Tissue-Level Effect of Andrographis and Ashwagandha Metabolites on Metabolic and Inflammatory Gene Expression in Skeletal Muscle and Adipose Tissue: An Ex Vivo/In Vitro Investigation
Abstract
1. Introduction
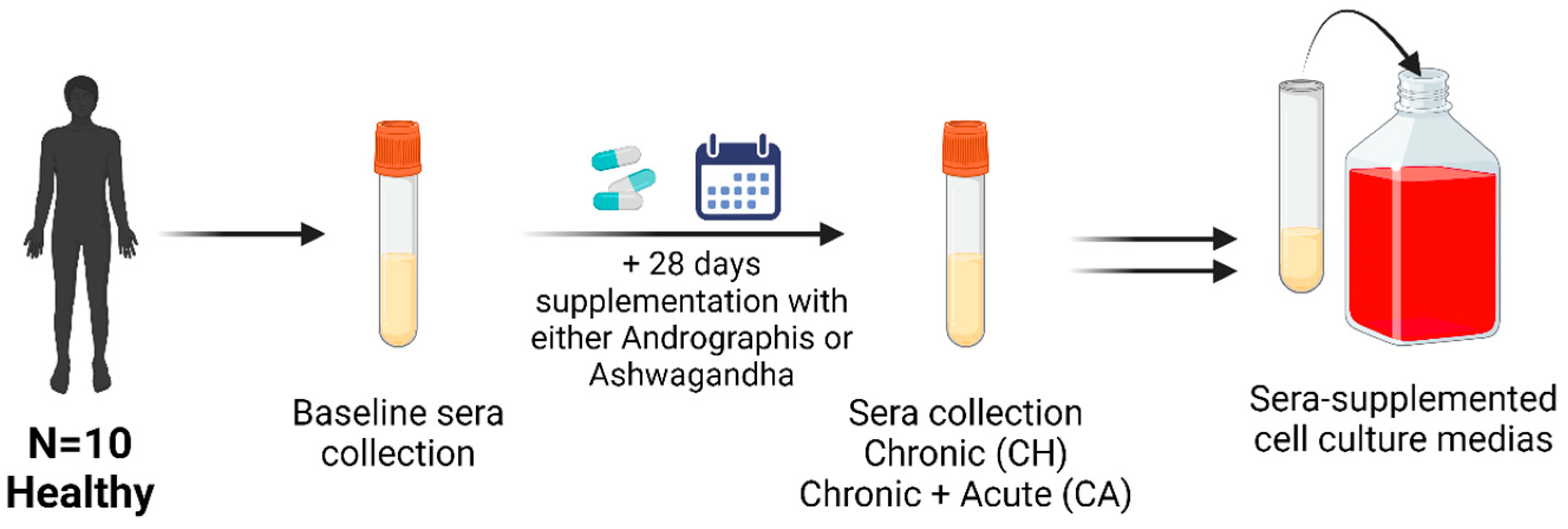
2. Materials and Methods
2.1. Participants
Adipose Tissue
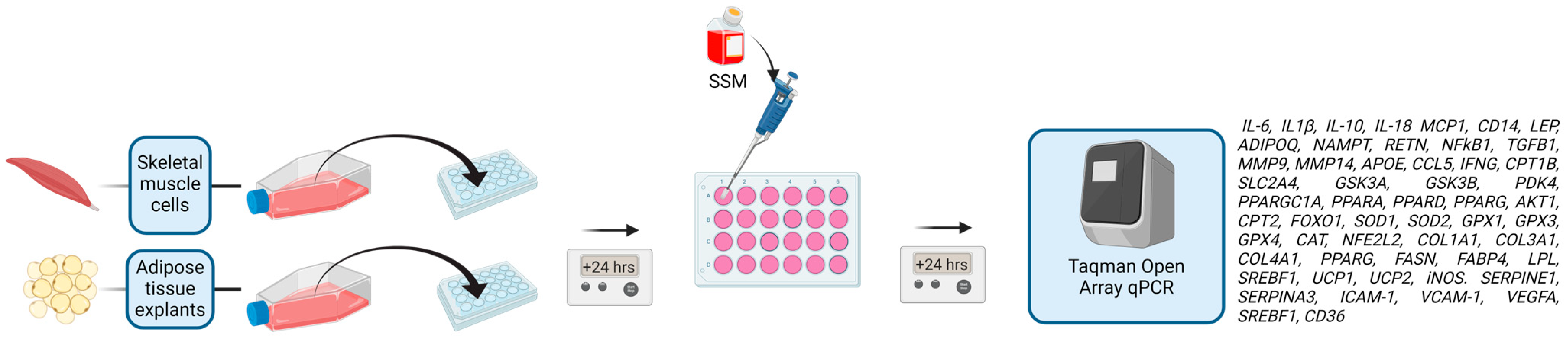
2.2. Primary Human Myogenic Cell Culture
2.3. Treatment
2.4. OpenArray
2.5. Data Analysis
3. Results
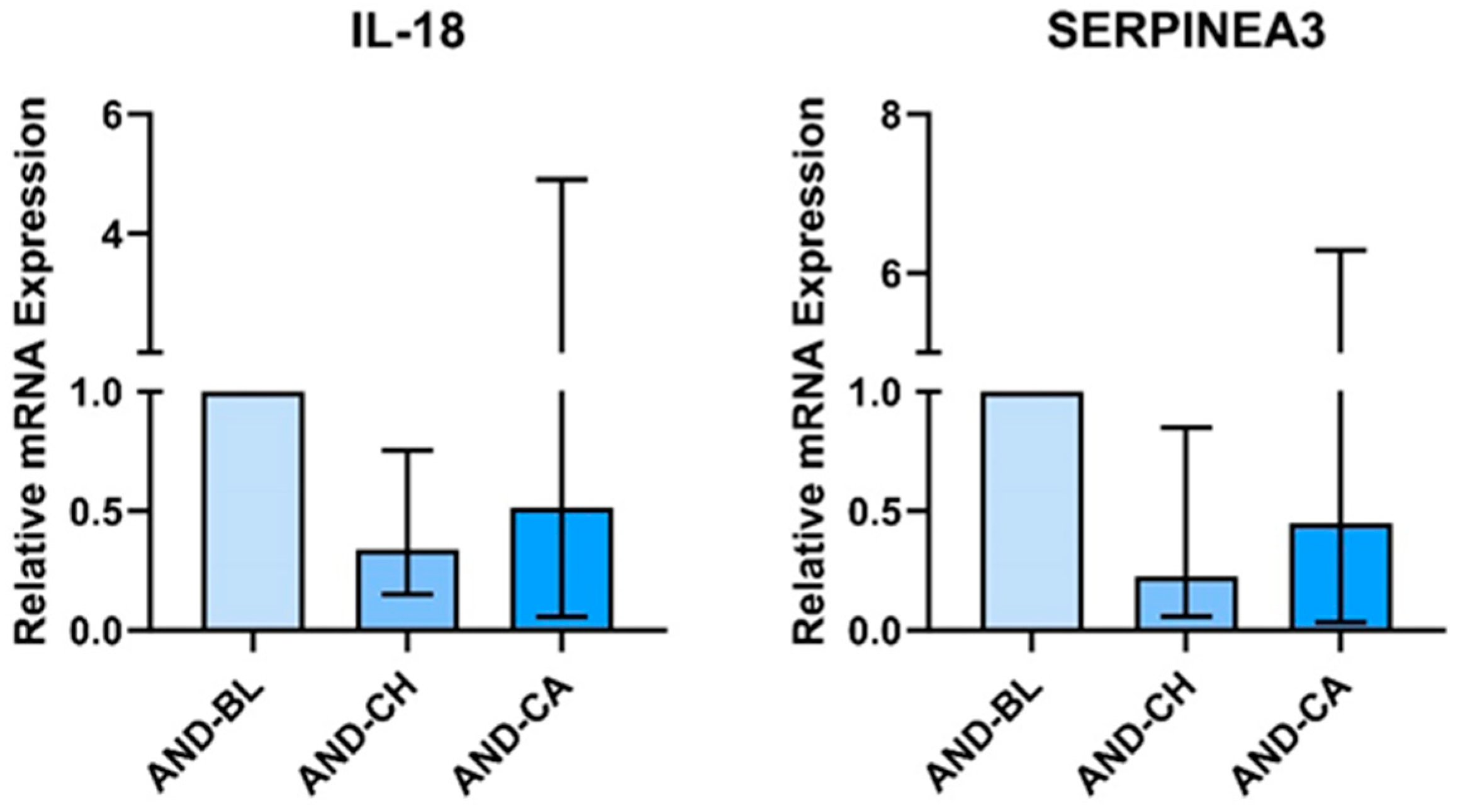
3.1. Andrographis
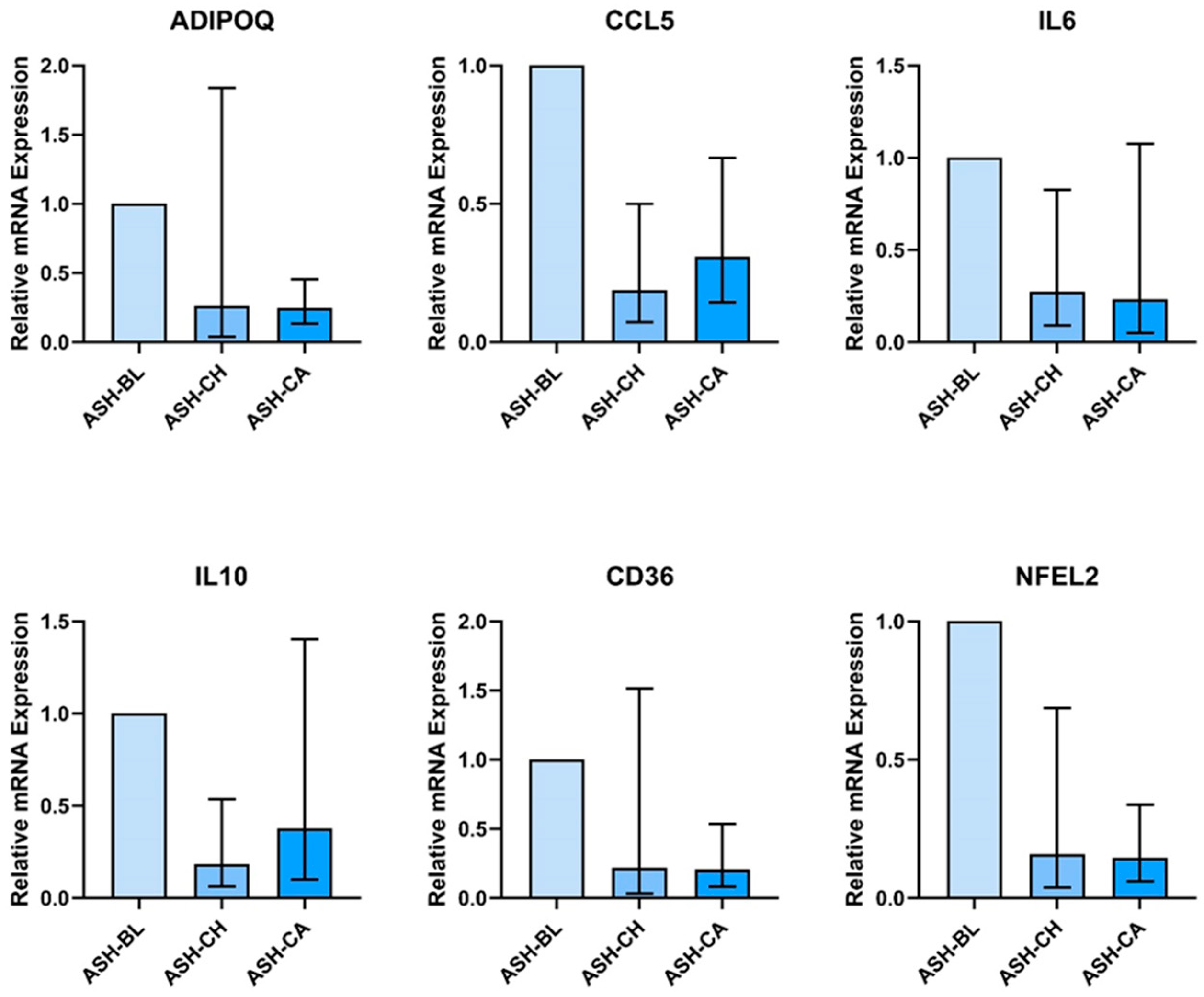
3.2. Ashwagandha
4. Discussion
4.1. Effect of Andrographis in AT
4.2. Effect of Ashwagandha on AT and SKM
4.3. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sattar, N.; McMurray, J.J.V.; McInnes, I.B.; Aroda, V.R.; Lean, M.E.J. Treating Chronic Diseases without Tackling Excess Adiposity Promotes Multimorbidity. Lancet Diabetes Endocrinol. 2023, 11, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflam-Mation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef]
- Lindhorst, A.; Raulien, N.; Wieghofer, P.; Eilers, J.; Rossi, F.M.V.; Bechmann, I.; Gericke, M. Adipocyte Death Triggers a Pro-Inflammatory Response and Induces Metabolic Activation of Resident Macrophages. Cell Death Dis. 2021, 12, 579. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, Y.; Song, J. Characterization and Treatment of Inflammation and Insulin Resistance in Obese Adipose Tissue. Diabetes Metab. Syndr. Obes. 2020, 13, 3449–3460. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Nikolajczyk, B.S. Tissue Immune Cells Fuel Obesity-Associated Inflammation in Adipose Tissue and Beyond. Front. Immunol. 2019, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dys-Function as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Trouwborst, I.; Bowser, S.M.; Goossens, G.H.; Blaak, E.E. Ectopic Fat Accumulation in Distinct Insulin Resistant Pheno-Types; Targets for Personalized Nutritional Interventions. Front. Nutr. 2018, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Mengeste, A.M.; Rustan, A.C.; Lund, J. Skeletal muscle energy metabolism in obesity. Obesity 2021, 29, 1582–1595. [Google Scholar] [CrossRef]
- Jalali, M.; Bahadoran, Z.; Mirmiran, P.; Azizi, F.; Hosseinpanah, F. Severity of Adipose Tissue Dysfunction Is Associated with Progression of Pre-Diabetes to Type 2 Diabetes: The Tehran Lipid and Glucose Study. BMC Public Health 2024, 24, 121. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Skeletal Muscle Inflammation and Insulin Resistance in Obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef]
- Silva Figueiredo, P.; Inada, A.C.; Ribeiro Fernandes, M.; Granja Arakaki, D.; Freitas, K.C.; Avellaneda Guimarães, R.C.; Nascimento, V.; Aiko Hiane, P. An Overview of Novel Dietary Supplements and Food Ingredients in Patients with Metabolic Syndrome and Non-Alcoholic Fatty Liver Disease. Molecules 2018, 23, 877. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, P.; Malinowska, M.; Ignacyk, M.; Szustowski, P.; Nowak, J.; Pesta, K.; Szeląg, M.; Szklanny, D.; Judasz, E.; Ka-czmarek, G.; et al. Ashwagandha (Withania somnifera)-Current Research on the Health-Promoting Activities: A Narrative Review. Pharmaceutics 2023, 15, 1057. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Muhammad, G.; Hussain, M.A.; Altaf, M.; Bukhari, S.N.A. Withania somnifera L.: Insights into the Phyto-Chemical Profile, Therapeutic Potential, Clinical Trials, and Future Prospective. Iran. J. Basic Med. Sci. 2020, 23, 1501–1526. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Rayalam, S.; Della-Fera, M.A.; Ambati, S.; Yang, J.-Y.; Baile, C.A. Withaferin a Induces Apoptosis and Inhibits Adipogenesis in 3t3-L1 Adipocytes. BioFactors 2008, 33, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Jafri, A.A.; Aggarwal, J.; Batra, J. Antidiabetic Effect of Withania somnifera Root in Stz Induced Diabetic Rats. Cardiometry 2022, 1278–1283. [Google Scholar] [CrossRef]
- Lee, J.; Liu, J.; Feng, X.; Salazar Hernández, M.A.; Mucka, P.; Ibi, D.; Choi, J.W.; Ozcan, U. Withaferin a Is a Leptin Sensitizer with Strong Antidiabetic Properties in Mice. Nat. Med. 2016, 22, 1023–1032. [Google Scholar] [CrossRef]
- Lee, D.H.; Ahn, J.; Jang, Y.J.; Seo, H.D.; Ha, T.Y.; Kim, M.J.; Huh, Y.H.; Jung, C.H. Withania somnifera Extract Enhances Energy Expenditure via Improving Mitochondrial Function in Adipose Tissue and Skeletal Muscle. Nutrients 2020, 12, 431. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, S.H.; Lee, E.; Seo, H.D.; Ahn, J.; Jang, Y.J.; Ha, T.Y.; Im, S.S.; Jung, C.H. Withaferin a Exerts an An-Ti-Obesity Effect by Increasing Energy Expenditure through Thermogenic Gene Expression in High-Fat Diet-Fed Obese Mice. Phytomedicine 2021, 82, 153457. [Google Scholar] [CrossRef]
- Chao, W.W.; Lin, B.F. Isolation and Identification of Bioactive Compounds in Andrographis paniculata (Chuanxinlian). Chin. Med. 2010, 5, 17. [Google Scholar] [CrossRef]
- Intharuksa, A.; Arunotayanun, W.; Yooin, W.; Sirisa-Ard, P. A Comprehensive Review of Andrographis paniculata (Burm. f.) Nees and Its Constituents as Potential Lead Compounds for Covid-19 Drug Discovery. Molecules 2022, 27, 4479. [Google Scholar] [CrossRef]
- Chen, C.C.; Chuang, W.T.; Lin, A.H.; Tsai, C.W.; Huang, C.S.; Chen, Y.T.; Chen, H.W.; Lii, C.K. Andrographolide Inhibits Adipogenesis of 3t3-L1 Cells by Suppressing c/Ebpβ Expression and Activation. Toxicol. Appl. Pharmacol. 2016, 307, 115–122. [Google Scholar] [CrossRef]
- Jin, L.; Fang, W.; Li, B.; Shi, G.; Li, X.; Yang, Y.; Yang, J.; Zhang, Z.; Ning, G. Inhibitory Effect of Andrographolide in 3t3-L1 Adipocytes Differentiation through the PPARγ Pathway. Mol. Cell. Endocrinol. 2012, 358, 81–87. [Google Scholar] [CrossRef]
- Villiers, D.; Potgieter, M.; Ambele, M.A.; Adam, L.; Durandt, C.; Pepper, M.S. The Role of Reactive Oxygen Species in Adipogenic Differentiation. Adv. Exp. Med. Biol. 2018, 1083, 125–144. [Google Scholar] [CrossRef]
- Kalamkar, S.D.; Bose, G.S.; Ghaskadbi, S.; Mittal, S. Andrographolide and Pterostilbene Inhibit Adipocyte Differentiation by Downregulating PPARγ through Different Regulators. Nat. Prod. Res. 2023, 37, 3145–3151. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, S.; Tang, J.; Ding, N.; Chu, X.; Cheng, L.; Ding, X.; Liang, T.; Feng, S.; Rahman, S.U.; et al. An-Drographolide Inhibits Inflammatory Cytokines Secretion in Lps-Stimulated Raw264.7 Cells through Suppression of Nf-Κb/Mapk Signaling Pathway. Evid. Based Complement. Altern. Med. 2017, 2017, 8248142. [Google Scholar] [CrossRef]
- Shen, T.; Yang, W.S.; Yi, Y.S.; Sung, G.H.; Rhee, M.H.; Poo, H.; Kim, M.Y.; Kim, K.W.; Kim, J.H.; Cho, J.Y. Ap-1/Irf-3 Targeted Anti-Inflammatory Activity of Andrographolide Isolated from Andrographis paniculata. Evid. Based Complement. Altern. Med. 2013, 2013, 210736. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Zheng, Y.R.; Deng, L.J.; Sun, P.H.; Ye, J.X.; Wei, X.D.; Liu, F.F.; Yu, L.Z.; Ye, W.C.; Fan, C.L.; et al. Diterpenoid Lactones with Anti-Inflammatory Effects from the Aerial Parts of Andrographis paniculata. Molecules 2019, 24, 2726. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, H.; Xu, Y.; Fan, W.; Yao, H.; Wang, Y.; Hu, W.; Lou, G.; Shi, Y.; Chen, X.; et al. Andrographolide promotes skeletal muscle regeneration after acute injury through epigenetic modulation. Eur. J. Pharmacol. 2020, 888, 173470. [Google Scholar] [CrossRef]
- Cabrera, D.; Gutiérrez, J.; Cabello-Verrugio, C.; Morales, M.G.; Mezzano, S.; Fadic, R.; Casar, J.C.; Hancke, J.L.; Brandan, E. Andrographolide Attenuates Skeletal Muscle Dystrophy in Mdx Mice and Increases Efficiency of Cell Therapy by Reducing Fibrosis. Skelet. Muscle 2014, 4, 6. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Xiang, L.; Zhou, F.; Li, H.; Su, Y.; Xu, X.; Wang, Q. Metabolites Identification of Bioactive Compounds Daturataturin a, Daturametelin i, n-Trans-Feruloyltyramine, and Cannabisin f from the Seeds of Datura metel in Rats. Front. Pharmacol. 2018, 9, 731. [Google Scholar] [CrossRef]
- Singh, S.K.; Valicherla, G.R.; Bikkasani, A.K.; Cheruvu, S.H.; Hossain, Z.; Taneja, I.; Ahmad, H.; Raju, K.S.R.; Sangwan, N.S.; Singh, S.K.; et al. Elucidation of Plasma Protein Binding, Blood Partitioning, Permeability, Cyp Phenotyping and Cyp Inhibition Studies of Withanone Using Validated Uplc Method: An Active Constituent of Neuroprotective Herb Ashwagandha. J. Ethnopharmacol. 2021, 270, 113819. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U. Evaluation of a Highly Standardized Withania Somnifera Extract on Endothelial Dysfunction and Biomarkers of Oxidative Stress in Patients with Type 2 Diabetes Mellitus: A Randomized, Double Blind, Placebo Controlled Study. Int. J. Ayu Pharm. Res. 2014, 2, 22–32. [Google Scholar]
- Sandborn, W.J.; Targan, S.R.; Byers, V.S.; Rutty, D.A.; Mu, H.; Zhang, X.; Tang, T. Andrographis Paniculata Extract (HMPL-004) for Active Ulcerative Colitis. Am. J. Gastroenterol. 2013, 108, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Hancke, J.L.; Srivastav, S.; Cáceres, D.D.; Burgos, R.A. A Double-Blind, Randomized, Placebo-Controlled Study to Assess the Efficacy of Andrographis paniculata Standardized Extract (ParActin®) on Pain Reduction in Subjects with Knee Osteoarthritis. Phytother. Res. 2019, 33, 1469–1479. [Google Scholar] [CrossRef]
- Ciampi, E.; Uribe-San-Martin, R.; Cárcamo, C.; Cruz, J.P.; Reyes, A.; Reyes, D.; Pinto, C.; Vásquez, M.; Burgos, R.A.; Hancke, J. Efficacy of Andrographolide in Not Active Progressive Multiple Sclerosis: A Prospective Exploratory Double-Blind, Parallel-Group, Randomized, Placebo-Controlled Trial. BMC Neurol. 2020, 20, 173. [Google Scholar] [CrossRef]
- Travers, R.L.; Motta, A.C.; Betts, J.A.; Bouloumié, A.; Thompson, D. The Impact of Adiposity on Adipose Tissue-Resident Lymphocyte Activation in Humans. Int. J. Obes. 2015, 39, 762–769. [Google Scholar] [CrossRef]
- Wangdi, J.T.; O’Leary, M.F.; Kelly, V.G.; Jackman, S.R.; Tang, J.C.Y.; Dutton, J.; Bowtell, J.L. Tart Cherry Supplement Enhances Skeletal Muscle Glutathione Peroxidase Expression and Functional Recovery after Muscle Damage. Med. Sci. Sports Exerc. 2022, 54, 609–621. [Google Scholar] [CrossRef]
- Shanely, R.A.; Zwetsloot, K.A.; Triplett, N.T.; Meaney, M.P.; Farris, G.E.; Nieman, D.C. Human Skeletal Muscle Biopsy Procedures Using the Modified Bergström Technique. J. Vis. Exp. 2014, 91, e51812. [Google Scholar] [CrossRef]
- O’Leary, M.F.; Wallace, G.R.; Bennett, A.J.; Tsintzas, K.; Jones, S.W. Il-15 Promotes Human Myogenesis and Mitigates the Detrimental Effects of Tnfα on Myotube Development. Sci. Rep. 2017, 7, 12997. [Google Scholar] [CrossRef]
- Prel, J.B.; Hommel, G.; Röhrig, B.; Blettner, M. Confidence Interval or P-Value?: Part 4 of a Series on Evaluation of Scientific Publications. Dtsch. Arztebl. Int. 2009, 106, 335–339. [Google Scholar] [CrossRef]
- Halsey, L.G. The Reign of the P-Value Is over: What Alternative Analyses Could We Employ to Fill the Power Vacuum? Biol. Lett. 2019, 15, 20190174. [Google Scholar] [CrossRef]
- Kalsheker, N.; Morley, S.; Morgan, K. Gene regulation of the serine proteinase inhibitors alpha1-antitrypsin and al-pha1-antichymotrypsin. Biochem. Soc. Trans. 2002, 30, 93–98. [Google Scholar] [CrossRef]
- Choi, Y.; Choi, H.; Yoon, B.K.; Lee, H.; Seok, J.W.; Kim, H.J.; Kim, J.W. Serpina3c regulates adipogenesis by modulating insulin growth factor 1 and integrin signaling. iScience 2020, 23, 100961. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Olivo, L.A.; Mejia-Elizondo, R.; Alonso-Castro, A.J.; Ponce-Noyola, P.; Maldonado-Lagunas, V.; Melendez-Zajgla, J.; Saavedra-Alanis, V.M. Serpina3g Participates in the Antiadipogenesis and Insulin-Resistance Induced by Tumor Necrosis Factor-α in 3t3-F442a Cells. Cytokine 2014, 69, 180–188. [Google Scholar] [CrossRef]
- Li, B.Y.; Guo, Y.Y.; Xiao, G.; Guo, L.; Tang, Q.Q. Serpina3c Ameliorates Adipose Tissue Inflammation through the Ca-Thepsin g/Integrin/Akt Pathway. Mol. Metab. 2022, 61, 101500. [Google Scholar] [CrossRef]
- Li, B.; Lei, Z.; Wu, Y.; Li, B.; Zhai, M.; Zhong, Y.; Ju, P.; Kou, W.; Shi, Y.; Zhang, X.; et al. The Association and Pathogenesis of Serpina3 in Coronary Artery Disease. Front. Cardiovasc. Med. 2021, 8, 756889. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, Z.; Wang, P.; Zheng, M.; Yang, X.; Liu, Y.; Ma, Z.; Chen, M.; Yang, X. Proteomics of Epicardial Adipose Tissue in Patients with Heart Failure. J. Cell. Mol. Med. 2020, 24, 511–520. [Google Scholar] [CrossRef]
- Trøseid, M.; Seljeflot, I.; Arnesen, H. The Role of Interleukin-18 in the Metabolic Syndrome. Cardiovasc. Diabetol. 2010, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Thomas, R.; Kochumon, S.; Sindhu, S. Increased Adipose Tissue Expression of Il-18r and Its Ligand Il-18 Associates with Inflammation and Insulin Resistance in Obesity. Immun. Inflamm. Dis. 2017, 5, 318–335. [Google Scholar] [CrossRef]
- Moriwaki, Y.; Yamamoto, T.; Shibutani, Y.; Aoki, E.; Tsutsumi, Z.; Takahashi, S.; Okamura, H.; Koga, M.; Fukuchi, M.; Hada, T. Elevated Levels of Interleukin-18 and Tumor Necrosis Factor-Alpha in Serum of Patients with Type 2 Diabetes Mellitus: Relationship with Diabetic Nephropathy. Metabolism 2003, 52, 605–608. [Google Scholar] [CrossRef]
- Yamaoka-Tojo, M.; Tojo, T.; Wakaume, K.; Kameda, R.; Nemoto, S.; Takahira, N.; Masuda, T.; Izumi, T. Circulating Interleukin-18: A Specific Biomarker for Atherosclerosis-Prone Patients with Metabolic Syndrome. Nutr. Metab. 2011, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, Y.; Dai, Z.; Sun, Y. Intermittent High Glucose Stimulate Mcp-l, Il-18, and Pai-1, but Inhibit Adiponectin Ex-Pression and Secretion in Adipocytes Dependent of Ros. Cell Biochem. Biophys. 2009, 55, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Chen, S.R.; Wang, K.; Peng, Y.; Wang, Y.T.; Wang, D.; Wang, Y.; Zhou, G.C. Attenuation of Innate Immunity by Andrographolide Derivatives through Nf-Κb Signaling Pathway. Sci. Rep. 2017, 7, 4738. [Google Scholar] [CrossRef] [PubMed]
- Wood, I.S.; Wang, B.; Jenkins, J.R.; Trayhurn, P. The Pro-Inflammatory Cytokine Il-18 Is Expressed in Human Adipose Tissue and Strongly Upregulated by Tnfalpha in Human Adipocytes. Biochem. Biophys. Res. Commun. 2005, 337, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, W.; Wu, J.; Zhen, J.; Sun, Q.; Yu, M. Andrographolide, a Natural Anti-Inflammatory Agent: An Update. Front. Pharmacol. 2022, 13, 920435. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.L. Naturalizing mouse models for immunology. Nat. Immunol. 2021, 22, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Wagar, L.E.; DiFazio, R.M.; Davis, M.M. Advanced Model Systems and Tools for Basic and Translational Human Immunology. Genome Med. 2018, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Lempesis, I.G.; Tsilingiris, D.; Liu, J.; Dalamaga, M. Of Mice and Men: Considerations on Adipose Tissue Physiology in Animal Models of Obesity and Human Studies. Metab. Open 2022, 15, 100208. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.A.; Vieira-Silva, S.; Liston, A.; Raes, J. How Informative Is the Mouse for Human Gut Microbiota Research? Dis. Mod. Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef]
- Curat, C.A.; Miranville, A.; Sengenès, C.; Diehl, M.; Tonus, C.; Busse, R.; Bouloumié, A. From Blood Monocytes to Adipose Tissue-Resident Macrophages: Induction of Diapedesis by Human Mature Adipocytes. Diabetes 2004, 53, 1285–1292. [Google Scholar] [CrossRef]
- Keophiphath, M.; Rouault, C.; Divoux, A.; Clément, K.; Lacasa, D. Ccl5 Promotes Macrophage Recruitment and Survival in Human Adipose Tissue. Arter. Thromb. Vasc. Biol. 2010, 30, 39–45. [Google Scholar] [CrossRef]
- Gupta, M.; Kaur, G. Aqueous Extract from the Withania somnifera Leaves as a Potential Anti-Neuroinflammatory Agent: A Mechanistic Study. J. Neuroinflammation 2016, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, Y.; Yan, M.; Zhang, G. Modulatory Action of Withaferin-a on Oxidative Damage through Regulation of Inflammatory Mediators and Apoptosis via Pi3k/Akt Signaling Pathway in High Cholesterol-Induced Atherosclerosis in Experimental Rats. J. Biochem. Mol. Toxicol. 2022, 36, 23154. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, M.H.; Azmi, M.N.; Shariff, K.A.; Tan, J.S. Withaferin a Protects against High-Fat Diet-Induced Obesity via Attenuation of Oxidative Stress, Inflammation, and Insulin Resistance. Appl. Biochem. Biotechnol. 2019, 188, 241–259. [Google Scholar] [CrossRef]
- Yanai, H.; Yoshida, H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int. J. Mol. Sci. 2019, 20, 1190. [Google Scholar] [CrossRef]
- Lee, J.; Ellis, J.M.; Wolfgang, M.J. Adipose Fatty Acid Oxidation Is Required for Thermogenesis and Potentiates Oxidative Stress-Induced Inflammation. Cell Rep. 2015, 10, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Schmid, B.; Rippmann, J.F.; Tadayyon, M.; Hamilton, B.S. Inhibition of Fatty Acid Synthase Prevents Preadipocyte Differentiation. Biochem. Biophys. Res. Commun. 2005, 328, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Yoo, M.J.; Kang, H.; Lee, S.R.; Kim, S.; Yu, J.S.; Kim, J.C.; Jang, T.S.; Pang, C.; Kim, K.H. Withasomniferol d, a New Anti-Adipogenic Withanolide from the Roots of Ashwagandha (Withania somnifera). Pharmaceuticals 2021, 14, 1017. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Rupérez, A.I.; Gomez-Llorente, C.; Gil, A.; Aguilera, C.M. Cell Models and Their Application for Stud-Ying Adipogenic Differentiation in Relation to Obesity: A Review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef]
- Li, J.; Jin, C.; Gustafsson, S.; Rao, A.; Wabitsch, M.; Park, C.Y.; Quertermous, T.; Knowles, J.W.; Bielczyk-Maczynska, E. Single-Cell Transcriptome Dataset of Human and Mouse in Vitro Adipogenesis Models. Sci. Data 2023, 10, 387. [Google Scholar] [CrossRef]
- Börgeson, E.; Boucher, J.; Hagberg, C.E. Of Mice and Men: Pinpointing Species Differences in Adipose Tissue Biology. Front. Cell Dev. Biol. 2022, 10, 1003118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.A.; Tao, C.; Gupta, R.K.; Scherer, P.E. Tracking Adipogenesis during White Adipose Tissue Development, Expansion and Regeneration. Nat. Med. 2013, 19, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Song, M.G.; Lee, H.J.; Jin, B.Y.; Gutierrez-Aguilar, R.; Shin, K.H.; Choi, S.H.; Um, S.H.; Kim, D.H. Depot-Specific Differences in Angiogenic Capacity of Adipose Tissue in Differential Susceptibility to Diet-Induced Obesity. Mol. Metab. 2016, 5, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, S.; Queguiner, I.; Msika, S.; Calderari, S.; Rufat, P.; Gasc, J.M.; Corvol, P.; Larger, E. Angiogenesis Associated with Visceral and Subcutaneous Adipose Tissue in Severe Human Obesity. Diabetes 2008, 57, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.N.; Peics, J.; Ma, T.; Karavaeva, I.; Dall, M.; Chubanava, S.; Basse, A.L.; Dmytriyeva, O.; Treebak, J.T.; Gerhart-Hines, Z. Nampt-Mediated Nad+ Biosynthesis Is Indispensable for Adipose Tissue Plasticity and Development of Obesity. Mol. Metab. 2018, 11, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, L.; Tchernof, A.; Deshaies, Y.; Marceau, S.; Lescelleur, O.; Biron, S.; Vohl, M.C. Zfp36: A promising candidate gene for obesity-related metabolic complications identified by converging genomics. Obes. Surg. 2007, 17, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Karelis, A.D.; St-Pierre, D.H.; Conus, F.; Rabasa-Lhoret, R.; Poehlman, E.T. Metabolic and body composition factors in subgroups of obesity: What do we know? J. Clin. Endocrinol. Metab. 2004, 89, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bossé, Y.; Marceau, P.; Biron, S.; Lebel, S.; Richard, D.; Vohl, M.C.; Tchernof, A. Gene expression variability in subcutaneous and omental adipose tissue of obese men. Gene Expr. 2007, 14, 35–46. [Google Scholar] [CrossRef] [PubMed]
- van Beek, E.A.; Bakker, A.H.; Kruyt, P.M.; Hofker, M.H.; Saris, W.H.; Keijer, J. Intra- and interindividual variation in gene expression in human adipose tissue. Eur. J. Physiol. 2007, 453, 851–861. [Google Scholar] [CrossRef]
- Pires, N.; Gota, V.; Gulia, A.; Hingorani, L.; Agarwal, M.; Puri, A. Safety and pharmacokinetics of withaferin-a in advanced stage high grade osteosarcoma: A phase i trial. J. Ayurveda Integr. Med. 2020, 11, 68–72. [Google Scholar] [CrossRef]
- Panossian, A.; Hovhannisyan, A.; Mamikonyan, G.; Abrahamian, H.; Hambardzumyan, E.; Gabrielian, E.; Goukasova, G.; Wikman, G.; Wagner, H. Pharmacokinetic and oral bioavailability of andrographolide from Andrographis paniculata fixed combination kan jang in rats and human. Phytomedicine 2000, 7, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.; McGinley, C.; Lee, M.J.; Saner, N.J.; Garnham, A.; Bishop, D.J. Interpretation of exercise-induced changes in human skeletal muscle mrna expression depends on the timing of the post-exercise biopsies. PeerJ 2022, 10, e12856. [Google Scholar] [CrossRef] [PubMed]


| Ashwagandha (N = 10) | Andrographis (N = 10) | |
|---|---|---|
| M/F | 4/6 | 4/6 |
| Age (years) (SD) | 24.6 (4.0) | 24.3 (3.9) |
| BMI (kg·m2) (SD) | 23.0 (2.3) | 21.6 (2.5) |
| Adipose Tissue Donors (N = 7) | |
|---|---|
| M/F | 4/3 |
| Age (years) (SD) | 25.3 (4.3) |
| BMI (kg·m2) (SD) | 24.0 (2.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugtmeijer, C.; Bowtell, J.L.; O’Leary, M. Tissue-Level Effect of Andrographis and Ashwagandha Metabolites on Metabolic and Inflammatory Gene Expression in Skeletal Muscle and Adipose Tissue: An Ex Vivo/In Vitro Investigation. Nutrients 2024, 16, 2291. https://doi.org/10.3390/nu16142291
Lugtmeijer C, Bowtell JL, O’Leary M. Tissue-Level Effect of Andrographis and Ashwagandha Metabolites on Metabolic and Inflammatory Gene Expression in Skeletal Muscle and Adipose Tissue: An Ex Vivo/In Vitro Investigation. Nutrients. 2024; 16(14):2291. https://doi.org/10.3390/nu16142291
Chicago/Turabian StyleLugtmeijer, Celeste, Joanna L. Bowtell, and Mary O’Leary. 2024. "Tissue-Level Effect of Andrographis and Ashwagandha Metabolites on Metabolic and Inflammatory Gene Expression in Skeletal Muscle and Adipose Tissue: An Ex Vivo/In Vitro Investigation" Nutrients 16, no. 14: 2291. https://doi.org/10.3390/nu16142291
APA StyleLugtmeijer, C., Bowtell, J. L., & O’Leary, M. (2024). Tissue-Level Effect of Andrographis and Ashwagandha Metabolites on Metabolic and Inflammatory Gene Expression in Skeletal Muscle and Adipose Tissue: An Ex Vivo/In Vitro Investigation. Nutrients, 16(14), 2291. https://doi.org/10.3390/nu16142291






