From Breastfeeding to Support in Mothers’ Feeding Choices: A Key Role in the Prevention of Postpartum Depression?
Abstract
1. Introduction
2. What Happens in the Postpartum Period: The Origin of PPD
3. The Gravity of the Issue: Prevalence of PPD
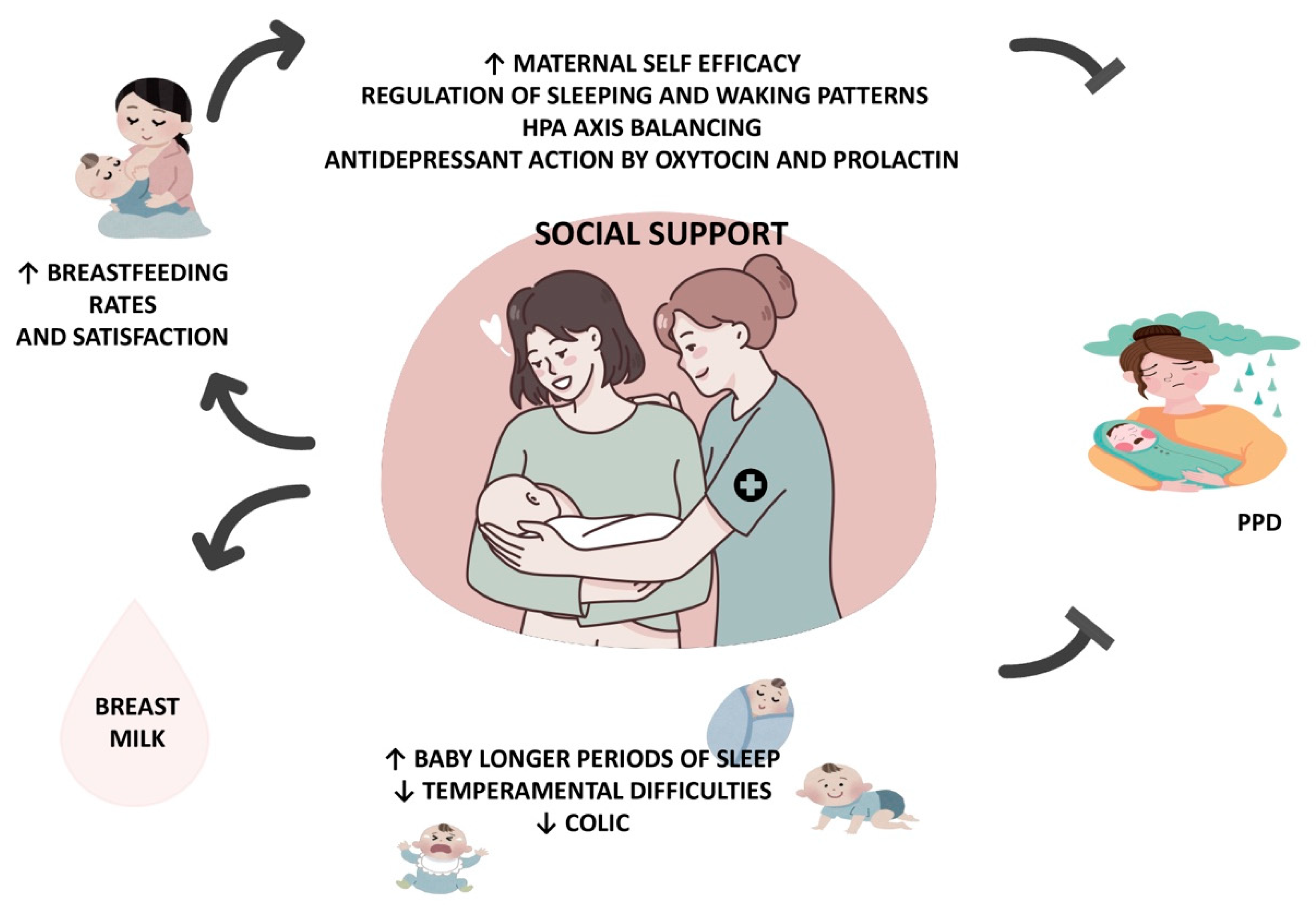
4. Breastfeeding in the Prevention of PPD
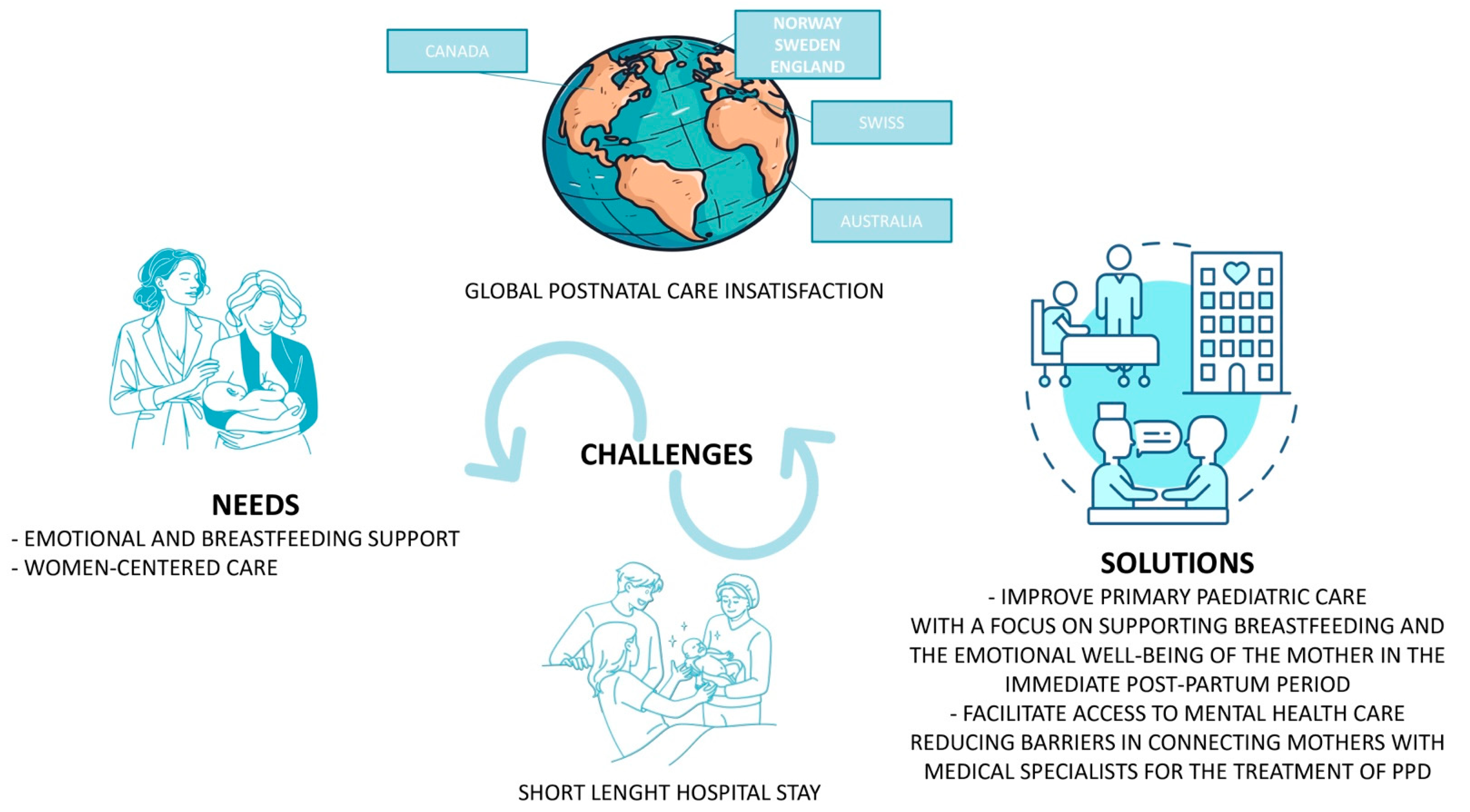
5. Support in the Postpartum Period
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mercer, R.T. Becoming a mother versus maternal role attainment. J. Nurs. Scholarsh. 2004, 36, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Leahy-Warren, P.; McCarthy, G. Maternal parental self-efficacy in the postpartum period. Midwifery 2011, 27, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.D.; Aldag, J.C. Maternal perceived quality of life following child-birth. J. Obstet. Gynecol. Neonatal Nurs. 2007, 36, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Coyle, S.B. Health-related quality of life of mothers: A review of the re- search. Health Care Women Int. 2009, 30, 484–506. [Google Scholar] [CrossRef] [PubMed]
- Rallis, S.; Skouteris, H.; Wertheim, E.J.; Paxton, S.J. Predictors of body image during the first year postpartum: A prospective study. Women Health 2007, 45, 87–104. [Google Scholar] [CrossRef]
- Britton, J.R. Maternal anxiety: Course and antecedents during the early postpartum period. Depress. Anxiety 2008, 25, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Baattaiah, B.A.; Alharbi, M.D.; Babteen, N.M.; Al-Maqbool, H.M.; Babgi, F.A.; Albatati, A.A. The relationship between fatigue, sleep quality, resilience, and the risk of postpartum depression: An emphasis on maternal mental health. BMC Psychol. 2023, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Tulman, L.; Fawcett, J.; Groblewski, L.; Silverman, L. Changes in functional status after childbirth. Nurs. Res. 1990, 39, 70–75. [Google Scholar] [CrossRef]
- Corwin, E.J.; Brownstead, J.; Barton, N.; Heckard, S.; Morin, K. The impact of fatigue on the development of postpartum depression. J. Obstet. Gynecol. Neonatal Nurs. 2005, 34, 577–586. [Google Scholar] [CrossRef]
- Beck, C.T. Predictors of postpartum depression: An update. Nurs. Res. 2001, 50, 275–282. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Fowles, E.R.; Walker, L.O. Postpartum maternal health care in the United States: A critical review. J. Perinat. Educ. 2006, 15, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.S.; Zuckerman, B.; Bauchner, H.; Homer, C.G.; Wise, P. Women’s health after pregnancy and child outcomes at age 3 years: A prospective cohort study. Am. J. Public Health 2002, 92, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Minkovitz, C.S.; Strobino, D.; Scharfstein, D.; Hou, W.; Miller, T. Maternal depressive symptoms and children’s receipt of health care in the first 3 years of life. Pediatrics 2005, 115, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Alimi, R.; Azmoude, E.; Moradi, M.; Zamani, M. The Association of Breastfeeding with a Reduced Risk of Postpartum Depression: A Systematic Review and Meta-Analysis. Breastfeed. Med. 2022, 17, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.S.; Caldeira, N.T.; Eugênio, D.S.; Lucca, M.M.D.; Silva, I.A. Breastfeeding self-efficacy and postpartum depression: A cohort study. Rev. Lat. Am. Enfermagem. 2018, 26, e3035. [Google Scholar] [CrossRef] [PubMed]
- Leeming, D.; Marshall, J.; Hinsliff, S. Self-conscious emotions and breastfeeding support: A focused synthesis of UK qualitative research. Matern. Child. Nutr. 2022, 18, e13270. [Google Scholar] [CrossRef] [PubMed]
- Dimcea, D.A.; Petca, R.C.; Dumitrașcu, M.C.; Șandru, F.; Mehedințu, C.; Petca, A. Postpartum Depression: Etiology, Treatment, and Consequences for Maternal Care. Diagnostics 2024, 14, 865. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Priest, M.F.; Nasenbeny, J.; Lu, T.; Kozorovitskiy, Y. Biased Oxytocinergic Modulation of Midbrain Dopamine Systems. Neuron 2017, 95, 368–384.e5. [Google Scholar] [CrossRef]
- Uvnäs-Moberg, K.; Ekström-Bergström, A.; Berg, M.; Buckley, S.; Pajalic, Z.; Hadjigeorgiou, E.; Dencker, A. Maternal plasma levels of oxytocin during physiological childbirth—A systematic review with implications for uterine contractions and central actions of oxytocin. BMC Pregnancy Childbirth 2019, 19, 285. [Google Scholar] [CrossRef]
- Nissen, E.; Lilja, G.; Widstrom, A.M.; Uvnas-Moberg, K. Elevation of oxytocin levels early post partum in women. Acta Obstet. Gynecol. Scand. 1995, 74, 530–533. [Google Scholar] [CrossRef]
- Uvnäs-Moberg, K.; Handlin, L.; Petersson, M. Self-soothing behaviors with particular reference to oxytocin release induced by non-noxious sensory stimulation. Front. Psychol. 2014, 5, 1529. [Google Scholar]
- Lagercrantz, H.; Bistoletti, P. Catecholamine release in the newborn infant at birth. Pediatr. Res. 1977, 11, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Blouquit, M.F.; Sturbois, G.; Bréart, G.; Grill, C.; Sureau, C.; Roffi, J. Catecholamine levels in newborn human plasma in normal and abnormal conditions and in maternal plasma at delivery. Experientia 1979, 35, 618–619. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, B.; Edman, G.; Widström, A.M.; Mathiesen, A.S.; Uvnäs-Moberg, K. Maternal foetal attachment and personality during first pregnancy. J. Reprod. Infant. Psychol. 2004, 22, 57–69. [Google Scholar] [CrossRef]
- Levin, G.; Ein-Dor, T. A unified model of the biology of peripartum depression. Transl. Psychiatry 2023, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Pawluski, J.L.; Li, M.; Lonstein, J.S. Serotonin and motherhood: From molecules to mood. Front. Neuroendocrinol. 2019, 53, 100742. [Google Scholar] [CrossRef] [PubMed]
- Thul, T.A.; Corwin, E.J.; Carlson, N.S.; Brennan, P.A.; Young, L.J. Oxytocin and postpartum depression: A systematic review. Psychoneuroendocrinology 2020, 120, 104793. [Google Scholar] [CrossRef]
- Serati, M.; Redaelli, M.; Buoli, M.; Altamura, A.C. Perinatal Major Depression Biomarkers: A systematic review. J. Affect. Disord. 2016, 193, 391–404. [Google Scholar] [CrossRef]
- Nguyen, A.J.; Hoyer, E.; Rajhans, P.; Strathearn, L.; Kim, S. A tumultuous transition to motherhood: Altered brain and hormonal responses in mothers with postpartum depression. J. Neuroendocrinol. 2019, 31, e12794. [Google Scholar] [CrossRef]
- Yu, Y.; Liang, H.F.; Chen, J.; Li, Z.B.; Han, Y.S.; Chen, J.X.; Li, J.C. Postpartum Depression: Current Status and Possible Identification Using Biomarkers. Front. Psychiatry 2021, 12, 620371. [Google Scholar] [CrossRef]
- Bränn, E.; Fransson, E.; White, R.A.; Papadopoulos, F.C.; Edvinsson, Å.; Kamali-Moghaddam, M.; Cunningham, J.L.; Sundström-Poromaa, I.; Skalkidou, A. Inflammatory markers in women with postpartum depressive symptoms. J. Neurosci. Res. 2020, 98, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Buglione-Corbett, R.; Deligiannidis, K.M.; Leung, K.; Zhang, N.; Lee, M.; Rosal, M.C.; Moore-Simas, T.A. Expression of inflammatory markers in women with perinatal depressive symptoms. Arch. Womens Ment. Health 2018, 21, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Pawluski, J.L.; Lonstein, J.S.; Fleming, A.S. The Neurobiology of Postpartum Anxiety and Depression. Trends Neurosci. 2017, 40, 106–120. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, M.W.; Wisner, K.L. Perinatal mental illness: Definition, description and aetiology. Best. Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 3–12. [Google Scholar] [CrossRef]
- Pearlstein, T.; Howard, M.; Salisbury, A.; Zlotnick, C. Postpartum depression. Am. J. Obstet. Gynecol. 2009, 200, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Shorey, S.; Chee, C.Y.I.; Ng, E.D.; Chan, Y.H.; Tam, W.W.S.; Chong, Y.S. Prevalence and incidence of postpartum depression among healthy mothers: A systematic review and meta-analysis. J. Psychiatr. Res. 2018, 104, 235–248. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Wang, G. Prevalence and Risk Factors of Postpartum Depression in Women: A Systematic Review and Meta-analysis. J. Clin. Nurs. 2022, 31, 2665–2677. [Google Scholar] [CrossRef]
- Huang, C.; Fan, Y.; Hu, S. The Prevalence and Influencing Factors of Postpartum Depression Between Primiparous and Secundiparous. J. Nerv. Ment. Dis. 2023, 211, 190–194. [Google Scholar] [CrossRef]
- Figueiredo, B.; Dias, C.C.; Brandão, S.; Canário, C.; Nunes-Costa, R. Breastfeeding and postpartum depression: State of the art review. J. Pediatr. 2013, 89, 332–338. [Google Scholar] [CrossRef]
- Brunton, P.J.; Russell, J.A.; Douglas, A.J. Adaptive responses of the maternal hypothalamic-pituitary-adrenal axis during pregnancy and lactation. J. Neuroendocrinol. 2008, 20, 764–776. [Google Scholar] [CrossRef]
- Heinrichs, M.; Meinlschmidt, G.; Neumann, I.; Wagner, S.; Kirschbaum, C.; Ehlert, U.; Hellhammer, D.H. Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. J. Clin. Endocrinol. Metab. 2001, 86, 4798–4804. [Google Scholar] [CrossRef] [PubMed]
- Altemus, M.; Deuster, P.A.; Galliven, E.; Carter, C.S.; Gold, P.W. Suppression of hypothalmic-pituitary-adrenal axis responses to stress in lactating women. J. Clin. Endocrinol. Metab. 1995, 80, 2954–2959. [Google Scholar] [PubMed]
- Tu, M.T.; Lupien, S.J.; Walker, C.D. Diurnal salivary cortisol levels in postpartum mothers as a function of infant feeding choice and parity. Psychoneuroendocrinology 2006, 31, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Handlin, L.; Jonas, W.; Petersson, M.; Ejdebäck, M.; Ransjö-Arvidson, A.B.; Nissen, E.; Uvnäs-Moberg, K. Effects of sucking and skin-to-skin contact on maternal ACTH and cortisol levels during the second day postpartum-influence of epidural analgesia and oxytocin in the perinatal period. Breastfeed. Med. 2009, 4, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Nagel, E.M.; Howland, M.A.; Pando, C.; Stang, J.; Mason, S.M.; Fields, D.A.; Demerath, E.W. Maternal Psychological Distress and Lactation and Breastfeeding Outcomes: A Narrative Review. Clin. Ther. 2022, 44, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Sibolboro Mezzacappa, E.; Endicott, J. Parity mediates the association between infant feeding method and maternal depressive symptoms in the postpartum. Arch. Womens Ment. Health. 2007, 10, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Zhang, Y.; Tang, Q.; Zhao, X.; Zhao, X.; Xiang, Y.; Geng, W.; Feng, Y.; Cai, W. Sleep duration of lactating mothers and its relationship with feeding pattern, milk macronutrients and related serum factors: A combined longitudinal cohort and cross-sectional study. Front. Nutr. 2022, 9, 973291. [Google Scholar] [CrossRef]
- Smith, J.P.; Forrester, R.I. Association between breastfeeding and new mothers’ sleep: A unique Australian time use study. Int. Breastfeed. J. 2021, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.O.; Shenassa, E. Understanding and meeting the needs of women in the postpartum period: The Perinatal Maternal Health Promotion Model. J. Midwifery Womens Health 2013, 58, 613–621. [Google Scholar] [CrossRef]
- Moran, C.F.; Holt, V.L.; Martin, D.P. What do women want to know after child- birth? Birth 1997, 24, 27–34. [Google Scholar] [CrossRef]
- Kurth, E.; Krahenbuhl, K.; Eicher, M.; Rodmann, S.; Folmli, L.; Conzelmann, C.; Zemp, E. Safe start at home: What parents of newborns need after early discharge from hospital—A focus group study. BMC Health Serv. Res. 2016, 16, 82. [Google Scholar] [CrossRef]
- Reece, S.M. The parent expectations survey: A measure of perceived self-efficacy. Clin. Nurs. Res. 1992, 1, 336–346. [Google Scholar] [CrossRef]
- Reece, S.M.; Harkless, G. Self-efficacy, stress, and parental adapta- tion: Applications to the care of childbearing families. J. Fam. Nurs. 1998, 4, 198–215. [Google Scholar] [CrossRef]
- Leahy-Warren, P.; McCarthy, G.; Corcoran, P. First-time mothers: Social support, maternal parental self-efficacy and postnatal depression. J. Clin. Nurs. 2012, 21, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Mercer, R.; Walker, L.O. A review of nursing interventions to foster becoming a mother. J. Obstet. Gynecol. Neonatal Nurs. 2006, 35, 568–582. [Google Scholar] [CrossRef]
- Bandura, A. Self-Efficacy: The Exercise of Control; Freeman: New York, NY, USA, 1997. [Google Scholar]
- Dennis, C.L. Identifying predictors of breastfeeding self-efficacy in the immediate postpartum period. Res. Nurs. Health 2006, 29, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Haga, S.M.; Ulleberg, P.; Slinning, K.; Kraft, P.; Steen, T.B.; Staff, A. A longitudinal study of postpartum depressive symptoms: Multilevel growth curve analyses of emotion regulation strategies, breastfeeding self-efficacy, and social support. Arch. Womens Ment. Health 2012, 15, 175–184. [Google Scholar] [CrossRef]
- Howell, E.A.; Mora, P.A.; DiBonaventura, M.D.; Leventhal, H. Modifiable factors associated with changes in postpartum depressive symptoms. Arch. Womens Ment. Health. 2009, 12, 113–120. [Google Scholar] [CrossRef]
- Jones, T.L.; Prinz, R.J. Potential roles of parental self-efficacy in parent and child adjustment: A review. Clin. Psychol. Rev. 2005, 25, 341–363. [Google Scholar] [CrossRef]
- Leerkes, E.M.; Crockenberg, S.C. The Development of Maternal Self-Efficacy and Its Impact on Maternal Behavior. Infancy 2002, 3, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.A.; McFall, B.A.; Diego, M.A. Patterns of brain electrical activity in infants of depressed mothers who breastfeed and bottle feed: The mediating role of infant temperament. Biol. Psychol. 2004, 67, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Cohen Engler, A.; Hadash, A.; Shehadeh, N.; Pillar, G. Breastfeeding may improve nocturnal sleep and reduce infantile colic: Potential role of breast milk melatonin. Eur. J. Pediatr. 2012, 171, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Sinha, B.; Sankar, M.J.; Taneja, S.; Bhandari, N.; Rollins, N.; Bahl, R.; Martines, J. Breastfeeding and maternal health outcomes: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 96–113. [Google Scholar] [CrossRef]
- Dias, C.C.; Figueiredo, B. Breastfeeding and depression: A systematic review of the literature. J. Affect. Disord. 2015, 171, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Luo, J.; Wang, J.; Liang, Y. Association between breastfeeding and postpartum depression: A meta-analysis. J. Affect. Disord. 2022, 308, 512–519. [Google Scholar] [CrossRef]
- Avilla, J.C.; Giugliani, C.; Bizon, A.M.B.L.; Martins, A.C.M.; Senna, A.F.K.; Giugliani, E.R.J. Association between maternal satisfaction with breastfeeding and postpartum depression symptoms. PLoS ONE 2020, 15, e0242333. [Google Scholar] [CrossRef]
- Yuen, M.; Hall, O.J.; Masters, G.A.; Nephew, B.C.; Carr, C.; Leung, K.; Griffen, A.; McIntyre, L.; Byatt, N.; Moore-Simas, T.A. The Effects of Breastfeeding on Maternal Mental Health: A Systematic Review. J. Womens Health 2022, 31, 787–807. [Google Scholar] [CrossRef]
- Hjalmhult, E.; Lomborg, K. Managing the first period at home with a new-born: A grounded theory study of mothers’ experiences. Scand. J. Caring Sci. 2012, 26, 654–662. [Google Scholar] [CrossRef]
- Paavilainen, R.; Astedt-Kurki, P. Self-reported family health and well-being after early discharge from maternity hospital: A phenomenological study. J. Adv. Nurs. 1997, 26, 266–272. [Google Scholar] [CrossRef]
- Lof, M.; Svalenius, E.C.; Persson, E.K. Factors that influence first-time mothers’ choice and experience of early discharge. Scand. J. Caring Sci. 2006, 20, 323–330. [Google Scholar] [CrossRef]
- Dennis, C.L.; Fung, K.; Grigoriadis, S.; Robinson, G.E.; Romans, S.; Ross, L. Traditional postpartum practices and rituals: A qualitative systematic review. Women Health 2007, 3, 487–502. [Google Scholar] [CrossRef]
- Benoit, C.; Stengel, C.; Phillips, R.; Zadoroznyj, M.; Berry, S. Privatisation & marketisation of post-birth care: The hidden costs for new mothers. Int. J. Equity Health 2012, 11, 61. [Google Scholar]
- Brown, S.J.; Davey, M.A.; Bruinsma, F.J. Women’s views and experiences of postnatal hospital care in the Victorian Survey of Recent Mothers 2000. Midwifery 2005, 21, 109–126. [Google Scholar] [CrossRef]
- Rudman, A.; Waldenström, U. Critical views on postpartum care expressed by new mothers. BMC Health Serv. Res. 2007, 7, 178. [Google Scholar] [CrossRef]
- Zadoroznyj, M.; Brodribb, W.E.; Young, K.; Kruske, S.; Miller, Y.D. ‘I really needed help’: What mothers say about their post-birth care in Queensland, Australia. Women Birth. 2015, 28, 246–251. [Google Scholar] [CrossRef]
- Johansson, K.; Aarts, C.; Darj, E. First-time parents’ experiences of home- based postnatal care in Sweden. Upsala J. Med. Sci. 2010, 115, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Bowman, K.G. Postpartum learning needs. J. Obstet. Gynecol. Neonatal Nurs. 2005, 34, 438–443. [Google Scholar] [CrossRef]
- Schobinger, E.; Vanetti, M.; Ramelet, A.S.; Horsch, A. Social support needs of first-time parents in the early-postpartum period: A qualitative study. Front Psychiatry 2022, 13, 1043990, Erratum in: Front Psychiatry 2023, 14, 1171192. [Google Scholar] [CrossRef]
- McLeish, J.; Harvey, M.; Redshaw, M.; Henderson, J.; Malouf, R.; Alderdice, F. First-Time Mothers’ Expectations and Experiences of Postnatal Care in England. Qual. Health Res. 2020, 30, 1876–1887. [Google Scholar] [CrossRef] [PubMed]
- Gavine, A.; Shinwell, S.C.; Buchanan, P.; Farre, A.; Wade, A.; Lynn, F.; Marshall, J.; Cumming, S.E.; Dare, S.; McFadden, A. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst. Rev. 2022, 10, CD001141. [Google Scholar] [PubMed]
- Forster, D.A.; McLachlan, H.L.; Rayner, J.; Yelland, J.; Gold, L.; Rayner, S. The early postnatal period: Exploring women’s views, expectations and experiences of care using focus groups in Victoria, Australia. BMC Pregnancy Childbirth 2008, 8, 27. [Google Scholar] [CrossRef]
- Earls, M.F. Committee on Psychosocial Aspects of Child and Family Health American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics 2010, 126, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Olin, S.C.; Kerker, B.; Stein, R.E.; Weiss, D.; Whitmyre, E.D.; Hoagwood, K.; Horwitz, S.M. Can Postpartum Depression Be Managed in Pediatric Primary Care? J. Women Health 2016, 25, 381–390. [Google Scholar] [CrossRef]
- Brino, K.A.S. Pediatric Mental Health and the Power of Primary Care: Practical Approaches and Validating Challenges. J. Pediatr. Health Care 2020, 34, e12–e20. [Google Scholar] [CrossRef] [PubMed]
- van Venrooij, L.T.; Rusu, V.; Vermeiren, R.R.J.M.; Koposov, R.A.; Skokauskas, N.; Crone, M.R. Clinical decision support methods for children and youths with mental health disorders in primary care. Fam. Pract. 2022, 39, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Dominiak, M.; Antosik-Wojcinska, A.Z.; Baron, M.; Mierzejewski, P.; Swiecicki, L. Recommendations for the prevention and treatment of postpartum depression. Ginekol. Pol. 2021, 92, 153–164. [Google Scholar] [CrossRef]
- Jannati, N.; Farokhzadian, J.; Ahmadian, L. The Experience of Healthcare Professionals Providing Mental Health Services to Mothers with Postpartum Depression: A qualitative study. Sultan Qaboos Univ. Med. J. 2021, 21, 554–562. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dessì, A.; Pianese, G.; Mureddu, P.; Fanos, V.; Bosco, A. From Breastfeeding to Support in Mothers’ Feeding Choices: A Key Role in the Prevention of Postpartum Depression? Nutrients 2024, 16, 2285. https://doi.org/10.3390/nu16142285
Dessì A, Pianese G, Mureddu P, Fanos V, Bosco A. From Breastfeeding to Support in Mothers’ Feeding Choices: A Key Role in the Prevention of Postpartum Depression? Nutrients. 2024; 16(14):2285. https://doi.org/10.3390/nu16142285
Chicago/Turabian StyleDessì, Angelica, Gaia Pianese, Paolo Mureddu, Vassilios Fanos, and Alice Bosco. 2024. "From Breastfeeding to Support in Mothers’ Feeding Choices: A Key Role in the Prevention of Postpartum Depression?" Nutrients 16, no. 14: 2285. https://doi.org/10.3390/nu16142285
APA StyleDessì, A., Pianese, G., Mureddu, P., Fanos, V., & Bosco, A. (2024). From Breastfeeding to Support in Mothers’ Feeding Choices: A Key Role in the Prevention of Postpartum Depression? Nutrients, 16(14), 2285. https://doi.org/10.3390/nu16142285




