The Effect of Mobile Lifestyle Intervention Combined with High-Protein Meal Replacement on Liver Function in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: A Pilot Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
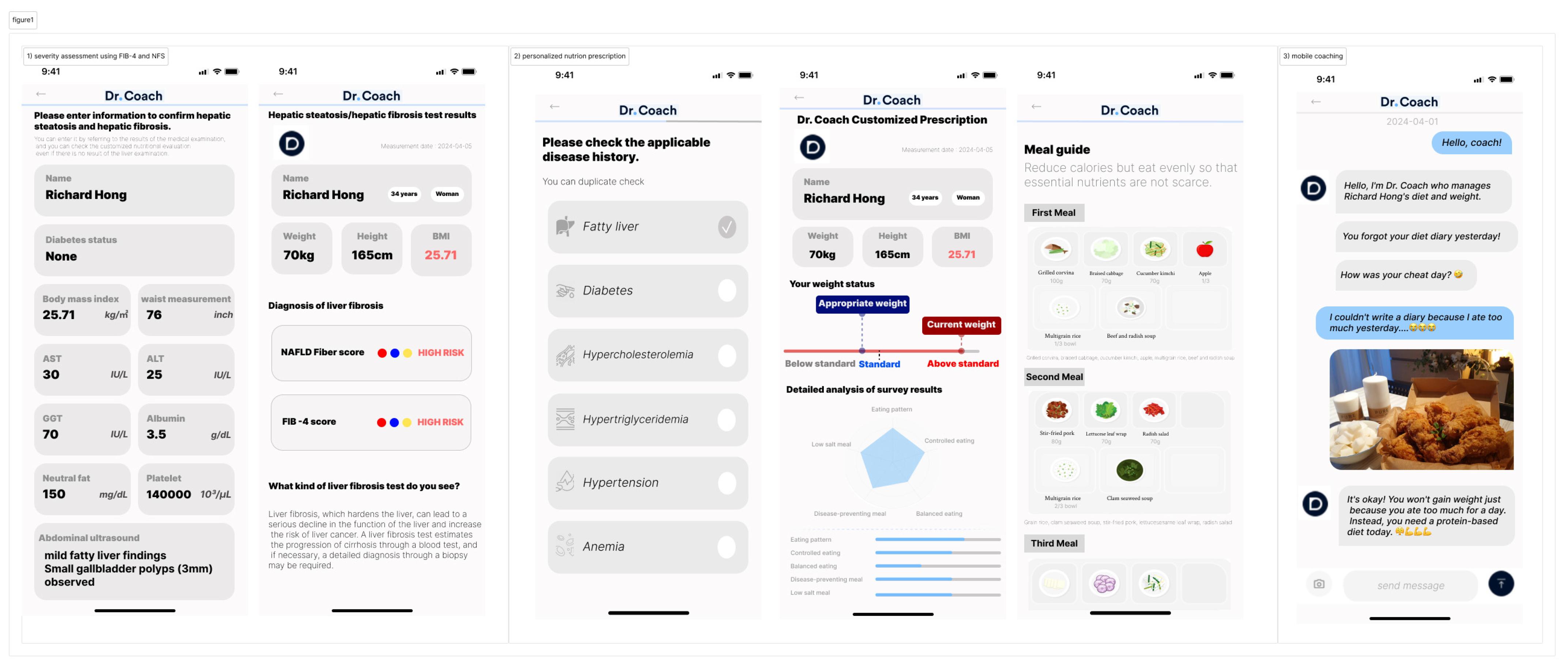
2.2. Intervention Program
2.3. Study Outcomes and Measurements
2.4. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Changes in Liver Enzymes and Body Weight
3.3. Adverse Events
4. Discussion
5. Conclusions
- Significant improvements in liver enzyme levels (ALT and GGT) were observed in the intervention group.
- The intervention did not cause significant changes in body weight or waist circumference.
- The combination of mobile lifestyle intervention and high-protein meal replacement shows potential as a therapeutic approach for MASLD.
- Further long-term studies are needed to substantiate and elaborate on these preliminary outcomes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, S.H.; Lee, H.W.; Yoo, J.J.; Cho, Y.; Kim, S.U.; Lee, T.H.; Jang, B.K.; Kim, S.G.; Ahn, S.B.; Kim, H.; et al. KASL clinical practice guidelines: Management of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2021, 27, 363–401. [Google Scholar] [CrossRef]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander Sharma, B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Obes. Facts 2016, 9, 65–90. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Soljak, M.; Boehm, B.O.; Car, J. Clinical relevance of smartphone apps for diabetes management: A global overview. Diabetes Metab. Res. Rev. 2018, 34, e2990. [Google Scholar] [CrossRef]
- Rivera, J.; McPherson, A.; Hamilton, J.; Birken, C.; Coons, M.; Iyer, S.; Agarwal, A.; Lalloo, C.; Stinson, J. Mobile apps for weight management: A scoping review. JMIR Mhealth Uhealth 2016, 4, e87. [Google Scholar] [CrossRef]
- Xie, L.F.; Itzkovitz, A.; Roy-Fleming, A.; Da Costa, D.; Brazeau, A.S. Understanding self-guided web-based educational interventions for patients with chronic health conditions: Systematic review of intervention features and adherence. J. Med. Internet Res. 2020, 22, e18355. [Google Scholar] [CrossRef]
- Sequi-Dominguez, I.; Alvarez-Bueno, C.; Martinez-Vizcaino, V.; Fernandez-Rodriguez, R.; Del Saz Lara, A.; Cavero-Redondo, I. Effectiveness of mobile health interventions promoting physical activity and lifestyle interventions to reduce cardiovascular risk among individuals with metabolic syndrome: Systematic review and meta-analysis. J. Med. Internet Res. 2020, 22, e17790. [Google Scholar] [CrossRef]
- Luis, D.; Primo, D.; Izaola, O.; Lopez, J.J. Effects of a short-term meal replacement hypocaloric diet in subjects with obesity and high fatty liver index. Nutrients 2022, 14, 5353. [Google Scholar] [CrossRef]
- Noronha, J.C.; Nishi, S.K.; Khan, T.A.; Blanco Mejia, S.; Kendall, C.W.C.; Kahleová, H.; Rahelić, D.; Salas-Salvadó, J.; Leiter, L.A.; Lean, M.E.J.; et al. Weight management using meal replacements and cardiometabolic risk reduction in individuals with pre-diabetes and features of metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2024, 25, e13751. [Google Scholar] [CrossRef]
- Deibert, P.; Lazaro, A.; Schaffner, D.; Berg, A.; Koenig, D.; Kreisel, W.; Baumstark, M.W.; Steinmann, D.; Buechert, M.; Lange, T. Comprehensive lifestyle intervention vs. soy protein-based meal regimen in non-alcoholic steatohepatitis. World J. Gastroenterol. 2019, 25, 1116–1131. [Google Scholar] [CrossRef]
- Lim, S.L.; Johal, J.; Ong, K.W.; Han, C.Y.; Chan, Y.H.; Lee, Y.M.; Loo, W.M. Lifestyle intervention enabled by mobile technology on weight loss in patients with nonalcoholic fatty liver disease: Randomized controlled trial. JMIR Mhealth Uhealth 2020, 8, e14802. [Google Scholar] [CrossRef]
- Gordon, S.C.; Kachru, N.; Parker, E.; Korrer, S.; Ozbay, A.B.; Wong, R.J. Health care use and costs among patients with nonalcoholic steatohepatitis with advanced fibrosis using the Fibrosis-4 score. Hepatol. Commun. 2020, 4, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef] [PubMed]
- McKay, F.H.; Slykerman, S.; Dunn, M. The app behavior change scale: Creation of a scale to assess the potential of apps to promote behavior change. JMIR Mhealth Uhealth 2019, 7, e11130. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Park, H.S.; Sung, S.W.; Ou, S.W.; Lee, K.Y.; Kim, B.S.; Han, J.H.; Kim, S.M.; Lee, H.R.; Yu, B.Y.; Lee, K.M.; et al. Development of Korean version of obesity-related quality of life scale. J. Korean Soc. Study Obes. 2003, 12, 280–293. [Google Scholar]
- Lee, Y.D.; Kim, K.W.; Choi, K.S.; Kim, M.; Cho, Y.J.; Sohn, C. Development of dietary pattern evaluation tool for adults and correlation with Dietary Quality Index. Nutr. Res. Pract. 2016, 10, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Liao, X.; Shao, C.; Lin, Y.; Wu, T.; Sun, Y.; Feng, S.T.; Ye, J.; Zhong, B. Normalization of γ-glutamyl transferase levels is associated with better metabolic control in individuals with nonalcoholic fatty liver disease. BMC Gastroenterol. 2021, 21, 215. [Google Scholar] [CrossRef] [PubMed]
- Drescher, H.K.; Weiskirchen, S.; Weiskirchen, R. Current status in testing for nonalcoholic fatty liver disease (nafld) and nonalcoholic steatohepatitis (NASH). Cells 2019, 8, 845. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Dalbeni, A. Treatments for NAFLD: State of art. Int. J. Mol. Sci. 2021, 22, 2350. [Google Scholar] [CrossRef]
- Moore, M.P.; Cunningham, R.P.; Dashek, R.J.; Mucinski, J.M.; Rector, R.S. A fad too far? Dietary strategies for the prevention and treatment of NAFLD. Obesity 2020, 28, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Farías, C.; Cisternas, C.; Gana, J.C.; Alberti, G.; Echeverría, F.; Videla, L.A.; Mercado, L.; Muñoz, Y.; Valenzuela, R. Dietary and nutritional interventions in nonalcoholic fatty liver disease in pediatrics. Nutrients 2023, 15, 4829. [Google Scholar] [CrossRef]
- Zeng, M.H.; Shi, Q.Y.; Xu, L.; Mi, Y.Q. Establishment and validation of an adherence prediction system for lifestyle interventions in non-alcoholic fatty liver disease. World J. Gastroenterol. 2024, 30, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Noronha, J.C.; Nishi, S.K.; Braunstein, C.R.; Khan, T.A.; Blanco Mejia, S.; Kendall, C.W.C.; Kahleová, H.; Rahelić, D.; Salas-Salvadó, J.; Leiter, L.A.; et al. The effect of liquid meal replacements on cardiometabolic risk factors in overweight/obese individuals with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Care 2019, 42, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Worm, N. Beyond body weight-loss: Dietary strategies targeting intrahepatic fat in NAFLD. Nutrients 2020, 12, 1316. [Google Scholar] [CrossRef]
- Kim, J.Y. Optimal diet strategies for weight loss and weight loss maintenance. J. Obes. Metab. Syndr. 2021, 30, 20–31. [Google Scholar] [CrossRef]
- Oliveira, C.L.P.; Boulé, N.G.; Berg, A.; Sharma, A.M.; Elliott, S.A.; Siervo, M.; Ghosh, S.; Prado, C.M. Consumption of a high-protein meal replacement leads to higher fat oxidation, suppression of hunger, and improved metabolic profile after an exercise session. Nutrients 2021, 13, 155. [Google Scholar] [CrossRef] [PubMed]
- Bezerra Duarte, S.M.; Faintuch, J.; Stefano, J.T.; Sobral de Oliveira, M.B.; de Campos Mazo, D.F.; Rabelo, F.; Vanni, D.; Nogueira, M.A.; Carrilho, F.J.; Marques Souza de Oliveira, C.P. Hypocaloric high-protein diet improves clinical and biochemical markers in patients with nonalcoholic fatty liver disease (NAFLD). Nutr. Hosp. 2014, 29, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Fakih El Khoury, C.; Karavetian, M.; Halfens, R.J.G.; Crutzen, R.; Khoja, L.; Schols, J.M.G.A. The effects of dietary mobile apps on nutritional outcomes in adults with chronic diseases: A systematic review and meta-analysis. J. Acad. Nutr. Diet. 2019, 119, 626–651. [Google Scholar] [CrossRef] [PubMed]
- Mazzotti, A.; Caletti, M.T.; Brodosi, L.; Di Domizio, S.; Forchielli, M.L.; Petta, S.; Bugianesi, E.; Bianchi, G.; Marchesini, G. An internet-based approach for lifestyle changes in patients with NAFLD: Two-year effects on weight loss and surrogate markers. J. Hepatol. 2018, 69, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.K.; Cole-Lewis, H.; Bernhardt, J.M. Mobile text messaging for health: A systematic review of reviews. Annu. Rev. Public Health 2015, 36, 393–415. [Google Scholar] [CrossRef] [PubMed]
- Kleine, H.D.; McCormack, L.A.; Drooger, A.; Meendering, J.R. Barriers to and facilitators of weight management in adults using a meal replacement program that includes health coaching. J. Prim. Care Community Health 2019, 10, 2150132719851643. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.Y.; Chen, C.; Magana, C.; Caballero Barajas, K.; Olayiwola, J.N. A mobile phone-based health coaching intervention for weight loss and blood pressure reduction in a national payer population: A retrospective study. JMIR Mhealth Uhealth 2017, 5, e80. [Google Scholar] [CrossRef]
- Tanaka, K.; Sasai, H.; Wakaba, K.; Murakami, S.; Ueda, M.; Yamagata, F.; Sawada, M.; Takekoshi, K. Professional dietary coaching within a group chat using a smartphone application for weight loss: A randomized controlled trial. J. Multidiscip. Healthc. 2018, 11, 339–347. [Google Scholar] [CrossRef]
- Smith, A. Smartphone Ownership-2013 Update; Pew Research Center: Washington, DC, USA, 2013; Volume 12. [Google Scholar]


| Intervention (N = 25) | Control (N = 24) | p-Value | |
|---|---|---|---|
| Age (years), mean (SD) | 41.32 (6.74) | 43.50 (6.67) | 0.261 |
| Male sex (%) | 25 (100.0) | 21 (87.5) | 0.068 |
| Body weight (kg), mean (SD) | 91.21 (12.37) | 88.43 (15.35) | 0.489 |
| BMI (kg/m2), mean (SD) | 30.04 (4.48) | 30.24 (3.70) | 0.866 |
| Waist circumference (cm), mean (SD) | 102.36 (10.64) | 101.73 (12.51) | 0.850 |
| Body fat percent (%), mean (SD) | 30.30 (5.58) | 31.58 (5.74) | 0.433 |
| Systolic blood pressure (mmHg), mean (SD) | 131.32 (15.23) | 133.42 (19.00) | 0.671 |
| Diastolic blood pressure (mmHg), mean (SD) | 81.38 (13.33) | 80.56 (8.09) | 0.796 |
| AST (IU/L), mean (SD) | 46.88 (20.00) | 40.12 (11.38) | 0.155 |
| ALT (IU/L), mean (SD) | 77.72 (38.20) | 64.29 (27.21) | 0.165 |
| GGT (IU/L), mean (SD) | 66.20 (43.08) | 64.29 (45.32) | 0.881 |
| Alkaline phosphatase (IU/L), mean (SD) | 69.08 (22.10) | 73.25 (20.04) | 0.497 |
| Albumin (IU/L), mean (SD) | 4.64 (0.18) | 4.74 (0.15) | 0.038 |
| Total cholesterol (mmol/L), mean (SD) | 204.88 (42.99) | 199.46 (39.66) | 0.649 |
| HDL-cholesterol (mmol/L), mean (SD) | 43.60 (7.92) | 45.58 (9.66) | 0.435 |
| LDL-cholesterol (mmol/L), mean (SD) | 122.74 (32.79) | 121.19 (35.24) | 0.874 |
| Triglycerides (mmol/L), mean (SD) | 192.72 (116.37) | 163.42 (87.46) | 0.326 |
| Fasting glucose (mmol/L), mean (SD) | 96.04 (6.72) | 97.46 (13.75) | 0.646 |
| WBC (×103/µL), mean (SD) | 6.67 (1.93) | 6.79 (1.93) | 0.83 |
| Hb (g/dL), mean (SD) | 16.34 (0.71) | 15.81 (1.24) | 0.075 |
| Platelet (×103/µL), mean (SD) | 251.08 (53.52) | 265.00 (57.23) | 0.383 |
| Smoking status | 0.91 | ||
| Non-smoker | 11 (44.0) | 12 (46.2) | |
| Ex-smoker | 9 (36.0) | 10 (38.5) | |
| Current smoker | 5 (20.0) | 4 (15.4) | |
| Household income, N (%) | 0.19 | ||
| <300,000 | 2 (8.0) | 0 (0.0) | |
| <500,000 | 4 (16.0) | 10 (38.5) | |
| <800,000 | 7 (28.0) | 9 (34.6) | |
| <100,000 | 5 (20.0) | 3 (11.5) | |
| >100,000 | 7 (28.0) | 4 (15.4) | |
| Educational level, N (%) | 0.492 | ||
| Less than high school | 1 (4.0) | 0 (0.0) | |
| High school | 1 (4.0) | 0 (0.0) | |
| College or more | 19 (76.0) | 20 (76.9) | |
| Graduate or more | 4 (16.0) | 6 (23.1) | |
| Subjective health, N (%) | 0.727 | ||
| Very good | 2 (8.0) | 2 (8.3) | |
| Good | 19 (76.0) | 16 (66.7) | |
| Bad | 4 (16.0) | 6 (25.0) | |
| Alcohol consumption, N (%) | 0.317 | ||
| None | 3 (12.0) | 1 (4.2) | |
| Moderate | 22 (88.0) | 23 (95.8) | |
| Hypertension, N (%) | 0.928 | ||
| Yes | 7 (28.0) | 7 (29.2) | |
| No | 18 (72.0) | 17 (70.8) | |
| Dyslipidemia, N (%) | 0.24 | ||
| Yes | 3 (12.0) | 6 (25.0) | |
| No | 22 (88.0) | 18 (75.0) | |
| Gout, N (%) | 0.302 | ||
| Yes | 0 (0.0) | 1 (4.2) | |
| No | 24 (96.0) | 23 (95.8) | |
| Thyroid disease, N (%) | 0.976 | ||
| Yes | 1 (4.0) | 1 (4.2) | |
| No | 24 (96.0) | 23 (95.8) |
| Intervention (N = 25) | Control (N = 24) | p-Value | |||
|---|---|---|---|---|---|
| Changes from Baseline | Within Group p-Value | Changes from Baseline | Within Group p-Value | ||
| Weight (kg), mean (SD) | −2.93 (2.58) | 0.405 | −2.74 (1.54) | 0.537 | 0.762 |
| BMI (kg/m2), mean (SD) | −0.98 (0.87) | 0.43 | −0.94 (0.51) | 0.377 | 0.843 |
| Body fat percent (%) | −1.36 (1.34) | 0.393 | −0.70 (1.50) | 0.674 | 0.115 |
| Waist circumference (cm), mean (SD) | −2.73 (2.38) | 0.357 | −2.22 (2.08) | 0.54 | 0.428 |
| Systolic blood pressure (mmHg), mean (SD) | 0.64 (17.21) | 0.872 | −1.71 (18.67) | 0.75 | 0.649 |
| Diastolic blood pressure (mmHg), mean (SD) | −0.08 (9.69) | 0.974 | −1.25 (12.27) | 0.726 | 0.712 |
| AST (IU/L), mean (SD) | −13.28 (20.30) | 0.009 | −4.25 (12.98) | 0.34 | 0.071 |
| ALT (IU/L), mean (SD) | −28.32 (25.93) | 0.002 | −10.67 (15.54) | 0.196 | 0.006 |
| GGT (IU/L), mean (SD) | −27.76 (25.02) | 0.007 | 2.79 (54.22) | 0.888 | 0.014 |
| Alkaline phosphatase (IU/L), mean (SD) | −0.96 (5.81) | 0.926 | 1.46 (7.49) | 0.813 | 0.218 |
| Albumin (IU/L), mean (SD) | −0.06 (0.15) | 0.286 | 0.07 (0.21) | 0.212 | 0.017 |
| Total cholesterol (mmol/L), mean (SD) | −12.92 (27.84) | 0.282 | −5.54 (22.49) | 0.656 | 0.314 |
| HDL-cholesterol (mmol/L), mean (SD) | −1.20 (6.18) | 0.601 | 1.04 (4.40) | 0.694 | 0.152 |
| LDL-cholesterol (mmol/L), mean (SD) | −2.95 (38.19) | 0.776 | −1.39 (21.95) | 0.899 | 0.862 |
| Triglycerides (mmol/L), mean (SD) | −43.84 (131.72) | 0.131 | −25.96 (65.92) | 0.221 | 0.553 |
| Fasting glucose (mmol/L), mean (SD) | −1.52 (8.02) | 0.475 | 0.17 (9.75) | 0.961 | 0.511 |
| WBC (×103/µL), mean (SD) | −0.25 (1.42) | 0.604 | 0.20 (1.13) | 0.72 | 0.228 |
| Hb (g/dL), mean (SD) | −0.14 (0.51) | 0.484 | 0.03 (0.69) | 0.943 | 0.346 |
| Platelet (×103/µL), mean (SD) | −14.20 (26.18) | 0.323 | 3.17 (30.61) | 0.852 | 0.038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, E.; Kim, S.; Kim, S.; Kim, J.Y.; Kim, H.J.; Go, Y.; Lee, Y.J.; Lee, H.; Gil, S.; Yoon, S.K.; et al. The Effect of Mobile Lifestyle Intervention Combined with High-Protein Meal Replacement on Liver Function in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: A Pilot Randomized Controlled Trial. Nutrients 2024, 16, 2254. https://doi.org/10.3390/nu16142254
Cho E, Kim S, Kim S, Kim JY, Kim HJ, Go Y, Lee YJ, Lee H, Gil S, Yoon SK, et al. The Effect of Mobile Lifestyle Intervention Combined with High-Protein Meal Replacement on Liver Function in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: A Pilot Randomized Controlled Trial. Nutrients. 2024; 16(14):2254. https://doi.org/10.3390/nu16142254
Chicago/Turabian StyleCho, Eunbyul, Sunwoo Kim, Soonkyu Kim, Ju Young Kim, Hwa Jung Kim, Yumi Go, Yu Jung Lee, Haesol Lee, Siye Gil, Sung Kwon Yoon, and et al. 2024. "The Effect of Mobile Lifestyle Intervention Combined with High-Protein Meal Replacement on Liver Function in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: A Pilot Randomized Controlled Trial" Nutrients 16, no. 14: 2254. https://doi.org/10.3390/nu16142254
APA StyleCho, E., Kim, S., Kim, S., Kim, J. Y., Kim, H. J., Go, Y., Lee, Y. J., Lee, H., Gil, S., Yoon, S. K., & Chu, K. (2024). The Effect of Mobile Lifestyle Intervention Combined with High-Protein Meal Replacement on Liver Function in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: A Pilot Randomized Controlled Trial. Nutrients, 16(14), 2254. https://doi.org/10.3390/nu16142254








