Parenteral Nutrition, Inflammatory Bowel Disease, and Gut Barrier: An Intricate Plot
Abstract
1. Introduction
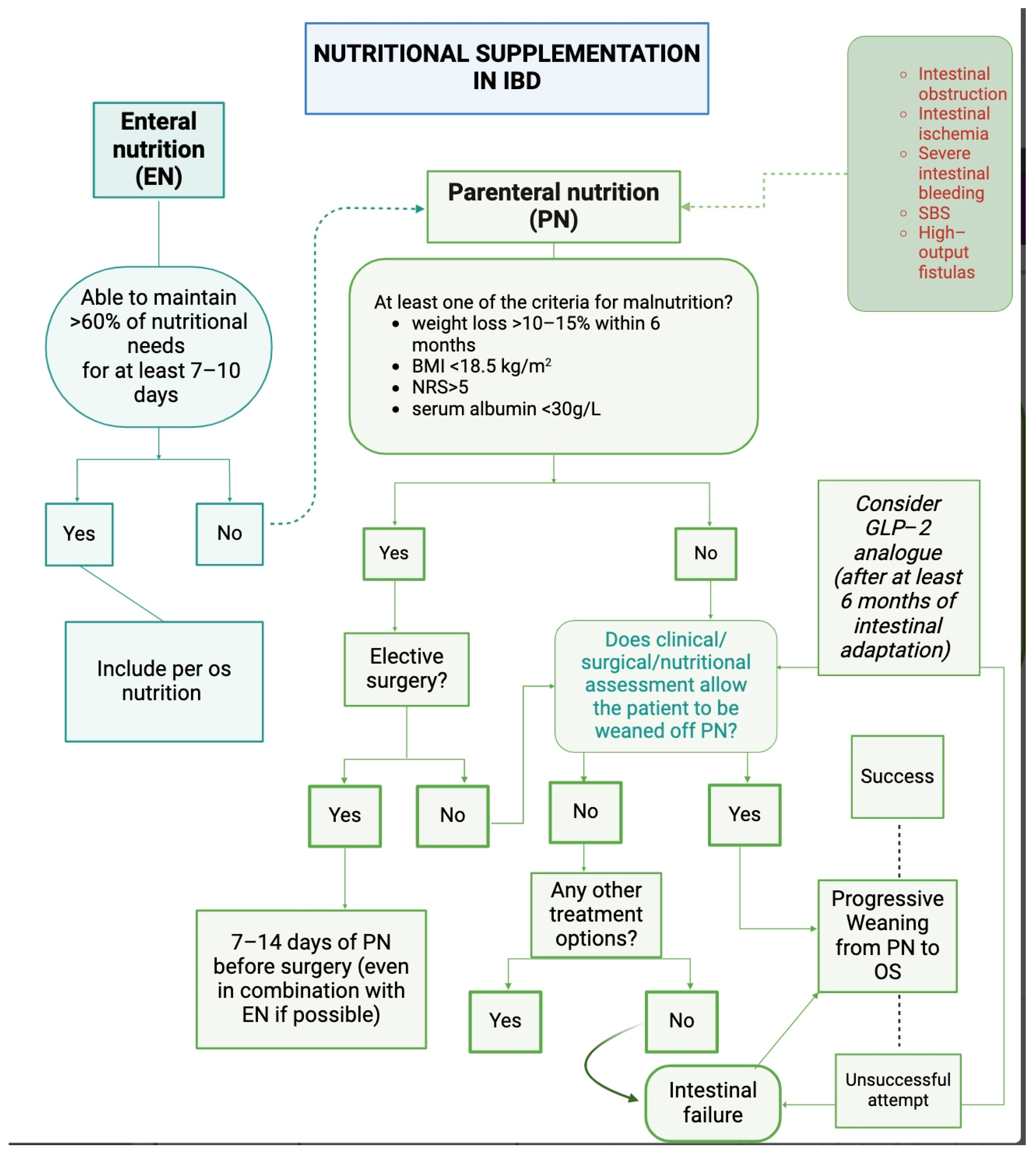
2. Parenteral Nutrition in Inflammatory Bowel Disease
3. Human Gut Barrier and Microbiota
3.1. Composition and Function of a Healthy Gut Barrier
3.2. Specific Barrier Changes in Inflammatory Bowel Disease and Short Bowel Syndrome
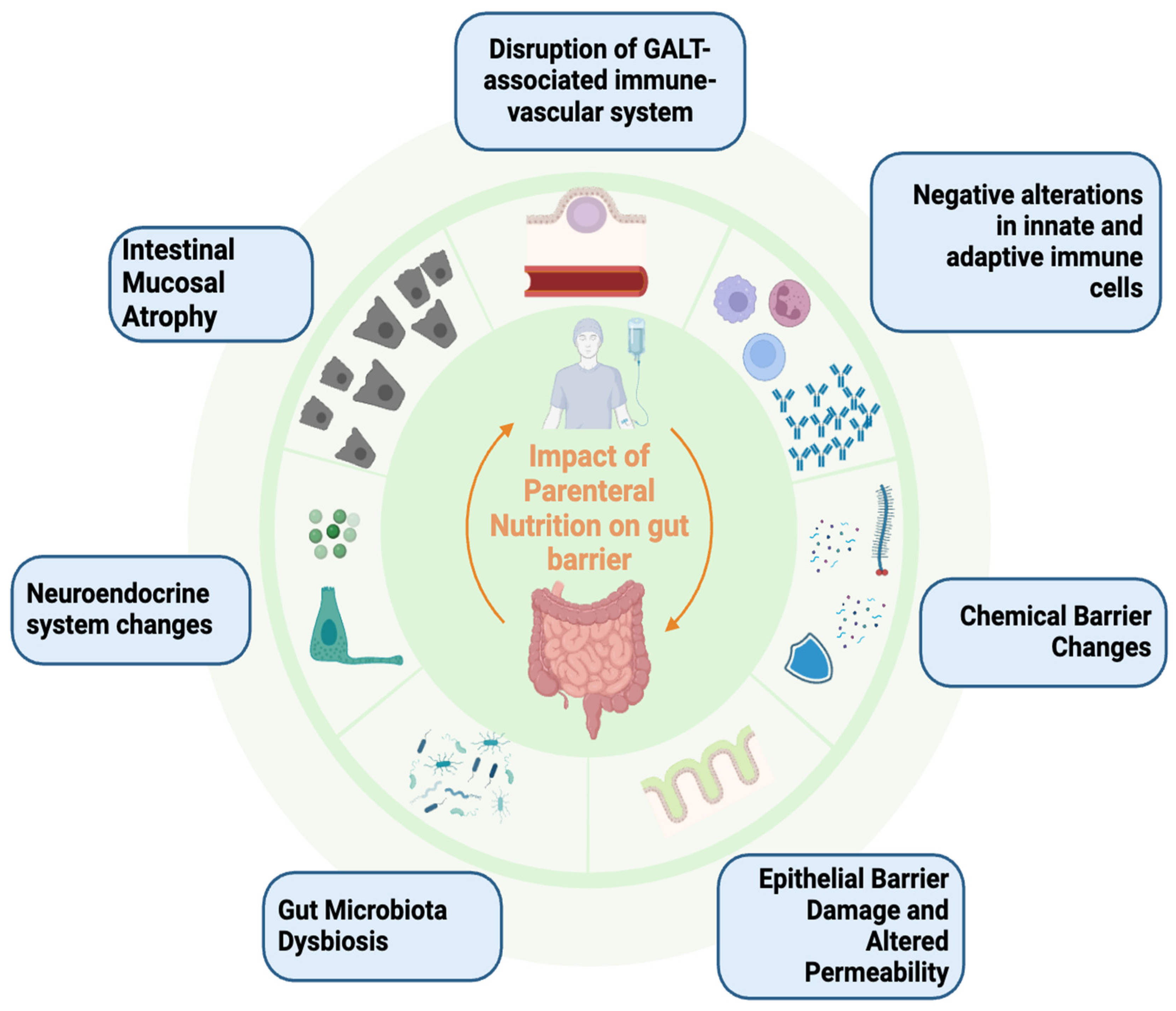
4. Potential Impact of Parenteral Nutrition on Gut Barrier and Microbiota
4.1. Intestinal Atrophic Damage and Role of Intestinotrophic Agents
4.2. Chemical and Immunological Barrier Hazard
4.3. Gut Dysbiosis
| TYPE OF STUDY | STUDY DESIGN | METHOD | RESULTS | REFERENCES |
|---|---|---|---|---|
| Animals | Rats receiving TPN for 3, 7, or 14 days compared to control rats | Ileal lumen contents and ileal biopsies | ↓↓ Firmicutes in TPN group No significant differences in Bacteroidetes | Hodin et al. (2012) [134] |
| Animals | WT or MyD88−/− mice receiving PN solution for 5 days Controls with free access to chow | Segments of small intestine and colon preservation of mucosa-associated bacteria | Domination of Proteobacteria and Bacteroidetes in the TPN group ↑↑ Salmonella, Escherichia, Proteus and Bacteroides | Miyasaka et al. (2013) [135] |
| Animals | Male mice randomized to chow or PN for 5 days | Segments of ileum 16S rRNA sequencing | PN group: ↑↑ Bacteroidetes and Lactobacillus ↓↓ Firmicutes | Heneghan et al. (2013) [136] |
| Animals | Neonatal piglets with PN for 7 days Piglets with EN Orally fed piglets | Mucosal samples of the ileum | TPN ileum was enriched in mucolytic bacteria ↑ C. perfringens in the TPN ileum than in the TEN ileum | Deplancke et al. (2002) [138] |
| Animals | Neonatal piglets with TPN for 14 days Healthy controls | Microbial composition in ileal mucosa 16S rRNA sequencing | Mixed Lipid piglets more similar to Soybean Oil ↑ Parabacteroides with Soybean Oil ↑ Enterobacteriaceae with Mixed Lipid | Lavallee et al. (2017) [139] |
| Humans | Children with IF
| Fecal samples Culture-independent phylogenetic DNA-based microarray analysis | ↓ Diversity and richness ↑ Lactobacilli, Proteobacteria and Actinobacteria, Clostridium clusters IX, XIII, and XV, Fusobacteria, Spirochetes ↓ Clostridium clusters III, IV, and XIVa | Korpela et al. (2017) [144] |
| Humans | SBS children weaned from parenteral SBS children on PN therapy + oral and/or enteral intake Healthy controls | Fecal samples 16S rRNA sequencing | ↓ Bacterial diversity in children with SBS receiving PN vs. children weaned off PN Patients with suspected SIBO: ↑ Enterobacteriaceae, patient receiving PN without suspected SIBO: ↑ Lactobacillaceae | Lilja et al. (2015) [143] |
| Humans | Children with SBS dependent on PN Healthy controls | Fecal samples 16S rRNA sequencing Measurement of SCFAs | ↓ Richness in all SBS groups ↓ Acetate in SBS groups Equal propionate and butyrate and total SCFAs | Wang et al. (2017) [140] |
| Humans | Adults with SBS on PN 24 months after the final digestive circuit modification Healthy controls | Fecal samples, culture-dependent method | ↓ Bacterial counts ↓ Bacteroidetes, Firmicutes, Bifidobacterium, and Methanobrevibacter smithii | Boccia et al. (2017) [85] |
| Humans | SBS children dependent on PN Children weaned from PN Healthy control subjects | Fecal samples 16S rRNA sequencing | ↓ Firmicutes order Clostridiales ↓ Firmicutes and ↑ Enterobacteriaceae in SBS group (and poor growth) | Piper et al. (2017) [148] |
4.4. Epithelial Barrier and Intestinal Permeability
5. Conclusions, Challenges, and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Corridoni, D.; Arseneau, K.O.; Cominelli, F. Inflammatory Bowel Disease. Immunol. Lett. 2014, 161, 231–235. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Haneishi, Y.; Furuya, Y.; Hasegawa, M.; Picarelli, A.; Rossi, M.; Miyamoto, J. Inflammatory Bowel Diseases and Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3817. [Google Scholar] [CrossRef] [PubMed]
- Dunleavy, K.A.; Raffals, L.E.; Camilleri, M. Intestinal Barrier Dysfunction in Inflammatory Bowel Disease: Underpinning Pathogenesis and Therapeutics. Dig. Dis. Sci. 2023, 68, 4306–4320. [Google Scholar] [CrossRef]
- Zeissig, S.; Bürgel, N.; Günzel, D.; Richter, J.; Mankertz, J.; Wahnschaffe, U.; Kroesen, A.J.; Zeitz, M.; Fromm, M.; Schulzke, J.-D. Changes in Expression and Distribution of Claudin 2, 5 and 8 Lead to Discontinuous Tight Junctions and Barrier Dysfunction in Active Crohn’s Disease. Gut 2007, 56, 61–72. [Google Scholar] [CrossRef]
- Vanuytsel, T.; Tack, J.; Farre, R. The Role of Intestinal Permeability in Gastrointestinal Disorders and Current Methods of Evaluation. Front. Nutr. 2021, 8, 717925. [Google Scholar] [CrossRef] [PubMed]
- Radziszewska, M.; Smarkusz-Zarzecka, J.; Ostrowska, L.; Pogodziński, D. Nutrition and Supplementation in Ulcerative Colitis. Nutrients 2022, 14, 2469. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hébuterne, X.; Hvas, C.L.; Joly, F.; Klek, S.; Krznaric, Z.; Ockenga, J.; et al. ESPEN Guideline on Clinical Nutrition in Inflammatory Bowel Disease. Clin. Nutr. 2023, 42, 352–379. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Munsell, M.; Harris, M.L. Nationwide Prevalence and Prognostic Significance of Clinically Diagnosable Protein-Calorie Malnutrition in Hospitalized Inflammatory Bowel Disease Patients. Inflamm. Bowel Dis. 2008, 14, 1105–1111. [Google Scholar] [CrossRef]
- Seres, D.S.; Valcarcel, M.; Guillaume, A. Advantages of Enteral Nutrition over Parenteral Nutrition. Ther. Adv. Gastroenterol. 2013, 6, 157–167. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN Guideline: Clinical Nutrition in Surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef] [PubMed]
- Koretz, R.L.; Lipman, T.O.; Klein, S. American Gastroenterological Association AGA Technical Review on Parenteral Nutrition. Gastroenterology 2001, 121, 970–1001. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.D.; Elkins, J.R.; Stocchi, L.; Farraye, F.A.; Hashash, J.G. Use and Misuse of Parenteral Nutrition in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2022, 28, 1592–1602. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Heyland, D.K.; Ortiz Reyes, L.A.; Laaf, E.; Wendt, S.; Elke, G.; Stoppe, C. Combination of Enteral and Parenteral Nutrition in the Acute Phase of Critical Illness: An Updated Systematic Review and Meta-Analysis. JPEN J. Parenter. Enteral Nutr. 2022, 46, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Boutté, H.J. Overview of Total Parenteral Nutrition in Patients with Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2022, 18, 50–53. [Google Scholar]
- Elia, M. Defining, Recognizing, and Reporting Malnutrition. Int. J. Low. Extrem. Wounds 2017, 16, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Viganò, C.; Palermo, A.; Pirola, L.; Mulinacci, G.; Allocca, M.; Peyrin-Biroulet, L.; Danese, S. Inflammation and Malnutrition in Inflammatory Bowel Disease. Lancet Gastroenterol. Hepatol. 2023, 8, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Wędrychowicz, A.; Zając, A.; Tomasik, P. Advances in Nutritional Therapy in Inflammatory Bowel Diseases: Review. World J. Gastroenterol. 2016, 22, 1045–1066. [Google Scholar] [CrossRef] [PubMed]
- Turkot, M.; Sobocki, J. Results of Home Parenteral Nutrition in Patients with Severe Inflammatory Bowel Disease—An Alternative for Surgery of Malnourished Patients. Pol. Przegl. Chir. 2017, 89, 23–28. [Google Scholar] [CrossRef]
- Papi, C.; Fascì-Spurio, F.; Rogai, F.; Settesoldi, A.; Margagnoni, G.; Annese, V. Mucosal Healing in Inflammatory Bowel Disease: Treatment Efficacy and Predictive Factors. Dig. Liver Dis. 2013, 45, 978–985. [Google Scholar] [CrossRef]
- Pironi, L. Definitions of Intestinal Failure and the Short Bowel Syndrome. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Arends, J.; Baxter, J.; Bozzetti, F.; Peláez, R.B.; Cuerda, C.; Forbes, A.; Gabe, S.; Gillanders, L.; Holst, M.; et al. ESPEN Endorsed Recommendations. Definition and Classification of Intestinal Failure in Adults. Clin. Nutr. 2015, 34, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Steiger, E.; Joly, F.; Wanten, G.J.A.; Chambrier, C.; Aimasso, U.; Sasdelli, A.S.; Szczepanek, K.; Jukes, A.; Theilla, M.; et al. Intravenous Supplementation Type and Volume Are Associated with 1-Year Outcome and Major Complications in Patients with Chronic Intestinal Failure. Gut 2020, 69, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Reiner, J.; Berlin, P.; Wobar, J.; Schäffler, H.; Bannert, K.; Bastian, M.; Vollmar, B.; Jaster, R.; Lamprecht, G.; Witte, M. Teduglutide Promotes Epithelial Tight Junction Pore Function in Murine Short Bowel Syndrome to Alleviate Intestinal Insufficiency. Dig. Dis. Sci. 2020, 65, 3521–3537. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Allard, J.P.; Joly, F.; Geransar, P.; Genestin, E.; Pape, U. Use of Teduglutide in Adults with Short Bowel Syndrome–Associated Intestinal Failure. Nutr. Clin. Pract. 2024, 39, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Lakananurak, N.; Gramlich, L. The Role of Preoperative Parenteral Nutrition. Nutrients 2020, 12, 1320. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN Practical Guideline: Clinical Nutrition in Inflammatory Bowel Disease. Clin. Nutr. 2020, 39, 632–653. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.G.; et al. ESPEN Practical Guideline: Clinical Nutrition in Surgery. Clin. Nutr. 2021, 40, 4745–4761. [Google Scholar] [CrossRef]
- Yoon, J.Y. Nutritional Approach as Therapeutic Manipulation in Inflammatory Bowel Disease. Intest. Res. 2019, 17, 463–475. [Google Scholar] [CrossRef]
- Jacobson, S. Early Postoperative Complications in Patients with Crohn’s Disease given and Not given Preoperative Total Parenteral Nutrition. Scand. J. Gastroenterol. 2012, 47, 170–177. [Google Scholar] [CrossRef]
- Ganaie, A.; Itoo, M.; Bhat, G. Effects of Perioperative Parenteral Nutrition on Wound Healing and Hospital Stay in Surgical Patients: A Randomized Controlled Study. Int. J. Res. Med. Sci. 2015, 3, 3156–3160. [Google Scholar] [CrossRef]
- Btaiche, I.F.; Khalidi, N. Metabolic Complications of Parenteral Nutrition in Adults, Part 1. Am. J. Health Syst. Pharm. 2004, 61, 1938–1949. [Google Scholar] [CrossRef] [PubMed]
- Lawiński, M.; Jachnis, A.; Ukleja, A.; Pertkiewicz, M. Cholelithiasis in Home Parenteral Nutrition (Hpn) Patients–Complications of the Clinical Nutrition: Diagnosis, Treatment, Prevention. Pol. Przegl Chir. 2014, 86, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Schoultz, I.; Keita, Å.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Kunze, W.A.A.; Clerc, N., II. The Intestine as a Sensory Organ: Neural, Endocrine, and Immune Responses. Am. J. Physiol.-Gastrointest. Liver Physiol. 1999, 277, G922–G928. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Van Itallie, C.M. Physiology and Function of the Tight Junction. Cold Spring Harb. Perspect. Biol. 2009, 1, a002584. [Google Scholar] [CrossRef] [PubMed]
- Chairatana, P.; Nolan, E.M. Defensins, Lectins, Mucins, and Secretory Immunoglobulin A: Microbe-Binding Biomolecules That Contribute to Mucosal Immunity in the Human Gut. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 45–56. [Google Scholar] [CrossRef]
- Mabbott, N.A.; Donaldson, D.S.; Ohno, H.; Williams, I.R.; Mahajan, A. Microfold (M) Cells: Important Immunosurveillance Posts in the Intestinal Epithelium. Mucosal Immunol. 2013, 6, 666–677. [Google Scholar] [CrossRef]
- Barker, N. Adult Intestinal Stem Cells: Critical Drivers of Epithelial Homeostasis and Regeneration. Nat. Rev. Mol. Cell Biol. 2014, 15, 19–33. [Google Scholar] [CrossRef]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of Stem Cells in Small Intestine and Colon by Marker Gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Cornick, S.; Tawiah, A.; Chadee, K. Roles and Regulation of the Mucus Barrier in the Gut. Tissue Barriers 2015, 3, e982426. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O. Fight Them or Feed Them: How the Intestinal Mucus Layer Manages the Gut Microbiota. Gastroenterol. Rep. 2019, 7, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Faderl, M.; Noti, M.; Corazza, N.; Mueller, C. Keeping Bugs in Check: The Mucus Layer as a Critical Component in Maintaining Intestinal Homeostasis. IUBMB Life 2015, 67, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Hooper, L.V. Antimicrobial Defense of the Intestine. Immunity 2015, 42, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Hoffert, U.; Hornef, M.W.; Henriques-Normark, B.; Axelsson, L.-G.; Midtvedt, T.; Pütsep, K.; Andersson, M. Secreted Enteric Antimicrobial Activity Localises to the Mucus Surface Layer. Gut 2008, 57, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.C.; Bevins, C.L. Paneth Cells: Maestros of the Small Intestinal Crypts. Annu. Rev. Physiol. 2013, 75, 289–311. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Lee, J.; Li, K.K.; Holland, D.; Maughan, H.; Guttman, D.S.; Yusta, B.; Drucker, D.J. Disruption of the Murine Glp2r Impairs Paneth Cell Function and Increases Susceptibility to Small Bowel Enteritis. Endocrinology 2012, 153, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Steenwinckel, V.; Louahed, J.; Lemaire, M.M.; Sommereyns, C.; Warnier, G.; McKenzie, A.; Brombacher, F.; Van Snick, J.; Renauld, J.-C. IL-9 Promotes IL-13-Dependent Paneth Cell Hyperplasia and up-Regulation of Innate Immunity Mediators in Intestinal Mucosa. J. Immunol. 2009, 182, 4737–4743. [Google Scholar] [CrossRef] [PubMed]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s Complex Roles in Immunity and Mucosal Homeostasis in the Gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]
- Fagarasan, S. Evolution, Development, Mechanism and Function of IgA in the Gut. Curr. Opin. Immunol. 2008, 20, 170–177. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional Specialization within the Intestinal Immune System. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Mörbe, U.M.; Jørgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human Gut-Associated Lymphoid Tissues (GALT); Diversity, Structure, and Function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut Biogeography of the Bacterial Microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut Microbiota, Intestinal Permeability, and Systemic Inflammation: A Narrative Review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Puca, P.; Lopetuso, L.R.; Petito, V.; Masi, L.; Bartocci, B.; Murgiano, M.; De Felice, M.; Petronio, L.; Gasbarrini, A.; et al. Bile Acid-Related Regulation of Mucosal Inflammation and Intestinal Motility: From Pathogenesis to Therapeutic Application in IBD and Microscopic Colitis. Nutrients 2022, 14, 2664. [Google Scholar] [CrossRef]
- Buffie, C.G.; Pamer, E.G. Microbiota-Mediated Colonization Resistance against Intestinal Pathogens. Nat. Rev. Immunol. 2013, 13, 790–801. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The Effect of Diet on the Human Gut Microbiome: A Metagenomic Analysis in Humanized Gnotobiotic Mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Itav, S.; Rothschild, D.; Meijer, M.T.; Levy, M.; Moresi, C.; Dohnalová, L.; Braverman, S.; Rozin, S.; Malitsky, S.; et al. Persistent Microbiome Alterations Modulate the Rate of Post-Dieting Weight Regain. Nature 2016, 540, 544–551. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and Consequences of Intestinal Dysbiosis. Cell Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, J.; Poniedziałek, B.; Adamski, Z.; Rzymski, P. The Effects of the Microbiota on the Host Immune System. Autoimmunity 2014, 47, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Lo, B.C.; Núñez, G. Host–Microbiota Interactions in Inflammatory Bowel Disease. Nat. Rev. Immunol. 2020, 20, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Janowitz, H.D.; Croen, E.C.; Sachar, D.B. The Role of the Fecal Stream in Crohn’s Disease: An Historical and Analytic Review. Inflamm. Bowel Dis. 1998, 4, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.J.; Ullman, T.A.; Ford, A.C.; Abreu, M.T.; Abadir, A.; Marshall, J.K.; Talley, N.J.; Moayyedi, P. Antibiotic Therapy in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2011, 106, 661–673. [Google Scholar] [CrossRef]
- Wang, S.-L.; Wang, Z.-R.; Yang, C.-Q. Meta-Analysis of Broad-Spectrum Antibiotic Therapy in Patients with Active Inflammatory Bowel Disease. Exp. Ther. Med. 2012, 4, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Harper, P.H.; Lee, E.C.; Kettlewell, M.G.; Bennett, M.K.; Jewell, D.P. Role of the Faecal Stream in the Maintenance of Crohn’s Colitis. Gut 1985, 26, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Rutgeerts, P.; Goboes, K.; Peeters, M.; Hiele, M.; Penninckx, F.; Aerts, R.; Kerremans, R.; Vantrappen, G. Effect of Faecal Stream Diversion on Recurrence of Crohn’s Disease in the Neoterminal Ileum. Lancet 1991, 338, 771–774. [Google Scholar] [CrossRef]
- Mehandru, S.; Colombel, J.-F. The Intestinal Barrier, an Arbitrator Turned Provocateur in IBD. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 83–84. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut Microbiota in the Pathogenesis of Inflammatory Bowel Disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Chiodini, R.J.; Van Kruiningen, H.J.; Thayer, W.R.; Merkal, R.S.; Coutu, J.A. Possible Role of Mycobacteria in Inflammatory Bowel Disease. I. An Unclassified Mycobacterium Species Isolated from Patients with Crohn’s Disease. Dig. Dis. Sci. 1984, 29, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Feller, M.; Huwiler, K.; Stephan, R.; Altpeter, E.; Shang, A.; Furrer, H.; Pfyffer, G.E.; Jemmi, T.; Baumgartner, A.; Egger, M. Mycobacterium Avium Subspecies Paratuberculosis and Crohn’s Disease: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2007, 7, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Sim, W.; Bishop, R.F.; Catto-Smith, A.G.; Cameron, D.J.S.; Kirkwood, C.D. Mycobacterium Avium Subspecies Paratuberculosis in Children with Early-Onset Crohn’s Disease: A Longitudinal Follow-up Study. Inflamm. Bowel Dis. 2011, 17, 1825–1826. [Google Scholar] [CrossRef] [PubMed]
- Timms, V.J.; Daskalopoulos, G.; Mitchell, H.M.; Neilan, B.A. The Association of Mycobacterium Avium Subsp. Paratuberculosis with Inflammatory Bowel Disease. PLoS ONE 2016, 11, e0148731. [Google Scholar] [CrossRef] [PubMed]
- Dharmani, P.; Strauss, J.; Ambrose, C.; Allen-Vercoe, E.; Chadee, K. Fusobacterium Nucleatum Infection of Colonic Cells Stimulates MUC2 Mucin and Tumor Necrosis Factor Alpha. Infect. Immun. 2011, 79, 2597–2607. [Google Scholar] [CrossRef] [PubMed]
- Zeller, G.; Tap, J.; Voigt, A.Y.; Sunagawa, S.; Kultima, J.R.; Costea, P.I.; Amiot, A.; Böhm, J.; Brunetti, F.; Habermann, N.; et al. Potential of Fecal Microbiota for Early-Stage Detection of Colorectal Cancer. Mol. Syst. Biol. 2014, 10, 766. [Google Scholar] [CrossRef]
- Schaubeck, M.; Clavel, T.; Calasan, J.; Lagkouvardos, I.; Haange, S.B.; Jehmlich, N.; Basic, M.; Dupont, A.; Hornef, M.; von Bergen, M.; et al. Dysbiotic Gut Microbiota Causes Transmissible Crohn’s Disease-like Ileitis Independent of Failure in Antimicrobial Defence. Gut 2016, 65, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Eftychi, C.; Schwarzer, R.; Vlantis, K.; Wachsmuth, L.; Basic, M.; Wagle, P.; Neurath, M.F.; Becker, C.; Bleich, A.; Pasparakis, M. Temporally Distinct Functions of the Cytokines IL-12 and IL-23 Drive Chronic Colon Inflammation in Response to Intestinal Barrier Impairment. Immunity 2019, 51, 367–380.e4. [Google Scholar] [CrossRef]
- Leibovitzh, H.; Lee, S.-H.; Xue, M.; Raygoza Garay, J.A.; Hernandez-Rocha, C.; Madsen, K.L.; Meddings, J.B.; Guttman, D.S.; Espin-Garcia, O.; Smith, M.I.; et al. Altered Gut Microbiome Composition and Function Are Associated with Gut Barrier Dysfunction in Healthy Relatives of Patients with Crohn’s Disease. Gastroenterology 2022, 163, 1364–1376.e10. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-Sensing Receptors GPR43 and GPR109A Facilitate Dietary Fibre-Induced Gut Homeostasis through Regulation of the Inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef]
- Lavelle, A.; Sokol, H. Gut Microbiota-Derived Metabolites as Key Actors in Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef]
- Kaneko, T.; Bando, Y.; Kurihara, H.; Satomi, K.; Nonoyama, K.; Matsuura, N. Fecal Microflora in a Patient with Short-Bowel Syndrome and Identification of Dominant Lactobacilli. J. Clin. Microbiol. 1997, 35, 3181–3185. [Google Scholar] [CrossRef]
- Khrais, A.; Ali, H.; Choi, S.; Ahmed, A.; Ahlawat, S. D-Lactic Acidosis in Short Bowel Syndrome. Cureus 2022, 14, e25471. [Google Scholar] [CrossRef] [PubMed]
- Davidovics, Z.H.; Carter, B.A.; Luna, R.A.; Hollister, E.B.; Shulman, R.J.; Versalovic, J. The Fecal Microbiome in Pediatric Patients with Short Bowel Syndrome. JPEN J. Parenter. Enteral Nutr. 2016, 40, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Boccia, S.; Torre, I.; Santarpia, L.; Iervolino, C.; Del Piano, C.; Puggina, A.; Pastorino, R.; Dragic, M.; Amore, R.; Borriello, T.; et al. Intestinal Microbiota in Adult Patients with Short Bowel Syndrome: Preliminary Results from a Pilot Study. Clin. Nutr. 2017, 36, 1707–1709. [Google Scholar] [CrossRef]
- Sommovilla, J.; Zhou, Y.; Sun, R.C.; Choi, P.M.; Diaz-Miron, J.; Shaikh, N.; Sodergren, E.; Warner, B.B.; Weinstock, G.M.; Tarr, P.I.; et al. Small Bowel Resection Induces Long-Term Changes in the Enteric Microbiota of Mice. J. Gastrointest. Surg. 2015, 19, 56–64; discussion 64. [Google Scholar] [CrossRef]
- Kumar, J.A.; Teckman, J.H. Controversies in the Mechanism of Total Parenteral Nutrition Induced Pathology. Children 2015, 2, 358–370. [Google Scholar] [CrossRef]
- Piper, H.G. Intestinal Microbiota in Short Bowel Syndrome. Semin. Pediatr. Surg. 2018, 27, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Avelar Rodriguez, D.; Ryan, P.M.; Toro Monjaraz, E.M.; Ramirez Mayans, J.A.; Quigley, E.M. Small Intestinal Bacterial Overgrowth in Children: A State-of-the-Art Review. Front. Pediatr. 2019, 7, 363. [Google Scholar] [CrossRef]
- Miazga, A.; Osiński, M.; Cichy, W.; Żaba, R. Current Views on the Etiopathogenesis, Clinical Manifestation, Diagnostics, Treatment and Correlation with Other Nosological Entities of SIBO. Adv. Med. Sci. 2015, 60, 118–124. [Google Scholar] [CrossRef]
- Lkhagva, E.; Chung, H.-J.; Hong, J.; Tang, W.H.W.; Lee, S.-I.; Hong, S.-T.; Lee, S. The Regional Diversity of Gut Microbiome along the GI Tract of Male C57BL/6 Mice. BMC Microbiol. 2021, 21, 44. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, W.; Schink, M.; Zopf, Y. Microbiota in the Gastrointestinal Tract. Med. Sci. 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.C.; Bhagatwala, J. Small Intestinal Bacterial Overgrowth: Clinical Features and Therapeutic Management. Clin. Transl. Gastroenterol. 2019, 10, e00078. [Google Scholar] [CrossRef] [PubMed]
- Agnes, A.; Puccioni, C.; D’Ugo, D.; Gasbarrini, A.; Biondi, A.; Persiani, R. The Gut Microbiota and Colorectal Surgery Outcomes: Facts or Hype? A Narrative Review. BMC Surg. 2021, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Lapthorne, S.; Pereira-Fantini, P.M.; Fouhy, F.; Wilson, G.; Thomas, S.L.; Dellios, N.L.; Scurr, M.; O’Sullivan, O.; Ross, R.P.; Stanton, C.; et al. Gut Microbial Diversity Is Reduced and Is Associated with Colonic Inflammation in a Piglet Model of Short Bowel Syndrome. Gut Microbes 2013, 4, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Devine, A.A.; Gonzalez, A.; Speck, K.E.; Knight, R.; Helmrath, M.; Lund, P.K.; Azcarate-Peril, M.A. Impact of Ileocecal Resection and Concomitant Antibiotics on the Microbiome of the Murine Jejunum and Colon. PLoS ONE 2013, 8, e73140. [Google Scholar] [CrossRef] [PubMed]
- Begley, M.; Gahan, C.G.M.; Hill, C. The Interaction between Bacteria and Bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohns Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Manithody, C.; Krebs, J.; Denton, C.; Besmer, S.; Rajalakshmi, P.; Jain, S.; Villalona, G.A.; Jain, A.K. Impaired Gut-Systemic Signaling Drives Total Parenteral Nutrition-Associated Injury. Nutrients 2020, 12, 1493. [Google Scholar] [CrossRef]
- Niinikoski, H.; Stoll, B.; Guan, X.; Kansagra, K.; Lambert, B.D.; Stephens, J.; Hartmann, B.; Holst, J.J.; Burrin, D.G. Onset of Small Intestinal Atrophy Is Associated with Reduced Intestinal Blood Flow in TPN-Fed Neonatal Piglets. J. Nutr. 2004, 134, 1467–1474. [Google Scholar] [CrossRef]
- Jain, A.K.; Stoll, B.; Burrin, D.G.; Holst, J.J.; Moore, D.D. Enteral Bile Acid Treatment Improves Parenteral Nutrition-Related Liver Disease and Intestinal Mucosal Atrophy in Neonatal Pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G218–G224. [Google Scholar] [CrossRef] [PubMed]
- Javid, P.J.; Collier, S.; Richardson, D.; Iglesias, J.; Gura, K.; Lo, C.; Kim, H.B.; Duggan, C.P.; Jaksic, T. The Role of Enteral Nutrition in the Reversal of Parenteral Nutrition–Associated Liver Dysfunction in Infants. J. Pediatr. Surg. 2005, 40, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Ekelund, M.; Kristensson, E.; Ekelund, M.; Ekblad, E. Total Parenteral Nutrition Causes Circumferential Intestinal Atrophy, Remodeling of the Intestinal Wall, and Redistribution of Eosinophils in the Rat Gastrointestinal Tract. Dig. Dis. Sci. 2007, 52, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Wen, J.X.; Arora, S.; Blomenkamp, K.S.; Rodrigues, J.; Blaufuss, T.A.; Liou, V.; Burrin, D.G.; Long, J.P.; Teckman, J.H. Validating Hyperbilirubinemia and Gut Mucosal Atrophy with a Novel Ultramobile Ambulatory Total Parenteral Nutrition Piglet Model. Nutr. Res. 2015, 35, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Basson, M.D.; Li, G.D.; Hong, F.; Han, O.; Sumpio, B.E. Amplitude-Dependent Modulation of Brush Border Enzymes and Proliferation by Cyclic Strain in Human Intestinal Caco-2 Monolayers. J. Cell Physiol. 1996, 168, 476–488. [Google Scholar] [CrossRef]
- Peterson, C.A.; Carey, H.V.; Hinton, P.L.; Lo, H.C.; Ney, D.M. GH Elevates Serum IGF-I Levels but Does Not Alter Mucosal Atrophy in Parenterally Fed Rats. Am. J. Physiol. 1997, 272, G1100–G1108. [Google Scholar] [CrossRef] [PubMed]
- Burrin, D.G.; Stoll, B.; Guan, X.; Cui, L.; Chang, X.; Holst, J.J. Glucagon-like Peptide 2 Dose-Dependently Activates Intestinal Cell Survival and Proliferation in Neonatal Piglets. Endocrinology 2005, 146, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Madnawat, H.; Welu, A.L.; Gilbert, E.J.; Taylor, D.B.; Jain, S.; Manithody, C.; Blomenkamp, K.; Jain, A.K. Mechanisms of Parenteral Nutrition–Associated Liver and Gut Injury. Nutr. Clin. Pract. 2020, 35, 63–71. [Google Scholar] [CrossRef]
- Ukleja, A. Weaning from Parenteral Nutrition. Gastroenterol. Clin. N. Am. 2019, 48, 525–550. [Google Scholar] [CrossRef]
- Scolapio, J.; Camilleri, M.; Fleming, C.; Oenning, L.; Burton, D.; Sebo, T.; Batts, K.; Kelly, D. Effect of Growth Hormone, Glutamine, and Diet on Adaptation in Short- Bowel Syndrome: A Randomized, Controlled Study. Gastroenterology 1997, 113, 1074–1081. [Google Scholar] [CrossRef]
- Szkudlarek, J.; Jeppesen, P.B.; Mortensen, P.B. Effect of High Dose Growth Hormone with Glutamine and No Change in Diet on Intestinal Absorption in Short Bowel Patients: A Randomised, Double Blind, Crossover, Placebo Controlled Study. Gut 2000, 47, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Seguy, D.; Vahedi, K.; Kapel, N.; Souberbielle, J.-C.; Messing, B. Low-Dose Growth Hormone in Adult Home Parenteral Nutrition-Dependent Short Bowel Syndrome Patients: A Positive Study. Gastroenterology 2003, 124, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Estall, J.L.; Drucker, D.J. Glucagon-like Peptide-2. Annu. Rev. Nutr. 2006, 26, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Vegge, A.; Thymann, T.; Lund, P.; Stoll, B.; Bering, S.B.; Hartmann, B.; Jelsing, J.; Qvist, N.; Burrin, D.G.; Jeppesen, P.B.; et al. Glucagon-like Peptide-2 Induces Rapid Digestive Adaptation Following Intestinal Resection in Preterm Neonates. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G277–G285. [Google Scholar] [CrossRef] [PubMed]
- Yusta, B.; Holland, D.; Koehler, J.A.; Maziarz, M.; Estall, J.L.; Higgins, R.; Drucker, D.J. ErbB Signaling Is Required for the Proliferative Actions of GLP-2 in the Murine Gut. Gastroenterology 2009, 137, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Bi, J.; Wang, X.; Jiang, T.; Wu, C.; Tian, F.; Gao, X.; Wan, X.; Zheng, H. GLP-2 Prevents Intestinal Mucosal Atrophy and Improves Tissue Antioxidant Capacity in a Mouse Model of Total Parenteral Nutrition. Nutrients 2016, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Billiauws, L.; Joly, F. Emerging Treatments for Short Bowel Syndrome in Adult Patients. Expert. Rev. Gastroenterol. Hepatol. 2019, 13, 241–246. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Hartmann, B.; Thulesen, J.; Graff, J.; Lohmann, J.; Hansen, B.S.; Tofteng, F.; Poulsen, S.S.; Madsen, J.L.; Holst, J.J.; et al. Glucagon-like Peptide 2 Improves Nutrient Absorption and Nutritional Status in Short-Bowel Patients with No Colon. Gastroenterology 2001, 120, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Amenta, F.; Vega, J.A.; Ricci, A.; Collier, W.L. Localization of 5-Hydroxytryptamine-like Immunoreactive Cells and Nerve Fibers in the Rat Female Reproductive System. Anat. Rec. 1992, 233, 478–484. [Google Scholar] [CrossRef]
- Pizzoferrato, M.; Puca, P.; Ennas, S.; Cammarota, G.; Guidi, L. Glucagon-like Peptide-2 Analogues for Crohn’s Disease Patients with Short Bowel Syndrome and Intestinal Failure. World J. Gastroenterol. 2022, 28, 6258–6270. [Google Scholar] [CrossRef]
- Kang, W.; Gomez, F.E.; Lan, J.; Sano, Y.; Ueno, C.; Kudsk, K.A. Parenteral Nutrition Impairs Gut-Associated Lymphoid Tissue and Mucosal Immunity by Reducing Lymphotoxin β Receptor Expression. Ann. Surg. 2006, 244, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kudsk, K.A.; Gocinski, B.; Dent, D.; Glezer, J.; Langkamp-Henken, B. Effects of Parenteral and Enteral Nutrition on Gut-Associated Lymphoid Tissue. J. Trauma 1995, 39, 44–51; discussion 51–52. [Google Scholar] [CrossRef]
- Jonker, M.A.; Heneghan, A.F.; Fechner, J.H.; Pierre, J.F.; Sano, Y.; Lan, J.; Kudsk, K.A. Gut Lymphocyte Phenotype Changes After Parenteral Nutrition and Neuropeptide Administration. Ann. Surg. 2015, 262, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Janu, P.; Li, J.; Renegar, K.B.; Kudsk, K.A. Recovery of Gut-Associated Lymphoid Tissue and Upper Respiratory Tract Immunity after Parenteral Nutrition. Ann. Surg. 1997, 225, 707–715; discussion 715–717. [Google Scholar] [CrossRef] [PubMed]
- Pierre, J.F. Gastrointestinal Immune and Microbiome Changes during Parenteral Nutrition. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G246–G256. [Google Scholar] [CrossRef] [PubMed]
- Busch, R.A.; Jonker, M.A.; Pierre, J.F.; Heneghan, A.F.; Kudsk, K.A. Innate Mucosal Immune System Response of BALB/c vs C57BL/6 Mice to Injury in the Setting of Enteral and Parenteral Feeding. JPEN J. Parenter. Enteral Nutr. 2016, 40, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, A.F.; Pierre, J.F.; Gosain, A.; Kudsk, K.A. IL-25 Improves Luminal Innate Immunity and Barrier Function during Parenteral Nutrition. Ann. Surg. 2014, 259, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of Diurnal Variation of Gut Microbes and High-Fat Feeding on Host Circadian Clock Function and Metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Busch, R.A.; Heneghan, A.F.; Pierre, J.F.; Neuman, J.C.; Reimer, C.A.; Wang, X.; Kimple, M.E.; Kudsk, K.A. Bombesin Preserves Goblet Cell Resistin-Like Molecule β During Parenteral Nutrition but Not Other Goblet Cell Products. JPEN J. Parenter. Enteral Nutr. 2016, 40, 1042–1049. [Google Scholar] [CrossRef]
- Bergstrom, K.S.B.; Morampudi, V.; Chan, J.M.; Bhinder, G.; Lau, J.; Yang, H.; Ma, C.; Huang, T.; Ryz, N.; Sham, H.P.; et al. Goblet Cell Derived RELM-β Recruits CD4+ T Cells during Infectious Colitis to Promote Protective Intestinal Epithelial Cell Proliferation. PLoS Pathog. 2015, 11, e1005108. [Google Scholar] [CrossRef]
- Heneghan, A.F.; Pierre, J.F.; Kudsk, K.A. IL-25 Improves IgA Levels during Parenteral Nutrition through the JAK-STAT Pathway. Ann. Surg. 2013, 258, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Omata, J.; Pierre, J.F.; Heneghan, A.F.; Tsao, F.H.C.; Sano, Y.; Jonker, M.A.; Kudsk, K.A. Parenteral Nutrition Suppresses the Bactericidal Response of the Small Intestine. Surgery 2013, 153, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Pierre, J.F.; Heneghan, A.F.; Meudt, J.M.; Shea, M.P.; Krueger, C.G.; Reed, J.D.; Kudsk, K.A.; Shanmuganayagam, D. Parenteral Nutrition Increases Susceptibility of Ileum to Invasion by E. Coli. J. Surg. Res. 2013, 183, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Hodin, C.M.; Visschers, R.G.J.; Rensen, S.S.; Boonen, B.; Olde Damink, S.W.M.; Lenaerts, K.; Buurman, W.A. Total Parenteral Nutrition Induces a Shift in the Firmicutes to Bacteroidetes Ratio in Association with Paneth Cell Activation in Rats. J. Nutr. 2012, 142, 2141–2147. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, E.A.; Feng, Y.; Poroyko, V.; Falkowski, N.R.; Erb-Downward, J.; Gillilland, M.G.; Mason, K.L.; Huffnagle, G.B.; Teitelbaum, D.H. Total Parenteral Nutrition-Associated Lamina Propria Inflammation in Mice Is Mediated by a MyD88-Dependent Mechanism. J. Immunol. 2013, 190, 6607–6615. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, A.F.; Pierre, J.F.; Tandee, K.; Shanmuganayagam, D.; Wang, X.; Reed, J.D.; Steele, J.L.; Kudsk, K.A. Parenteral Nutrition Decreases Paneth Cell Function and Intestinal Bactericidal Activity While Increasing Susceptibility to Bacterial Enteroinvasion. JPEN J. Parenter. Enteral Nutr. 2014, 38, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.B.; Andrews, K.; Droleskey, R.E.; Kansagra, K.V.; Stoll, B.; Burrin, D.G.; Sheffield, C.L.; Anderson, R.C.; Nisbet, D.J. Qualitative and Quantitative Comparison of Gut Bacterial Colonization in Enterally and Parenterally Fed Neonatal Pigs. Curr. Issues Intest. Microbiol. 2006, 7, 61–64. [Google Scholar]
- Deplancke, B.; Vidal, O.; Ganessunker, D.; Donovan, S.M.; Mackie, R.I.; Gaskins, H.R. Selective Growth of Mucolytic Bacteria Including Clostridium perfringens in a Neonatal Piglet Model of Total Parenteral Nutrition. Am. J. Clin. Nutr. 2002, 76, 1117–1125. [Google Scholar] [CrossRef]
- Lavallee, C.M.; MacPherson, J.A.R.; Zhou, M.; Gao, Y.; Wizzard, P.R.; Wales, P.W.; Turner, J.M.; Willing, B.P. Lipid Emulsion Formulation of Parenteral Nutrition Affects Intestinal Microbiota and Host Responses in Neonatal Piglets. JPEN J. Parenter. Enteral Nutr. 2017, 41, 1301–1309. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Lu, L.; Yan, W.; Tao, Y.; Zhou, K.; Jia, J.; Cai, W. Alterations in Intestinal Microbiota Relate to Intestinal Failure-Associated Liver Disease and Central Line Infections. J. Pediatr. Surg. 2017, 52, 1318–1326. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, F.; Li, Y.; Wang, J.; Li, J. Fecal Microbiota Signatures of Adult Patients with Different Types of Short Bowel Syndrome. J. Gastroenterol. Hepatol. 2017, 32, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Joly, F.; Mayeur, C.; Bruneau, A.; Noordine, M.-L.; Meylheuc, T.; Langella, P.; Messing, B.; Duée, P.-H.; Cherbuy, C.; Thomas, M. Drastic Changes in Fecal and Mucosa-Associated Microbiota in Adult Patients with Short Bowel Syndrome. Biochimie 2010, 92, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Engstrand Lilja, H.; Wefer, H.; Nyström, N.; Finkel, Y.; Engstrand, L. Intestinal Dysbiosis in Children with Short Bowel Syndrome Is Associated with Impaired Outcome. Microbiome 2015, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; Mutanen, A.; Salonen, A.; Savilahti, E.; de Vos, W.M.; Pakarinen, M.P. Intestinal Microbiota Signatures Associated with Histological Liver Steatosis in Pediatric-Onset Intestinal Failure. JPEN J. Parenter. Enteral Nutr. 2017, 41, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Neelis, E.; de Koning, B.; Rings, E.; Wijnen, R.; Nichols, B.; Hulst, J.; Gerasimidis, K. The Gut Microbiome in Patients with Intestinal Failure: Current Evidence and Implications for Clinical Practice. J. Parenter. Enter. Nutr. 2019, 43, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Siggers, R.H.; Siggers, J.; Thymann, T.; Boye, M.; Sangild, P.T. Nutritional Modulation of the Gut Microbiota and Immune System in Preterm Neonates Susceptible to Necrotizing Enterocolitis. J. Nutr. Biochem. 2011, 22, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Gillard, L.; Mayeur, C.; Robert, V.; Pingenot, I.; Le Beyec, J.; Bado, A.; Lepage, P.; Thomas, M.; Joly, F. Microbiota Is Involved in Post-Resection Adaptation in Humans with Short Bowel Syndrome. Front. Physiol. 2017, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Piper, H.G.; Fan, D.; Coughlin, L.A.; Ho, E.X.; McDaniel, M.M.; Channabasappa, N.; Kim, J.; Kim, M.; Zhan, X.; Xie, Y.; et al. Severe Gut Microbiota Dysbiosis Is Associated with Poor Growth in Patients with Short Bowel Syndrome. JPEN J. Parenter. Enteral Nutr. 2017, 41, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Budinska, E.; Gojda, J.; Heczkova, M.; Bratova, M.; Dankova, H.; Wohl, P.; Bastova, H.; Lanska, V.; Kostovcik, M.; Dastych, M.; et al. Microbiome and Metabolome Profiles Associated with Different Types of Short Bowel Syndrome: Implications for Treatment. JPEN J. Parenter. Enteral Nutr. 2020, 44, 105–118. [Google Scholar] [CrossRef]
- Feng, Y.; Sun, X.; Yang, H.; Teitelbaum, D.H. Dissociation of E-Cadherin and Beta-Catenin in a Mouse Model of Total Parenteral Nutrition: A Mechanism for the Loss of Epithelial Cell Proliferation and Villus Atrophy. J. Physiol. 2009, 587, 641–654. [Google Scholar] [CrossRef]
- Wildhaber, B.E.; Yang, H.; Spencer, A.U.; Drongowski, R.A.; Teitelbaum, D.H. Lack of Enteral Nutrition–Effects on the Intestinal Immune System. J. Surg. Res. 2005, 123, 8–16. [Google Scholar] [CrossRef]
- Demehri, F.R.; Barrett, M.; Teitelbaum, D.H. Changes to the Intestinal Microbiome with Parenteral Nutrition: Review of a Murine Model and Potential Clinical Implications. Nutr. Clin. Pract. 2015, 30, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Teitelbaum, D.H. Tumour Necrosis Factor--Induced Loss of Intestinal Barrier Function Requires TNFR1 and TNFR2 Signalling in a Mouse Model of Total Parenteral Nutrition. J. Physiol. 2013, 591, 3709–3723. [Google Scholar] [CrossRef]
- Yang, H.; Fan, Y.; Teitelbaum, D.H. Intraepithelial Lymphocyte-Derived Interferon-Gamma Evokes Enterocyte Apoptosis with Parenteral Nutrition in Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G629–G637. [Google Scholar] [CrossRef]
- Clayburgh, D.R.; Shen, L.; Turner, J.R. A Porous Defense: The Leaky Epithelial Barrier in Intestinal Disease. Lab. Investig. 2004, 84, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; McDunn, J.E.; Teitelbaum, D.H. Decreased Phospho-Akt Signaling in a Mouse Model of Total Parenteral Nutrition: A Potential Mechanism for the Development of Intestinal Mucosal Atrophy. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 298, G833–G841. [Google Scholar] [CrossRef]
- Sun, X.; Yang, H.; Nose, K.; Nose, S.; Haxhija, E.Q.; Koga, H.; Feng, Y.; Teitelbaum, D.H. Decline in Intestinal Mucosal IL-10 Expression and Decreased Intestinal Barrier Function in a Mouse Model of Total Parenteral Nutrition. Am. J. Physiol.-Gastrointest. Liver Physiol. 2008, 294, G139–G147. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Teitelbaum, D.H. Epidermal Growth Factor/TNF-α Transactivation Modulates Epithelial Cell Proliferation and Apoptosis in a Mouse Model of Parenteral Nutrition. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 302, G236–G249. [Google Scholar] [CrossRef]
- Yang, H.; Kiristioglu, I.; Fan, Y.; Forbush, B.; Bishop, D.K.; Antony, P.A.; Zhou, H.; Teitelbaum, D.H. Interferon-Gamma Expression by Intraepithelial Lymphocytes Results in a Loss of Epithelial Barrier Function in a Mouse Model of Total Parenteral Nutrition. Ann. Surg. 2002, 236, 226–234. [Google Scholar] [CrossRef]
- Yang, H.; Finaly, R.; Teitelbaum, D.H. Alteration in Epithelial Permeability and Ion Transport in a Mouse Model of Total Parenteral Nutrition. Crit. Care Med. 2003, 31, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Kudsk, K.A.; Croce, M.A.; Fabian, T.C.; Minard, G.; Tolley, E.A.; Poret, H.A.; Kuhl, M.R.; Brown, R.O. Enteral versus Parenteral Feeding Effects on Septic Morbidity after Blunt and Penetrating Abdominal Trauma. Ann. Surg. 1992, 215, 503–511; discussion 511-3. [Google Scholar] [CrossRef]
- Moore, F.A.; Moore, E.E.; Jones, T.N.; McCroskey, B.L.; Peterson, V.M. TEN versus TPN Following Major Abdominal Trauma–Reduced Septic Morbidity. J. Trauma 1989, 29, 916–922; discussion 922–923. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.A. Preventing Parenteral Nutrition Liver Disease. Early Hum. Dev. 2010, 86, 683–687. [Google Scholar] [CrossRef]
- Lacaille, F.; Gupte, G.; Colomb, V.; D’Antiga, L.; Hartman, C.; Hojsak, I.; Kolacek, S.; Puntis, J.; Shamir, R. Intestinal Failure–Associated Liver Disease. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 272–283. [Google Scholar] [CrossRef]
- Pironi, L.; Sasdelli, A.S. Intestinal Failure-Associated Liver Disease. Clin. Liver Dis. 2019, 23, 279–291. [Google Scholar] [CrossRef]
- Gaitantzi, H.; Hakenberg, P.; Theobald, J.; Heinlein, H.; Cai, C.; Loff, S.; Wölfl, S.; Ebert, M.P.; Breitkopf-Heinlein, K.; Subotic, U. Di (2-Ethylhexyl) Phthalate and Its Role in Developing Cholestasis. J. Pediatr. Gastroenterol. Nutr. 2018, 66, e28–e35. [Google Scholar] [CrossRef]
- Balestrieri, P.; Ribolsi, M.; Guarino, M.P.L.; Emerenziani, S.; Altomare, A.; Cicala, M. Nutritional Aspects in Inflammatory Bowel Diseases. Nutrients 2020, 12, 372. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covello, C.; Becherucci, G.; Di Vincenzo, F.; Del Gaudio, A.; Pizzoferrato, M.; Cammarota, G.; Gasbarrini, A.; Scaldaferri, F.; Mentella, M.C. Parenteral Nutrition, Inflammatory Bowel Disease, and Gut Barrier: An Intricate Plot. Nutrients 2024, 16, 2288. https://doi.org/10.3390/nu16142288
Covello C, Becherucci G, Di Vincenzo F, Del Gaudio A, Pizzoferrato M, Cammarota G, Gasbarrini A, Scaldaferri F, Mentella MC. Parenteral Nutrition, Inflammatory Bowel Disease, and Gut Barrier: An Intricate Plot. Nutrients. 2024; 16(14):2288. https://doi.org/10.3390/nu16142288
Chicago/Turabian StyleCovello, Carlo, Guia Becherucci, Federica Di Vincenzo, Angelo Del Gaudio, Marco Pizzoferrato, Giovanni Cammarota, Antonio Gasbarrini, Franco Scaldaferri, and Maria Chiara Mentella. 2024. "Parenteral Nutrition, Inflammatory Bowel Disease, and Gut Barrier: An Intricate Plot" Nutrients 16, no. 14: 2288. https://doi.org/10.3390/nu16142288
APA StyleCovello, C., Becherucci, G., Di Vincenzo, F., Del Gaudio, A., Pizzoferrato, M., Cammarota, G., Gasbarrini, A., Scaldaferri, F., & Mentella, M. C. (2024). Parenteral Nutrition, Inflammatory Bowel Disease, and Gut Barrier: An Intricate Plot. Nutrients, 16(14), 2288. https://doi.org/10.3390/nu16142288







