol. Trace Elem. Res. 2024, 202, 2100–2110. [Google Scholar] [CrossRef]
- Daiwile, A.P.; Sivanesan, S.; Tarale, P.; Naoghare, P.K.; Bafana, A.; Parmar, D.; Kannan, K. Role of fluoride induced histone trimethylation in development of skeletal fluorosis. Environ. Toxicol. Pharmacol. 2018, 57, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, Y.; Iqbal, M.; Mehmood, K.; Li, Y.; Tang, Z.; Zhang, H. Challenges of fluoride pollution in environment: Mechanisms and pathological significance of toxicity—A review. Environ. Pollut. 2022, 304, 119241. [Google Scholar] [CrossRef]
- Park, Y.A.; Plehwe, W.E.; Varatharajah, K.; Hale, S.; Christie, M.; Yates, C.J. Skeletal fluorosis secondary to methoxyflurane use for chronic pain. JBMR Plus 2024, 8, ziae032. [Google Scholar] [CrossRef]
- Shanthakumari, D.; Srinivasalu, S.; Subramanian, S. Effect of fluoride intoxication on lipidperoxidation and antioxidant status in experimental rats. Toxicology 2004, 204, 219–228. [Google Scholar] [CrossRef]
- Li, Y.; Yang, F.; Liu, J.; Jiang, M.; Yu, Y.; Zhou, Q.; Sun, L.; Zhang, Z.; Zhou, L. Protective effects of sodium butyrate on fluorosis in rats by regulating bone homeostasis and serum metabolism. Ecotoxicol. Environ. Saf. 2024, 276, 116284. [Google Scholar] [CrossRef]
- Collins, M.T.; Marcucci, G.; Anders, H.J.; Beltrami, G.; Cauley, J.A.; Ebeling, P.R.; Kumar, R.; Linglart, A.; Sangiorgi, L.; Towler, D.A.; et al. Skeletal and extraskeletal disorders of biomineralization. Nat. Rev. Endocrinol. 2022, 18, 473–489. [Google Scholar] [CrossRef]
- Pei, J.; Li, B.; Gao, Y.; Xu, J.; Darko, G.M.; Sun, D. Relationship between fluoride exposure and osteoclast markers during RANKL-induced osteoclast differentiation. Environ. Toxicol. Pharmacol. 2016, 46, 241–245. [Google Scholar]
- Bar-Shavit, Z. The osteoclast: A multinucleated, hematopoietic-origin, bone-resorbing osteoimmune cell. J. Cell. Biochem. 2007, 102, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, M.; Huynh, N.C.; Okamoto, K.; Muro, R.; Terashima, A.; Kurikawa, Y.; Komatsu, N.; Pluemsakunthai, W.; Nitta, T.; Abe, T.; et al. Stepwise cell fate decision pathways during osteoclastogenesis at single-cell resolution. Nat. Metab. 2020, 2, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gui, F.; Li, D.; Zhang, R.; Sun, Q.; Guo, X. Fluoride regulates the expression of extracellular matrix HSPG and related signaling pathways FGFR3 and Ihh/PTHrP feedback loop during endochondral ossification. Environ. Toxicol. Pharmacol. 2020, 73, 103275. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, A.; Mehmood, K.; Hussain, R.; Abbas, R.Z.; Javed, M.T.; Chang, Y.F.; Hu, L.; Pan, J.; Li, Y.; et al. Long-term exposure to the fluoride blocks the development of chondrocytes in the ducks: The molecular mechanism of fluoride regulating autophagy and apoptosis. Ecotoxicol. Environ. Saf. 2021, 217, 112225. [Google Scholar] [CrossRef]
- Otasek, D.; Morris, J.H.; Bouças, J.; Pico, A.R.; Demchak, B. Cytoscape automation: Empowering workflow-based network analysis. Genome Biol. 2019, 20, 185. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape string app: Network analysis and visualization of proteomics data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Duan, M.; Zhang, C.; Wang, K.; Feng, L.; Song, L.; Wu, S.; Chen, X. Identification of potential biomarkers associated with acute myocardial infarction by weighted gene coexpression network analysis. Oxid. Med. Cell. Longev. 2021, 2021, 5553811. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wang, M.; Luo, K.; Sun, L.; Yu, S.; Zuo, J.; Wang, Y. Expression profiles of long non-coding RNAs in the articular cartilage of rats exposed to T-2 toxin. Int. J. Mol. Sci. 2023, 24, 13703. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.; Cao, Y. Effects of fluoride on bone morphogenetic protein expression in osteoblast. Chin. J. Public Health 2003, 19, 34–35. [Google Scholar]
- Zhang, W.; Cui, Y.; Gao, S.; Zhang, X.; Li, G. Expression of proto-oncogenes c-fos and c-jun in osteoblasts activated by excessive fluoride. Chin. J. Prev. Med. 2003, 37, 30–34+92. [Google Scholar]
- Xu, J.; Chen, J.; Cai, H.; Cao, Y. Influence of fluoride on bone morphogenetic protein expression and the antagonistic effect of zinc in rat osteoblasts: All in vitro study. Chin. J. Endem. 2004, 23, 20–22. [Google Scholar]
- Jing, L. Expression of Osteogenic Phenotype in Fibroblasts induced by Fluoride and its Role in the Development Ofextraperiosteal Ossification of Skeletal Fluorosis. Ph.D. Thesis, Jilin University, Changchun, China, 2006. [Google Scholar]
- Jing, L.; Qi, L.; Li, T.; Li, G. Effect of fluoride on the expression of core-binding factor αl in fibroblast and osteoblast. Chin. J. Endem. 2006, 25, 629–632. [Google Scholar]
- Li, D. The role of Runx2 in the process of activating osteoblasts by excessive fluoride. Ph.D. Thesis, Jilin University, Changchun, China, 2006. [Google Scholar]
- Wang, C. The effects of fluoride on marrow stromal cells, their products OPG, RANKL, and related cytokines. Ph.D. Thesis, Jilin University, Changchun, China, 2006. [Google Scholar]
- Zhang, W.; Xue, L.; Cui, Y.; Li, G. The effect of different dosage of fluoride intake on activation of osteoblasts and the expression of Bmp-2, Bmp-4 and Smad-4. Chin. J. Endem. 2006, 25, 125–128. [Google Scholar]
- Zhao, Y.; Qi, L.; Xu, H.; Jing, L. Effects of fluoride on expressions of TGF-β and Smad2/3 in fibroblasts and osteoblasts. J. Jilin Univ. (Med.) 2006, 32, 956–959. [Google Scholar]
- Zhong, J. Effect of Proliferation and MCM3 Gene Expression in Osteoblasts Exposed to Fluoride. Ph.D. Thesis, Xinjiang Medical University, Urumqi, China, 2006. [Google Scholar]
- Liu, Y. Effect of Ras Gene Expression on Osteoblasts Cultured Invitro after Exposed to Fluoride. Master’s Thesis, Xinjiang Medical University, Urumqi, China, 2007. [Google Scholar]
- Wang, Y.; Tan, Q.; Yu, Y. Effect of fluoride on expression of calmodulin in rat osteoblasts in vitro. Chin. J. Public Health 2007, 23, 333–336. [Google Scholar]
- Wei, H. Expression and Effects of lGF-1 and bFGF in Fibroblast and Osteoblast Induced by Fluoride. Master’s Thesis, Jilin University, Changchun, China, 2007. [Google Scholar]
- Zhou, T. Effect on the Proliferation of Osteoblasts Cultured In Vitro and the Expression of TM9SF1 Gene after Exposed to Fluoride. Master’s Thesis, Xinjiang Medical University, Urumqi, China, 2007. [Google Scholar]
- Han, B. Influence of Subchronic Exposure to Excessive Fluoride on Level of Bone Sialoprotein Expression in Rat Bone Tissue. Master’s Thesis, China Medical University, Shenyang, China, 2008. [Google Scholar]
- Chi, G. Effects of fluoride on the gene expression of c-fos and c-jun in fibroblast and osteoblast. J. Inn. Mong. Univ. Natl. 2008, 23, 184–188. [Google Scholar]
- Liu, Y.; Liu, K.; Yang, X.; Liu, J.; Zhong, J.; Zhou, T. Effect of protein expression of Ras gene in osteoblasts cultured in vitro and exposed to fluoride. J. Xinjiang Med. Univ. 2008, 31, 940–942. [Google Scholar]
- Wang, M.; Yu, F.; Wu, P.; Zhao, D.; Su, J.; Han, B. Regulation of fluoride on osteocalcin gene expression in osteoblasts cultured in vitro. Sci. Agric. Sin. 2009, 42, 2629–2632. [Google Scholar]
- Dan, Y.; Yu, Y.; Deng, C. Effect of fluoride on expression of OSX mRNA and protein in bone tissue of rats. China Public Health 2011, 27, 1008–1009. [Google Scholar]
- Zhu, H.; Yu, Y.; Deng, C.; Yang, D. Effect of fluoride on expression of phosphoinositide 3-kinase, protein kinase bl mRNA and protein in bone tissue of rats. Chin. J. Endem. 2011, 30, 261–265. [Google Scholar]
- Guo, X.; Wu, S.; He, Y.; Zhang, Z.; Sun, G. Effect of subchronic fluoride exposure on pathologic change and β-catenin expression in rat bone tissue. J. Hyg. Res. 2011, 40, 304–307. [Google Scholar]
- Guo, X.; Cai, R.; Sun, G. Effects of fluoride and aluminum on mRNA expression of OPG and RANKL in MC3T3-El cells. Chin. J. Integr. Med. 2011, 24, 3–5+8. [Google Scholar]
- Zhong, Y. The Effects of Fluoride on Nrf2-ARE Signal Pathway in Rat Osteoblasts. Master’s Thesis, Guangdong Pharmaceutical University, Guangzhou, China, 2011. [Google Scholar]
- Zhang, X.; Lv, P.; Zhang, J.; Zhao, Z.; Xu, H.; Li, G. Immunoglobulin binding protein gene and protein expression in femur tissue of fluorosis rats. Chin. J. Endem. 2011, 30, 502–505. [Google Scholar]
- Yang, S.; Wang, Z.; Farquharson, C.; Alkasir, R.; Zahra, M.; Ren, G.; Han, B. Sodium fluoride induces apoptosis and alters bcl-2 family protein expression in MC3T3-El osteoblastic cells. Biochem. Biophys. Res. Commun. 2011, 410, 910–915. [Google Scholar] [CrossRef]
- Wan, L.; Yu, Y.; Wan, C.; Xie, Y.; Chen, X. Effects of chronic fluorosis on expression of DLX5 protein and mRNA in bone tissue of rats. Chin. J. Prev. Med. 2012, 13, 641–644. [Google Scholar]
- Wan, W.; Wan, L.; Yu, Y.; Wan, C.; Guan, Z. Effect of fluoride on the expression of twist mRNA and protein in bone tissue of rats. J. GuiYang Med. Coll. 2012, 37, 221–224. [Google Scholar]
- Xie, Y.; Yu, Y.; Wan, L.; Chen, X. Effect of fluoride on expression of Can mRNA and protein in bone tissue of rats. Chin. J. Pathol. 2012, 41, 761–764. [Google Scholar]
- Yu, Y.; Yang, D.; Zhu, H.; Deng, C.; Guan, Z. Expression of mRNA and protein of p38, Osx, PI3K and Akt1 in rat bone with chronic fluorosis. Chin. J. Pathol. 2012, 41, 622–626. [Google Scholar]
- Guo, X.; Yang, M.; Liang, D.; Guo, B.; Yang, L.; Cao, J. Fluoride induces autophagy and apoptosis and the interaction in MC3T3-El cells. Chin. J. Endem. 2012, 27, 165–168. [Google Scholar]
- Hu, C.; Ren, L.; Li, X.; Wu, N.; Li, G.; Liu, Q.; Xu, H. Effect of fluoride on insulin level of rats and insulin receptor expression in the MC3T3-El cells. Biol. Trace Elem. Res. 2012, 150, 297–305. [Google Scholar] [CrossRef]
- Chen, X.; Yu, Y.; Yi, W.; Wan, L.; Xie, Y. Effect of fluoride on expression of mRNA and protein of wnt3a and beta-catenin in osteoblast of rats. Chin. J. Endem. 2013, 32, 140–145. [Google Scholar]
- Li, X. Role of ER Stress and PERK Signaling Pathway in the Mechanism of Skeletal Fluorosis. Ph.D. Thesis, Jilin University, Changchun, China, 2014. [Google Scholar]
- Deng, C.; Yu, Y.; Zhang, Y. Expressions of transforming growth factor-beta1 and interleukin 6 mRNA and protein in bone of rats with chronic fluorosis. Chin. J. Endem. 2014, 33, 609–614. [Google Scholar]
- Duan, X.; Xu, H.; Wang, Y.; Wang, H.; Li, G.; Jing, L. Expression of core-binding factor α1 and osteocalcin in fluoride-treated fibroblasts and osteoblasts. J. Trace Elem. Med. Biol. 2014, 28, 278–283. [Google Scholar] [CrossRef]
- Lv, P. Roles for Insulin and Insulin Receptor in the Mechanism Underlying the Process of Skeletal Fluorosis. Ph.D. Thesis, Jilin University, Changchun, China, 2015. [Google Scholar]
- Wang, Y. PTH, PTH-rp, CaSR Expression and Its Role in the pathogenesis of Skeletal Fluorosis. Ph.D. Thesis, Jilin University, Changchun, China, 2015. [Google Scholar]
- Yan, X.; Hao, X.; Nie, Q.; Feng, C.; Wang, H.; Sun, Z.; Niu, R.; Wang, J. Effects of fluoride on the ultrastructure and expression of type I collagen in rat hard tissue. Chemosphere 2015, 128, 36–41. [Google Scholar] [CrossRef]
- Liu, X.; Song, J.; Liu, K.; Wang, W.; Xu, C.; Zhang, Y.; Liu, Y. Role of inhibition of osteogenesis function by Sema4d/Plexin-B1 signaling pathway in skeletal fluorosis in vitro. J. Huazhong Univ. Sci. Technolog Med. Sci. 2015, 35, 712–715. [Google Scholar] [CrossRef]
- Chen, X.; Wan, C.; Xie, C.; Wei, Y.; Wu, Y.; Wan, W. Fluoride lnhibits Expressions of Notch3 and Jagl Proteins in Rat Bone Tissues. J. Environ. Occup. Med. 2016, 33, 494–498. [Google Scholar]
- Chen, X.; Wan, C.; Xie, C. Influence of fluoride on RBPJ and related genes in bone tissue of rats. China J. Public Health 2016, 32, 195–198. [Google Scholar]
- Yin, F. Effects of Exogenous Calcium on Proliferation and Wnt Signaling Pathway of Mouse Osteoblasts Exposed to Fluoride. Master’s Thesis, Shanxi Agricultural University, Taigu, China, 2016. [Google Scholar]
- Chen, S.; Zhang, A.; Pan, X. The effects of fluoride on hypermethylation, transcription and expression of p16 gene in osteoblasts of rats. Chin. J. Endem. 2016, 35, 89–93. [Google Scholar]
- Gu, X.; Han, D.; Chen, W.; Zhang, L.; Lin, Q.; Gao, J.; Fanning, S.; Han, B. Sirt1-mediated FoxOs pathways protect against apoptosis by promoting autophagy in osteoblast-like MC3T3-El cells exposed to sodium fluoride. Oncotarget 2016, 7, 65218–65230. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Q.; Zhang, T.; Gao, X.; Yu, F.; Sun, B. Effect of inositol-requiring enzyme-1 signaling pathway on the differentiation of osteoblasts induced by fluoride. Chin. J. Endem. 2016, 35, 863–868. [Google Scholar]
- Li, Y.; Bian, S.; Wang, J.; Wang, J. Effects of fluoride and chitosan on the gene expressions of bone morphogenic protein 2 and collagen type-1 alpha 1 chain in the mouse femur. Fluoride 2016, 49, 47–55. [Google Scholar]
- Zhao, Y.; Huo, M.; Liu, Y.; Xie, Y.; Wang, J.; Li, Y.; Wang, J. Effects of fluoride on the expression of Bmp-2 and Smad 1 in rat osteoblasts in vitro. Fluoride 2016, 49, 13–22. [Google Scholar]
- Zhao, Y.; Li, Y.; Gao, Y.; Yuan, M.; Manthari, R.K.; Wang, J.; Wang, J. TGF-β1 acts as mediator in fluoride-induced autophagy in the mouse osteoblast cells. Food Chem. Toxicol. 2018, 115, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, X. Effect of Combined Expose of Fluorine and Arsenic on Osteoclast Differentiation and the RANK/TRAF-6/NF-kB1 Pathway in a Co-culture System. Master’s Thesis, Guizhou Medical University, Guiyang, China, 2019. [Google Scholar]
- Wang, J.; Gao, Y.; Chen, X.; Yang, J.; Xu, H.; Zhao, Y.; Li, Y. Effects of GSTO1 gene silencing on autophagy and apoptosis of fluoride-induced osteoblasts. Acta Vet. Aootechnica Sin. 2019, 50, 183–192. [Google Scholar]
- Yang, X.; Hong, F.; Xie, W.; Zhang, J.; Jin, X.; Qin, Z. Effects of joint exposure to fluoride and arsenic on OPG/RANKL regulating osteoclast differentiation. J. Environ. Health 2019, 36, 305–310. [Google Scholar]
- Gu, X.; Wang, Z.; Gao, J.; Han, D.; Zhang, L.; Chen, P.; Luo, G.; Han, B. Sirt1 suppresses p53-dependent apoptosis by modulation of p21 in osteoblast-like MC3T3-El cells exposed to fluoride. Toxicol. Vitr. 2019, 57, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, N.; Yu, H.; Yu, X.; Guo, F.; Zhao, Z.; Xu, H. Requirement of TGF-β signaling for effect of fluoride on osteoblastic differentiation. Biol. Trace Elem. Res. 2019, 187, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yu, Y.; Xu, L.; Ming, P.; Shao, S.; Qiu, J. Regulation of osteoblast behaviors via cross-talk between Hippo/YAP and MAPK signaling pathway under fluoride exposure. J. Mol. Med. 2019, 97, 1003–1017. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhang, Y.; Xu, L.; Zhao, L.; Ling, H.; Yu, Y. Expressions of Ihh, Shh and Smo mRNA and protein in rats’ bone exposed to different doses of fluoride and the significance. Chin. J. Endem. 2020, 39, 630–635. [Google Scholar]
- Deng, C.; Zhang, Y.; Xu, L.; Zhao, L.; Ling, H.; Yu, Y. Change and relationship between gli1 and beta-catenin on rats’ bone formation with chronic fluorosis. Chin. J. Pathol. 2020, 49, 168–173. [Google Scholar]
- Chen, L.; Zhang, M.; Ding, Y.; Li, M.; Zhong, J.; Feng, S. Fluoride induces hypomethylation of Bmp2 and activates osteoblasts through the Wnt/β-catenin signaling pathway. Chem. Biol. Interact. 2022, 356, 109870. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Xu, W.; Zhang, Z.; Jin, H.; Yang, Y.; Zhang, J.; Xu, H. Role of TGF-β1 in fluoride-treated osteoblasts at different stages. Biol. Trace Elem. Res. 2022, 200, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, W.; Liu, Y.; Gui, C.; Wang, L.; Chen, Y.; Deng, M.; Nan, N.; Duan, X.; Guan, Z. Effect of L-NMMA on NO/iNOS expression in MC3T3-El cells induced by excessive fluoride exposure. J. GuiZhou Med. Univ. 2023, 48, 266–271. [Google Scholar]
- Ding, H.; Yin, C.; Yang, M.; Zhou, R.; Wang, X.; Pan, X. Screening of differentially methylated genes in skeletal fluorosis of rats with different types and involvement of aberrant methylation of Cthrc1. Environ. Pollut. 2023, 332, 121931. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, C.; Gui, Y.; Zou, T.; Xi, S.; Guo, X. Fluoride regulates the differentiation and atrophy through FGF21/ERK signaling pathway in C2C12 cells. Ecotoxicol. Environ. Saf. 2023, 252, 114626. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Y.; Zhao, S.; Wu, J.; Lu, C.; Jiang, L.; Ran, S.; Wang, J.; Sun, F.; Liu, B. Fluoride resistance capacity in mammalian cells involves global gene expression changes associate with ferroptosis. Chem. Biol. Interact. 2023, 381, 110555. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Deng, C.; He, L.; Wu, Q.; Xu, L.; Yu, Y. Fluoride induces osteoblast autophagy by inhibiting the PI3K/Akt/mTOR signaling pathway in vivo and in vitro. Exp. Biol. Med. 2023, 248, 1159–1172. [Google Scholar]
- Sun, D.; Gao, Y.; Zhou, L.; Yu, J.; Li, Y.; Yu, W. Effects of sodium fluoride on matrix metal proteinases-13 mRNA and tissue inhibitor of metal protease-1 mRNA in rat bone tissue. Chin. J. Endem. 2008, 27, 364–367. [Google Scholar]
- Pei, J.; Gao, Y.; Li, B.; Zhou, L.; Zhang, Z.; Sun, D. Effect of fluoride on osteoclast formation at various levels of receptor activator of nuclear factor kappa-b ligand (RANKL). Fluoride 2012, 45, 86–93. [Google Scholar]
- Xie, Y.; Yu, Y.; Chen, X. Expression of NFAT mRNA and protein in osteoclasts of rats with chronic fluorosis. China Public Health 2013, 29, 530–533. [Google Scholar]
- Pei, J.; Yao, Y.; Li, B.; Wei, W.; Gao, Y.; Darko, G.M.; Sun, D. Excessive fluoride stimulated osteoclast formation through up-regulation of receptor activator for nuclear factor-κb ligand (RANKL) in C57bL/6 mice. Int. J. Clin. Exp. Med. 2017, 10, 15260–15268. [Google Scholar]
- Yu, H.; Jiang, N.; Yu, X.; Zhao, Z.; Zhang, X.; Xu, H. The role of TGFβ receptor1-smad3 signaling in regulating the osteoclastic mode affected by fluoride. Toxicology 2018, 393, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, W.; Li, L.; Chai, B.; Xu, X.; Bai, S. Effect of fluoride on osteoclast autophagy through the AKT/mTOR/ULK1 signaling pathway. Acad. J. Chin. PLA Med. Sch. 2022, 43, 960–965. [Google Scholar]
- Ding, H. Screening of Differentially Methylated Genes in Skeletal Fluorosis of Rats with Different Types and Involvement of Aberrant Methylation of Cthrc1. Ph.D. Thesis, Guizhou Medical University, Guiyang, China, 2023. [Google Scholar]
- Pei, J.; Li, B.; Gao, Y.; Wei, Y.; Zhou, L.; Yao, H.; Wang, J.; Sun, D. Fluoride decreased osteoclastic bone resorption through the inhibition of NFATC1 gene expression. Environ. Toxicol. 2014, 29, 588–595. [Google Scholar] [CrossRef]
- Lv, Y.; Kang, L.; Wu, G. Fluorosis increases the risk of postmenopausal osteoporosis by stimulating interferon γ. Biochem. Biophys. Res. Commun. 2016, 479, 372–379. [Google Scholar] [CrossRef]
- Liu, B.-C.; Xu, Z.-L.; Miao, Q.; Xu, Y.-Y.; Xu, M.; Qian, X.-J.; You, B.-R.; Yuan, B.-H.; Kang, N. Expression of type II collagen gene and structural change in bone tissues of rats with experimental fluorosis. Chin. J. Prev. Med. 2003, 37, 243–245. [Google Scholar]
- Li, T.; Guo, Q.; Liao, L.; Li, Y.; Zhang, Y. Effect of Fluoride on the Expression of Caspasel4 in Human Osteoblasts and Bone Tissue in Mice. Prog. Mod. Blomedlcine 2017, 17, 1631–1634. [Google Scholar]
- Zhu, Z.; Yu, Y.; Tao, X. Role of Hh signaling pathway in fluoride-induced primary chondrocyte damage in rats. China J. Public Health. 2015, 31, 574–578. [Google Scholar]
- Wang, W.; Peng, H. Expression of bone morphogenetic protein-2 and bone morphogenetic protein-7 in the posterior longitudinal ligament of fluorosis rat and its effect on ossification. Chin. J. Exp. Surg. 2016, 33, 761–764. [Google Scholar]
- Li, T.; Bai, S.; Zhang, Y.; Liu, K.; Zhang, Y.; Zhong, J. Experimental study of cartilage lesions and COLIXA3 protein expression in rats cartilage with chronic fluorosis. Chin. J. Endem. 2011, 30, 389–392. [Google Scholar]
- Yang, Q.; Chu, Y.; Jiang, W.; Li, J.; Li, Y.; Bao, Y.; Chen, F.; Li, B.; Yang, Y.; Gao, Y. Effects of different doses of sodium fluoride on cartilage lesion and expression of interleukin-6 in Balb/c mice. Chin. J. Endem. 2017, 36, 408–413. [Google Scholar]
- Zhang, L. Effect of Fluoride on Endochondral Ossification in Rat Growth Plate Cartilage and the regulatory role of EGFR Signal Pathway. Master’s Thesis, China Medical University, Shenyang, China, 2018. [Google Scholar]
- Zhu, Z.; Yu, Y.; Chen, R. Role of hedgehog signaling pathway on cartilage tissue damage in chronic fluorosis rats. China J. Public Health 2018, 34, 241–245. [Google Scholar]
- Zhang, R. Fluoride Inhibits the Proliferation and Differentiation of ATDC5 Cells via the Pl3K/AKT/mTOR Signaling Pathway. Master’s Thesis, China Medical University, Shenyang, China, 2020. [Google Scholar]
- Ma, L.; Zhang, R.; Li, D.; Qiao, T.; Guo, X. Fluoride regulates chondrocyte proliferation and autophagy via PI3K/AKT/mTOR signaling pathway. Chem. Biol. Interact. 2021, 349, 109659. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zuo, J.; Fu, X.; Gao, M.; Sun, L.; Yu, S.; Li, Z.; Zhou, G.; Ba, Y. Role of the hippo signaling pathway in the extracellular matrix degradation of chondrocytes induced by fluoride exposure. Ecotoxicol. Environ. Saf. 2021, 225, 112796. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, J.; Zhao, J.; Zhou, B.; Zhang, Y.; Wang, H. Hif-1α-mediated autophagy and canonical wnt/β-catenin signaling activation are involved in fluoride-induced osteosclerosis in rats. Environ. Pollut. 2022, 315, 120396. [Google Scholar] [CrossRef]
- Meng, H.; Zhang, T.; Liu, W.; Wang, H.; Wang, C.; Zhao, Z.; Liu, N.; Wang, W. Sodium fluoride induces apoptosis through the downregulation of hypoxia-inducible factor-1α in primary cultured rat chondrocytes. Int. J. Mol. Med. 2014, 33, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Guo, F.; Sun, B.; Zhang, X.; Xu, H. Different effects of fluoride exposure on the three major bone cell types. Biol. Trace Elem. Res. 2020, 193, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.S.; Silva, T.L.; Buzalaf, M.A.; Rodrigues, A.C.; de Oliveira, R.C. Differential effects of fluoride during osteoblasts mineralization in C57BL/6J and C3h/HeJ inbred strains of mice. Biol. Trace Elem. Res. 2014, 161, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, H.; Cheng, X.; Yang, J.; Yan, Z.; Ma, H.; Zhao, Y.; Ommati, M.M.; Manthari, R.K.; Wang, J. Calcium relieves fluoride-induced bone damage through the PI3K/AKT pathway. Food Funct. 2020, 11, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, P.; Gao, Y.; Ma, Z.; Wang, H.; Long, Y.; Ma, Z.; Liu, R. Effects of the combination of epimedii folium and ligustri lucidi fructus on apoptosis and autophagy in sop rats and osteoblasts via PI3K/AKT/mTOR pathway. Biomed. Pharmacother. 2024, 173, 116346. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Xia, K.; Zheng, D.; Gong, C.; Guo, W. RILP inhibits tumor progression in osteosarcoma via Grb10-mediated inhibition of the PI3K/AKT/mTOR pathway. Mol. Med. 2023, 29, 133. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.; Huang, L.; Zhang, X.; Wang, N.; Wang, H.; Mu, Y.; Li, K.; Liu, Z. Molecular characteristics and promoter analysis of porcine COL1A1. Genes 2022, 13, 1971. [Google Scholar] [CrossRef] [PubMed]
- Corbeau, J.; Grohs, C.; Jourdain, J.; Boussaha, M.; Besnard, F.; Barbat, A.; Plassard, V.; Rivière, J.; Hamelin, C.; Mortier, J.; et al. A recurrent de novo missense mutation in COL1A1 causes osteogenesis imperfecta type II and preterm delivery in Normande cattle. Genet. Sel. Evol. 2024, 56, 39. [Google Scholar] [CrossRef]
- Moradifard, S.; Hoseinbeyki, M.; Emam, M.M.; Parchiniparchin, F.; Ebrahimi-Rad, M. Association of the Sp1 binding site and -1997 promoter variations in COL1A1 with osteoporosis risk: The application of meta-analysis and bioinformatics approaches offers a new perspective for future research. Mutat. Res. Rev. Mutat. Res. 2020, 786, 108339. [Google Scholar] [CrossRef]
- Ding, D.; Xu, C.; Zhang, J.; Zhang, Y.; Xue, L.; Song, J.; Luo, Z.; Hong, X.; Wang, J.; Liang, W.; et al. Revealing underlying regulatory mechanisms of LINC00313 in Osimertinib-resistant LUAD cells by ceRNA network analysis. Transl. Oncol. 2024, 43, 101895. [Google Scholar] [CrossRef]
- Asada, H.; Tani, A.; Sakuma, H.; Hirabayashi, M.; Matsumoto, Y.; Watanabe, K.; Tsuboi, M.; Yoshida, S.; Harada, K.; Uchikai, T.; et al. Whole exome and transcriptome analysis revealed the activation of ERK and Akt signaling pathway in canine histiocytic sarcoma. Sci. Rep. 2023, 13, 8512. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, P.; Jin, M.; Huang, S.; Chen, H.; Chen, L.; Yang, J.; Su, N. Fgfr1 deficiency in osteocytes leads to increased bone mass by enhancing wnt/β-catenin signaling. Bone 2023, 174, 116817. [Google Scholar] [CrossRef]
- Xu, J.; Ze, X.; Zhao, L.; Sheng, L.; Ze, Y. Titanium dioxide nanoparticles oral exposure induce osteoblast apoptosis, inhibit osteogenic ability and increase lipogenesis in mouse. Ecotoxicol. Environ. Saf. 2024, 277, 116367. [Google Scholar] [CrossRef]
- Park, K.C.; Kwon, Y.; Lee, Y.; Kim, D.K.; Jang, Y.; Lee, S. Low selenium levels are associated with decreased bone mineral densities. J. Trace Elem. Med. Biol. 2020, 61, 126534. [Google Scholar] [CrossRef] [PubMed]
- Gökçe, A.B.; Eren, B.; Sağir, D.; Yilmaz, B.D. Inhibition of acrolein-induced apoptosis by the antioxidant selenium. Toxicol. Ind. Health 2020, 36, 84–92. [Google Scholar] [CrossRef]
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of osteoclast differentiation by cytokine networks. Immune Netw. 2018, 18, e8. [Google Scholar] [CrossRef]
- Peng, R.; Dong, Y.; Zheng, M.; Kang, H.; Wang, P.; Zhu, M.; Song, K.; Wu, W.; Li, F. Il-17 promotes osteoclast-induced bone loss by regulating glutamine-dependent energy metabolism. Cell Death Dis. 2024, 15, 111. [Google Scholar] [CrossRef]
- Zhu, G.; Chen, W.; Tang, C.Y.; McVicar, A.; Edwards, D.; Wang, J.; McConnell, M.; Yang, S.; Li, Y.; Chang, Z.; et al. Knockout and double knockout of cathepsin k and Mmp9 reveals a novel function of cathepsin k as a regulator of osteoclast gene expression and bone homeostasis. Int. J. Biol. Sci. 2022, 18, 5522–5538. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, E.; Scapolan, M.; Pecolo, M.; Wassermann, B.; Abu-Rumeileh, I.; Balestreri, L.; Borsatti, E.; Tripodo, C.; Colombatti, A.; Spessotto, P. MMP-13 stimulates osteoclast differentiation and activation in tumour breast bone metastases. Breast Cancer Res. 2011, 13, R105. [Google Scholar] [CrossRef]
- Behrends, M.; Wagner, S.; Kopka, K.; Schober, O.; Schäfers, M.; Kumbhar, S.; Waller, M.; Haufe, G. New matrix metalloproteinase inhibitors based on γ-fluorinated α-aminocarboxylic and α-aminohydroxamic acids. Bioorg Med. Chem. 2015, 23, 3809–3818. [Google Scholar] [CrossRef]
- Beederman, M.; Lamplot, J.D.; Nan, G.; Wang, J.; Liu, X.; Yin, L.; Li, R.; Shui, W.; Zhang, H.; Kim, S.H.; et al. Bmp signaling in mesenchymal stem cell differentiation and bone formation. J. Biomed. Sci. Eng. 2013, 6, 32–52. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGF-β/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 15005. [Google Scholar] [CrossRef] [PubMed]
- Edderkaoui, B. Chemokines in cartilage regeneration and degradation: New insights. Int. J. Mol. Sci. 2023, 25, 381. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Shi, R.; Zhang, G.; Wang, Y.; Ye, L.; Peng, L.; Guo, S.; He, J.; Yang, H.; Jiang, Y. miR-539-5p targets BMP2 to regulate Treg activation in B-cell acute lymphoblastic leukemia through TGF-β/Smads/MAPK. Exp. Biol. Med. 2024, 249, 10111. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Habib, R.; Shafiq, S.; Abbas, S.S.; Khan, S.; Eqani, S.; Nepovimova, E.; Khan, M.S.; Kuca, K.; Nurulain, S.M. Influence of the chronic groundwater fluoride consumption on cholinergic enzymes, ACHE and BCHE gene SNPs and pro-inflammatory cytokines: A study with Pakistani population groups. Sci. Total Environ. 2023, 880, 163359. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ha, S.; Xu, L.; Liu, C.; Liu, Y.; Wu, X.; Li, Z.; Wu, S.; Yang, B.; Chen, Z. Fluorinated hydroxyapatite conditions a favorable osteo-immune microenvironment via triggering metabolic shift from glycolysis to oxidative phosphorylation. J. Transl. Med. 2024, 22, 437. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xia, B.; Mai, S.; Feng, Z.; Wang, X.; Liu, Y.; Liu, R.; Li, Z.; Xiao, Y.; Chen, Z.; et al. Sodium fluoride under dose range of 2.4-24 μM, a promising osteoimmunomodulatory agent for vascularized bone formation. ACS Biomater. Sci. Eng. 2019, 5, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Sun, K.Y.; Zhu, Y.; Zhang, X.; Zhou, Y.H.; Zou, X. Metformin alleviates inflammation through suppressing FASN-dependent palmitoylation of Akt. Cell Death Dis. 2021, 12, 934. [Google Scholar] [CrossRef]
- Christodoulou, M.; Aspray, T.J.; Piec, I.; Fraser, W.D.; Schoenmakers, I. Alterations in regulators of the renal-bone axis, inflammation and iron status in older people with early renal impairment and the effect of vitamin d supplementation. Age Ageing 2024, 53, afae096. [Google Scholar] [CrossRef]
- Éva Sikura, K.; Combi, Z.; Potor, L.; Szerafin, T.; Hendrik, Z.; Méhes, G.; Gergely, P.; Whiteman, M.; Beke, L.; Fürtös, I.; et al. Hydrogen sulfide inhibits aortic valve calcification in heart via regulating runx2 by nf-κb, a link between inflammation and mineralization. J. Adv. Res. 2021, 27, 165–176. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, F.; Liu, K.; Xin, J.; Chen, J. HMGB1 could restrict 1,3-β-glucan induced mice lung inflammation by affecting Beclin1 and Bcl2 interaction and promoting the autophagy of epithelial cells. Ecotoxicol. Environ. Saf. 2021, 222, 112460. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, T.; Luo, Q.; Chen, Y.; Leung, V.Y.; Wen, C.; Shah, M.F.; Pan, H.; Chiu, K.; Cao, X.; et al. Cartilage degeneration and excessive subchondral bone formation in spontaneous osteoarthritis involves altered TGF-β signaling. J. Orthop. Res. 2016, 34, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, J.E.; Lee, S.B.; Lee, N.Y.; Park, S.Y. Gulp1 regulates chondrocyte growth arrest and differentiation via the TGF-β/Smad2/3 pathway. FEBS Lett. 2024, 598, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Gyemi, L.; Selznick, A.; Petrisor, B.; Ghert, M. Time to full weight-bearing with the use of a calcium sulfate-calcium phosphate bone substitute as a bone void filler following extended curettage in the treatment of primary benign bone tumours. J. Orthop. Surg. 2024, 32, 10225536241254200. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).