Nutrition in Gilbert’s Syndrome—A Systematic Review of Clinical Trials According to the PRISMA Statement
Abstract
1. Introduction
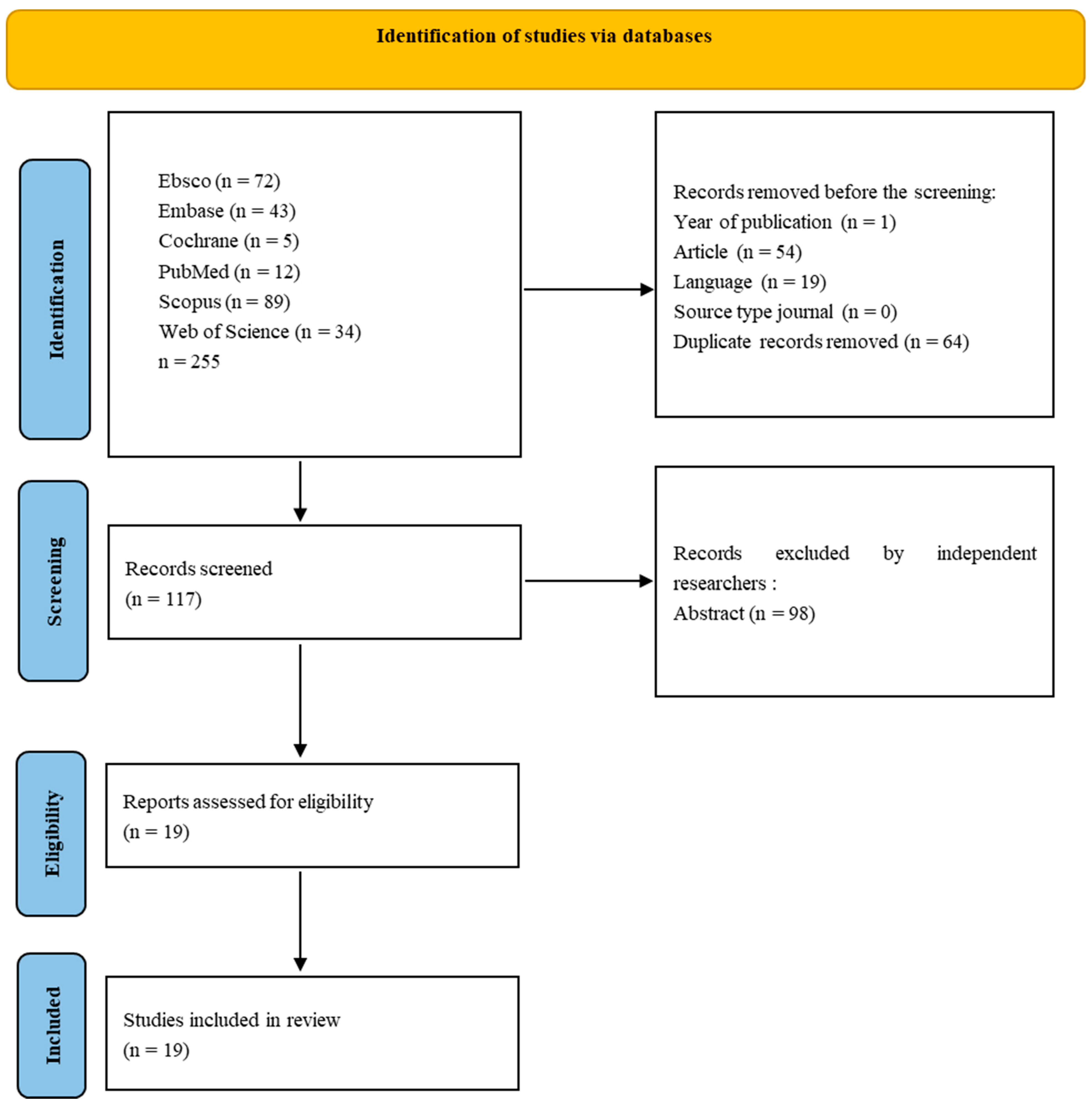
2. Materials and Methods
3. Results
3.1. Caloric Restriction in Gilbert’s Syndrome
3.2. Consumption of Various Diet Variants, Vegetables, Fruits, and Alcohol in Gilbert’s Syndrome
4. Discussion
5. Strength
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Radu, P.; Atsmon, J. Gilbert’s Syndrome—Clinical and Pharmacological Implications. Isr. Med. Assoc. J. 2001, 3, 593–598. [Google Scholar] [PubMed]
- Fretzayas, A.; Moustaki, M.; Liapi, O.; Karpathios, T. Gilbert Syndrome. Eur. J. Pediatr. 2012, 171, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Kamal, S.; Abdelhakam, S.; Ghoraba, D.; Massoud, Y.; Aziz, K.A.; Hassan, H.; Hafez, T.; Abdel Sallam, A. The Frequency, Clinical Course, and Health Related Quality of Life in Adults with Gilbert’s Syndrome: A Longitudinal Study. BMC Gastroenterol. 2019, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Roy-Chowdhury, N.; Deocharan, B.; Bejjanki, H.R.; Roy-Chowdhury, J.; Koliopoulos, C.; Petmezaki, S.; Valaes, T. Presence of the Genetic Marker for Gilbert Syndrome Is Associated with Increased Level and Duration of Neonatal Jaundice. Acta Paediatr. 2002, 91, 100–101. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.H.; Shiels, R.G.; Lang, C.A.; Seyed Khoei, N.; Bulmer, A.C. Diagnostic Criteria and Contributors to Gilbert’s Syndrome. Crit. Rev. Clin. Lab. Sci. 2018, 55, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Muraca, M.; Fevery, J. Influence of Sex and Sex Steroids on Bilirubin Uridine Diphosphate-Glucuronosyltransferase Activity of Rat Liver. Gastroenterology 1984, 87, 308–313. [Google Scholar] [CrossRef]
- Fevery, J. Bilirubin in Clinical Practice: A Review. Liver Int. 2008, 28, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Raijmakers, M.T.M.; Jansen, P.L.M.; Steegers, E.A.P.; Peters, W.H.M. Association of Human Liver Bilirubin UDP-Glucuronyltransferase Activity with a Polymorphism in the Promoter Region of the UGT1A1 Gene. J. Hepatol. 2000, 33, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Borlak, J.; Thum, T.; Landt, O.; Erb, K.; Hermann, R. Molecular Diagnosis of a Familial Nonhemolytic Hyperbilirubinemia (Gilbert’s Syndrome) in Healthy Subjects. Hepatology 2000, 32, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Ki, C.-S.; Lee, K.-A.; Lee, S.-Y.; Kim, H.-J.; Cho, S.S.; Park, J.-H.; Cho, S.; Sohn, K.M.; Kim, J.-W. Haplotype Structure of the UDP-Glucuronosyltransferase 1A1 (UGT1A1) Gene and Its Relationship to Serum Total Bilirubin Concentration in a Male Korean Population. Clin. Chem. 2003, 49, 2078–2081. [Google Scholar] [CrossRef] [PubMed]
- Peters, W.H.M.; te Morsche, R.H.M.; Roelofs, H.M.J. Combined Polymorphisms in UDP-Glucuronosyltransferases 1A1 and 1A6: Implications for Patients with Gilbert’s Syndrome. J. Hepatol. 2003, 38, 3–8. [Google Scholar] [CrossRef] [PubMed]
- de Morais, S.M.; Uetrecht, J.P.; Wells, P.G. Decreased Glucuronidation and Increased Bioactivation of Acetaminophen in Gilbert’s Syndrome. Gastroenterology 1992, 102, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Esteban, A.; Pérez-Mateo, M. Heterogeneity of Paracetamol Metabolism in Gilbert’s Syndrome. Eur. J. Drug Metab. Pharmacokinet. 1999, 24, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.T.; Santos, S.M.L.D.; da Costa, J.S.M.B.; Bernardo, R.C. Anesthesia in a patient with Gilbert’s syndrome: Case report. Rev. Bras. Anestesiol. 2004, 54, 399–403. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strassburg, C.P. Hyperbilirubinemia Syndromes (Gilbert-Meulengracht, Crigler-Najjar, Dubin-Johnson, and Rotor Syndrome). Best Pract. Res. Clin. Gastroenterol. 2010, 24, 555–571. [Google Scholar] [CrossRef] [PubMed]
- Więcek, S.; Jankowska, I.; Kwiecień, J.; Liberek, A. Gilbert’s Syndrome—Diagnostic and Therapeutic Standard. Stand. Med. Pediatr. 2019, 16, 50–55. [Google Scholar]
- Haverfield, E.V.; McKenzie, C.A.; Forrester, T.; Bouzekri, N.; Harding, R.; Serjeant, G.; Walker, T.; Peto, T.E.A.; Ward, R.; Weatherall, D.J. UGT1A1 Variation and Gallstone Formation in Sickle Cell Disease. Blood 2005, 105, 968–972. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Clark, H.D.; Wells, G.A.; Huët, C.; McAlister, F.A.; Salmi, L.R.; Fergusson, D.; Laupacis, A. Assessing the Quality of Randomized Trials: Reliability of the Jadad Scale. Control Clin. Trials 1999, 20, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Olivo, S.A.; Macedo, L.G.; Gadotti, I.C.; Fuentes, J.; Stanton, T.; Magee, D.J. Scales to Assess the Quality of Randomized Controlled Trials: A Systematic Review. Phys. Ther. 2008, 88, 156–175. [Google Scholar] [CrossRef] [PubMed]
- Felsher, B.F.; Rickard, D.; Redeker, A.G. The Reciprocal Relation between Caloric Intake and the Degree of Hyperbilirubinemia in Gilbert’s Syndrome. N. Engl. J. Med. 1970, 283, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.; Sherlock, S. Diagnosis of Gilbert’s Syndrome: Role of Reduced Caloric Intake Test. Br. Med. J. 1973, 3, 559. [Google Scholar] [CrossRef] [PubMed]
- Bensinger, T.A.; Maisels, M.J.; Carlson, D.E.; Conrad, M.E. Effect of Low Caloric Diet on Endogenous Carbon Monoxide Production: Normal Adults and Gilbert’s Syndrome. Proc. Soc. Exp. Biol. Med. 1973, 144, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Felsher, B.F.; Carpio, N.M. Caloric Intake and Unconjugated Hyperbilirubinemia. Gastroenterology 1975, 69, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Felsher, B.F. Effect of Changes in Dietary Components on the Serum Bilirubin in Gilbert’s Syndrome. Am. J. Clin. Nutr. 1976, 29, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Briheim, G.; Fryden, A.; Tobiasson, P. Serum Bile Acids in Gilbert ’ s Syndrome before and after Keduced Caloric Intake. Scand. J. Gastroenterol. 1982, 7, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.L.; Ricci, R.R. Effect of an Intraluminal Food-Bulk on Low Calorie Induced Hyperbilirubinaemia. Clin. Sci. 1984, 66, 493–496. [Google Scholar] [CrossRef]
- Gentile, S.; Orzes, N.; Persico, M.; Marmo, R.; Bronzino, P.; Tiribelli, C. Comparison of Nicotinic Acid- and Caloric Restriction-Induced Hyperbilirubinaemia in the Diagnosis of Gilbert’s Syndrome. J. Hepatol. 1985, 1, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Kapıcıoğlu, S.; Çetiner, M.; Bald, A. Measurement of Unconjugated Hyperbilirubinemia in Gilbert’s Syndrome during Compulsory Fasting. Marmara Med. J. 1999, 12, 171–174. [Google Scholar]
- Rodrigues, C.; Costa, E.; Vieira, E.; De Carvalho, J.; Santos, R.; Rocha-Pereira, P.; Santos-Silva, A.; Bronze-Da-Rocha, E. Bilirubin Dependence on UGT1A1 Polymorphisms, Hemoglobin, Fasting Time and Body Mass Index. Am. J. Med. Sci. 2012, 343, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Mölzer, C.; Wallner, M.; Kern, C.; Tosevska, A.; Schwarz, U.; Zadnikar, R.; Doberer, D.; Marculescu, R.; Wagner, K.H. Features of an Altered AMPK Metabolic Pathway in Gilbert’s Syndrome, and Its Role in Metabolic Health. Sci. Rep. 2016, 6, 30051. [Google Scholar] [CrossRef] [PubMed]
- Vajro, P.; DeVincenzo, A.; Lucariello, S.; Migliaro, F.; Sokal, E.; Bernard, O.; Vilei, T.; Muraca, M. Unusual Early Presentation of Gilbert Syndrome in Pediatric Recipients of Liver Transplantation. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Gollan, J.L.; Bateman, C.; Billing, B.H. Effect of Dietary Composition on the Unconjugated Hyperbilirubinaemia of Gilbert’s Syndrome. Gut 1976, 17, 335–340. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peterson, S.; Bigler, J.; Horner, N.K.; Potter, J.D.; Lampe, J.W. Cruciferase Interact with the UGT1A1*28 Polymorphism to Determine Serum Bilirubin Levels in Humans. J. Nutr. 2005, 135, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.L.; Peterson, S.; Chen, C.; Makar, K.W.; Schwarz, Y.; King, I.B.; Li, S.S.; Li, L.; Kestin, M.; Lampe, J.W. Cruciferous Vegetable Feeding Alters UGT1A1 Activity: Diet- and Genotype-Dependent Changes in Serum Bilirubin in a Controlled Feeding Trial. Cancer Prev. Res. 2009, 2, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Saracino, M.R.; Bigler, J.; Schwarz, Y.; Chang, J.; Li, S.; Li, L.; White, E.; Potter, J.D.; Lampe, J.W. Citrus Fruit Intake Is Associated with Lower Serum Bilirubin Concentration among Women. J. Nutr. 2009, 139, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, V.; Vukavić, T. Hyperbilirubinemia and Intermittent Lower Urinary Tract Dysfunction. Indian J. Pediatr. 2011, 78, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, L.M.; Gunsilius, L.; Lardi, C.; Weinmann, W.; Thierauf-emberger, A. Influence of Gilbert ’ s Syndrome on the Formation of Ethyl Glucuronide. Int. Acad. Leg. Med. 2015, 129, 1005–1010. [Google Scholar] [CrossRef]
- Izzy, M.; Angirekula, M.; Abu Dayyeh, B.K.; Bazerbachi, F.; Watt, K.D. Bariatric Surgery Proves Long-Term Benefit in Patients with Cirrhosis. Gastroenterol. Rep. 2021, 9, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Teich, N.; Lehmann, I.; Rosendahl, J.; Tröltzsch, M.; Mössner, J.; Schiefke, I. The Inverse Starving Test Is Not a Suitable Provocation Test for Gilbert’s Syndrome. BMC Res. Notes 2008, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Hallal, H.; Egea, J.M.; Mas, P.; García, M.D.; Pérez-Cuadrado, E.; Carballo, F. A Shortened, 2-Hour Rifampin Test: A Useful Tool in Gilbert’s Syndrome. Gastroenterol. Hepatol. 2006, 29, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Erdil, A.; Kadayifci, A.; Ates, Y.; Bagci, S.; Uygun, A.; Dagalp, K. Rifampicin Test in the Diagnosis of Gilbert’s Syndrome. Int. J. Clin. Pract. 2001, 55, 81–83. [Google Scholar] [CrossRef]
- Kringen, M.K.; Piehler, A.P.; Grimholt, R.M.; Opdal, M.S.; Haug, K.B.F.; Urdal, P. Serum Bilirubin Concentration in Healthy Adult North-Europeans Is Strictly Controlled by the UGT1A1 TARepeat Variants. PLoS ONE 2014, 9, e90248. [Google Scholar] [CrossRef] [PubMed]
- Moyer, A.M.; Skierka, J.M.; Kotzer, K.E.; Kluge, M.L.; Black, J.L.; Baudhuin, L.M. Clinical UGT1A1 Genetic Analysis in Pediatric Patients: Experience of a Reference Laboratory. Mol. Diagn. Ther. 2017, 21, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Hana, C.A.; Tran, L.V.; Mölzer, C.; Müllner, E.; Hörmann-Wallner, M.; Franzke, B.; Tosevska, A.; Zöhrer, P.A.; Doberer, D.; Marculescu, R.; et al. Serum Metabolomics Analysis Reveals Increased Lipid Catabolism in Mildly Hyperbilirubinemic Gilbert’s Syndrome Individuals. Metabolism 2021, 125, 154913. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, A.C.; Blanchfield, J.T.; Toth, I.; Fassett, R.G.; Coombes, J.S. Improved Resistance to Serum Oxidation in Gilbert’s Syndrome: A Mechanism for Cardiovascular Protection. Atherosclerosis 2008, 199, 390–396. [Google Scholar] [CrossRef] [PubMed]
- DInicolantonio, J.J.; McCarty, M.F.; O’Keefe, J.H. Antioxidant Bilirubin Works in Multiple Ways to Reduce Risk for Obesity and Its Health Complications. Open Hear. 2018, 5, 914. [Google Scholar] [CrossRef] [PubMed]
- Vítek, L.; Schwertner, H.A. The Heme Catabolic Pathway and Its Protective Effects on Oxidative Stress-Mediated Diseases. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2007; Volume 43, pp. 1–57. [Google Scholar] [CrossRef]
- Vítek, L. The Role of Bilirubin in Diabetes, Metabolic Syndrome, and Cardiovascular Diseases. Front. Pharmacol. 2012, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Vítek, L. Bilirubin and Atherosclerotic Diseases. Physiol. Res. 2017, 66, S11–S20. [Google Scholar] [CrossRef] [PubMed]
- Schwertner, H.A.; Vítek, L. Gilbert Syndrome, UGT1A1*28 Allele, and Cardiovascular Disease Risk: Possible Protective Effects and Therapeutic Applications of Bilirubin. Atherosclerosis 2008, 198, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wallner, M.; Bulmer, A.C.; Mölzer, C.; Müllner, E.; Marculescu, R.; Doberer, D.; Wolzt, M.; Wagner, O.F.; Wagner, K.H. Haem Catabolism: A Novel Modulator of Inflammation in Gilbert’s Syndrome. Eur. J. Clin. Investig. 2013, 43, 912–919. [Google Scholar] [CrossRef]
- Bulmer, A.C.; Verkade, H.J.; Wagner, K.H. Bilirubin and beyond: A Review of Lipid Status in Gilbert’s Syndrome and Its Relevance to Cardiovascular Disease Protection. Prog. Lipid Res. 2013, 52, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.-H.; Wallner, M.; Mölzer, C.; Gazzin, S.; Bulmer, A.C.; Tiribelli, C.; Vitek, L. Looking to the Horizon: The Role of Bilirubin in the Development and Prevention of Age-Related Chronic Diseases. Clin. Sci. 2015, 129, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Lee, Y.J.; Park, B.J.; Hong, K.W.; Jung, D.H. Total Serum Bilirubin and 8-Year Incident Type 2 Diabetes Mellitus: The Korean Genome and Epidemiology Study. Diabetes Metab. 2018, 44, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.; Hamoud, A.R.; Stec, D.E.; Hinds, T.D. Biliverdin Reductase and Bilirubin in Hepatic Disease. Am. J. Physiol.—Gastrointest. Liver Physiol. 2018, 314, G668–G676. [Google Scholar] [CrossRef] [PubMed]
- Hjelkrem, M.; Morales, A.; Williams, C.D.; Harrison, S.A. Unconjugated Hyperbilirubinemia Is Inversely Associated with Non-Alcoholic Steatohepatitis (NASH). Aliment. Pharmacol. Ther. 2012, 35, 1416–1423. [Google Scholar] [CrossRef]
- Ching, S.; Ingram, D.; Hahnel, R.; Beilby, J.; Rossi, E. Serum Levels of Micronutrients, Antioxidants and Total Antioxidant Status Predict Risk of Breast Cancer in a Case Control Study. J. Nutr. 2002, 132, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Jirásková, A.; Novotný, J.; Novotný, L.; Vodička, P.; Pardini, B.; Naccarati, A.; Schwertner, H.A.; Hubáček, J.A.; Punčochářová, L.; Šmerhovský, Z.; et al. Association of Serum Bilirubin and Promoter Variations in HMOX1 and UGT1A1 Genes with Sporadic Colorectal Cancer. Int. J. Cancer 2012, 131, 1549–1555. [Google Scholar] [CrossRef]
- Tosevska, A.; Moelzer, C.; Wallner, M.; Janosec, M.; Schwarz, U.; Kern, C.; Marculescu, R.; Doberer, D.; Weckwerth, W.; Wagner, K.H. Longer Telomeres in Chronic, Moderate, Unconjugated Hyperbilirubinaemia: Insights from a Human Study on Gilbert’s Syndrome. Sci. Rep. 2016, 6, 22300. [Google Scholar] [CrossRef] [PubMed]
- Zöhrer, P.A.; Hana, C.A.; Seyed Khoei, N.; Mölzer, C.; Hörmann-Wallner, M.; Tosevska, A.; Doberer, D.; Marculescu, R.; Bulmer, A.C.; Herbold, C.W.; et al. Gilbert’s Syndrome and the Gut Microbiota—Insights From the Case-Control BILIHEALTH Study. Front. Cell. Infect. Microbiol. 2021, 11, 701109. [Google Scholar] [CrossRef] [PubMed]
- Vítek, L.; Tiribelli, C. Gilbert ’ s Syndrome Revisited. J. Hepatol. 2023, 79, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. “Iatrogenic Gilbert Syndrome”—A Strategy for Reducing Vascular and Cancer Risk by Increasing Plasma Unconjugated Bilirubin. Med. Hypotheses 2007, 69, 974–994. [Google Scholar] [CrossRef] [PubMed]
- Kiranmayi, P.; Polisetty, L.; Krishna, Y.R. Food Faddism Complicating Jaundice in Gilbert’s Syndrome PatientsA Prospective Cohort Study. J. Clin. Diagn. Res. 2022, 16, 35–38. [Google Scholar] [CrossRef]
| Jadad Scale | Participants | Group | Duration | Intervention | Analysis | Results | Total Bilirubin | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Adults, n = 21 Age: no data Sex: no data Ethnicity: no data Country: USA | GS (n = 7) Relatives of people with GS (n = 6) Healthy (n = 8) | 2–7 days: Base-line diet 48 to 72 h Fasting diet 36 to 72 h Refeeding diet | Base-line diet 2000–3000 cal Fasting < 400 cal Refeeding 2000–3000 cal | Plasma: TB, CB | The concentration of unconjugated bilirubin (TB) in individuals with Gilbert’s syndrome significantly differed before, during, and after caloric restrictions (1.7 vs. 4.7 vs. 2.1 mg/100 mL). A relationship between calorie intake and TB levels in the blood was observed. A sudden increase in conjugated bilirubin (CB) occurred within 24 h of starting short-term fasting, followed by a significant decrease during the 12–48 h of refeeding. | TB > 1.7 mg/dL | Felsher et al. [22] |
| 1 | Adults, n = 37 Age: no data Sex: no data Ethnicity: no data Country: UK Rats Sprague-Dawley (n = 14) | Healthy (n = 12) GS (n = 10) Liver disease (n = 12) Hemolytic anemia (n = 3) Rat basal diet (n = 7) Rats starved (n = 7) | 5 days | People: 2-day basal diet, reduced to 400 cal/day for 3 days indocyanine green intravenously Rats: first group basal diet, second group 3 days was starved | Venous blood: HGB, Plasma: TB, UCB, CB haptoglobin, cortisol, Hepatic UDPGT | After caloric restriction in the diet, the following changes were observed:
| TB > 1.8 mg/dL | Owens and Sherlock [23] |
| 2 | Adults, n = 14 Age: 19–34 years Sex: 3 women; 11 men Ethnicity: no data Country: USA | Healthy (n = 5: 3 women, 2 men) GS (n = 9: men) | 2 days | Diet 400 cal/day (46 g of carbohydrates, 16.5 g of protein, and 14.5 g of fat) | LDH, ALP, ALT, ASP, TB, CB, HGB, CO production Liver biopsies | In both control group patients and those with GS, significant increases in serum total bilirubin (TB) levels were observed following caloric restriction, while there were no differences in carbon monoxide (CO) production. | TB > 1.3 mg/dL | Bensinger et al. [24] |
| 2 | Adults, n = 34 Age: no data Sex: no data Ethnicity: no data Country: USA | GS (n = 11) Hemolysis and unconjugated hyperbilirubinemia (n= 10) Healthy (n = 13) | 4–5 days | Dieta 2 or 3-day baseline of 2500–3000 cal/day, the caloric intake was abruptly reduced to approximately 300 cal/day for 2 days | Serum: TB, CB, Hepatic UDPG-T | Forty-eight hour fasting significantly increased TB levels in serum among individuals with Gilbert’s syndrome (from 1.5 to 3.5 mg/100 mL) and healthy individuals (from 0.5 to 0.9 mg/100 mL). Serum CB levels remained unchanged in all three patient groups. Reduced hepatic UDPG-T activity to levels observed in GS patients was observed only in two patients with hemolysis. | TB > 1.5 mg/dL | Felsher and Carpio [25] |
| 2 | Adults, n = 4 Age: 21–46 years Sex: no data Ethnicity: no data Country: USA | GS (n = 4) | About 2 weeks | Base-line balanced diet for 2 days Tested diet: low carbohydrate; low protein; low fat; isocaloric high fat, low carbohydrate, low protein; isocaloric low carbohydrate diet and a low protein | Anthropometric measurements Plasma: TB, CB | Higher TB levels were observed in individuals with GS after a low-calorie diet compared to a balanced diet. TB levels in individuals on various isocaloric diets with reduced macronutrient content were, on average, 1.1 mg/dL lower than after a low-calorie diet. A low-protein and low-carbohydrate isocaloric diet did not increase TB levels in the blood. None of the diets affected CB levels in the blood. Changes in body weight were observed only after the low-calorie diet. | TB > 1.3 mg/dL | Felsher [26] |
| 2 | Youth, adults, n = 12 Age: 14–46 years Sex: 3 women; 9 men Ethnicity: no data Country: Sweden | GS (n = 12) | 3 days | Reduced caloric intake (1.7 MJ/24 h) fluid meal (50 g of fat) | Serum: bile acids, UCB | The increase in UCB concentration after caloric restriction was 140%. In fasting, before and after calorie reduction, patients had normal serum bile acid levels. Postprandial concentrations of bile acids in serum were within the normal range before and after calorie restriction. | TB > 1.5 mg/dL | Briheim et al. [27] |
| 3 | Adults, n = 28 Age: 19–37 years Sex: 5 women; 25 men Ethnicity: no data Country: Italy | GS (n = 18: 15 men, 3 women) Control (n = 10: 8 men; n = 2 women) | Of at least 2 weeks | Caloric restriction 400 cal: Diet A: 100 g of sucrose/24 h Diet B: 250 g of bran (and 100–250 cm3 of barium sulfate solution | Plasma: TB | In the control group, there were no differences in TB concentration after diets A and B. TB concentration significantly increased under diet A compared to diet B in individuals with GS. TB concentration did not differ significantly between groups depending on the diet applied. | TB > 1 mg/dL | Ricci and Ricci [28] |
| 3 | Adolescents/Adults n = 60 Age: 16–48 years Sex: 27 women; 33 men Ethnicity: no data Country: Italy | GS1 (n = 10 men, n = 10 women) with high TB GS2 (n = 13 men, n = 7 women) with normal TB. Control (n= 10 men, n = 10 women) | The 2 tests were performed with an interval of at least 2 weeks. | Test with nicotinic acids (NA) Test fasting/hyperbilirubinemia (FH) patients 3 days after a control normocaloric diet were given a restricted diet (400 cal/48 h) | Blood: UCB and CB | In the NA test, significant differences were observed between individuals with GS and controls in UCB, maximal increment in AUC, and retention at 240 min (excluding sex). Following calorie restriction, TB levels were higher in GS1 compared to GS2 and the control group. Serum bilirubin increased after 24 h of caloric restriction in GS1, GS2, and normal subjects but not after 48 h. Bilirubin concentrations after 24 and 48 h were higher in males with GS compared to females. The NA test was more accurate in diagnosing GS in both males and females. | TB > 1.35 mg/dL | Gentile et al. [29] |
| 1 | Adults, n = 10 Age: no data Sex: no data Ethnicity: no data Country: Turkey | GS (n = 10) | 30 days | Compulsory 16 h fasting daily from 4:00 to 20:00 during Ramadan and consuming 2500 calories (30 g carbohydrates, 20 g protein, and 10 g fat) | Serum: TB, CB, UCB | After the first day of fasting, the concentrations of TB and UCB were significantly higher than before, while CB remained similar. In the following days of fasting (7, 14, 30), TB and UCB concentrations decreased to pre-fasting levels, while CB remained consistent throughout the fasting period. | TB > 1.4 mg/dL | Kapıcıoğlu et al. [30] |
| 3 | Adults, n = 146 Age: 20.7 years Sex: 146 women Ethnicity: White Country: Portuguese | Bilirubin concentration (μmmol/L) I group ≤6 (n = 49) II group 6–9 (n = 49) III group ≥ 9 (n = 48) | No data | 11 h fasting | Venous blood: hematological data, Plasma: bilirubin, Buffy coat-DNA extraction, screening of TA duplication in the UGT1A1 gene, BIA, International Physical Activity Questionnaire. | Women in the second and third tertiles exhibited higher Hb, Ht, MCH, MCHC concentrations and lower platelet counts than those in the first tertile. In the third tertile, RCB was the highest. There were no differences in total and differential white blood cell counts and non-genetic factors (physical activity, smoking, oral contraception, body fat content, alcohol consumption, and fasting time). The lowest BMI was observed in the third tertile. The genetic allele c.-41_-40dupTA, Hb, BMI, and fasting time were identified as the main factors associated with bilirubin concentration in the blood. | TB > 1 mg/dL | Rodrigues et al. [31] |
| 4 | Adults, n = 120 Age 20–80 years Sex: 40 women, 80 men Ethnicity: no data Country: Austria | Group < 35 years (n = 66: GS and Control: 9 women, 24 men each) Group ≥ 35 years (n = 54: GS and Control 11 women, 16 men each) | No data | 16 h fasting with a diet of 400 cal | Anthropometric measurements, BIA, questionnaire food consumption, Biomarkers of energy, carbohydrate, lipids metabolism | There were no differences between the GS and control groups in the consumption of healthy foods, snacks, red meat, alcohol, physical activity, liver enzymes, iron levels, and AMPKα1 gene expression. Individuals with GS had lower BMI, blood glucose, insulin, C-peptide, and TG levels and were less susceptible to metabolic diseases. In GS individuals, levels of p-AMPK α1/α2, -PPAR α/γ, and PgC1α were significantly higher, indicating an enhanced metabolic response to fasting. | TB > 1.0 mg/dL | Mölzer et al. [32] |
| 2 | Children, young, n = 46; Age: no data Sex: 25 girls, 21 boys Ethnicity: no data Country: Italy | Group OLT (n = 46) Control group (n = 20) non-GS OLT | 1 year | 1 day reduced caloric intake prolonged (14–16 h) fasting | Venous blood: hematologic parameters Serum: TB, Cb, UCB IXa, monoglucuronide, sex hormones, liver dysfunction, 2-bp (TA) insertion in the promoter of the gene | Four patients with OLT had hyperbilirubinemia, but CB levels were normal. Tests involving caloric restriction and fasting showed an increase in bilirubin levels. High relative amounts of UCB IXa and a predominance of monoglucuronide over diglucuronide were observed. Hematologic parameters and hormone levels in the blood were normal. Adult liver donors had gene mutations related to the 2-bp (TA) insertion. | TB > 1.3 mg/dL | Vajro et al. [33] |
| Jadad Scale | Participants | Group | Duration | Intervention | Analysis | Results | Total Bilirubin | References |
|---|---|---|---|---|---|---|---|---|
| 4 | Young adults, n = 29 Age:16–43 years Sex: 8 women, 21 men Ethnicity: no data Country: UK | About 10 days: 2 days normal diet Tested diet 1–2 day basal diet | Normal diet Tested diet: Low-energy, standard Low-energy, standard + IV glucose Low-energy, standard + IV lipid High-carbohydrate, low-lipid (fluid) High-carbohydrate, reduced-lipid Low-energy, high-lipid | Plasma: TB | The highest increase in TB concentration was observed in patients following Diet II (low-energy standard) and Diet III (low-energy, standard + IV glucose). The lowest increase in TB concentration occurred with Diet VI (high-carbohydrate, reduced-lipid). The level of hyperbilirubinemia achieved after oral carbohydrate intake was significantly lower than that observed after intravenous intake or Diet II (low-energy, standard). Diet VII (low-energy, high-lipid) significantly reduced hyperbilirubinemia to a level lower than Diet II-induced (low-energy, 400 cal). | TB > 1.7 mg/dL | Gollan et al. [34] | |
| 3 | Adults, n = 191 Age: 20–40 years Sex: 112 women, 79 men Ethnicity: no data Country: USA | UGT1A1 genotype: Group 6/6 and 6/7 (n = 169) Group 7/7 (n = 22) | Assessment of the consumption of 6 groups of botanical vegetables and fruits | Demographic survey, health history, 3-D food record Indices: BMI Venous blood: TB, CB, UGT1A1 promoter genotypes. | Men had higher serum levels of TB, CB, and UCB than women. An inverse relationship was demonstrated between all bilirubin levels and the interaction of the UGT1A1*28 genotype with the intake of the botanical group Cruciferae. Individuals with the 7/7 genotype had reduced blood bilirubin levels with increased consumption of cruciferous vegetables. | TB > 1.6 mg/dL | Peterson et al. [35] | |
| 4 | Adults, n = 70 Age: 20–40 years Sex: 35 women, 35 men Ethnicity: Caucasian, Asian, Other Country: USA | groups of genotypes UGT1A1*1/*1 (n = 29) UGT1A1*1/*28 (n = 36) UGT1A1*28/*28 (n = 5) (1) GSTM1+/GSTT1+ (n = 26) (2) GSTM1-null/GSTT1+ (n = 30) (3) GSTM1-null/GSTT1-null) (n = 14) | Each of the four diet periods lasted 14 days, with a 21-day washout period between diet periods | (1) Basal diet without fruit and vegetables; (2) Single-dose cruciferous diet; (3) Double-dose cruciferous diet; (4) Single-dose cruciferous plus apiaceous vegetable diet | GSTM1, GSTT1 genotypes UGT genotyping 3-d food record Plasma: TB, CB Urine: total isothiocyanates Indices: BMI | The decrease in serum bilirubin concentration in response to all three diets compared to the basal diet was particularly pronounced in individuals with the UGT1A1*28/*28 genotype and those consuming a double dose of cruciferous plants, which enhances UGT1A1 activity. Although UGT1A1 activity, measured by serum bilirubin, was higher in individuals with the GSTM1-null genotype than those with GSTM1+, this response was not statistically significant. | TB > 1.2 mg/dL | Navarro et al. [36] |
| 3 | Adults, n = 239 Age: 20–40 years Sex: 147 women, 146 men Ethnicity: Caucasian, Asian, Other Country: USA | UGT1A1 genotype: Group 6/6 Group 6/7 Group 7/7 | No data | Week | Food Frequency Questionnaire 6 botanical families 3-D food record; anthropometric measurements Plasma: TB, UGT genotyping | Gender (men), race (Asian), and the UGT1A1 genotype were associated with serum concentrations of TB, CB, and UCB. There was no association between the consumption of vegetables and fruits (assessed by FFQ) and TB levels. In women, an interaction was found between the UGT1A1 (7/7) genotype and the consumption of citrus fruits (assessed by FFQ) and Rutaceae (assessed by 3DFR), which was related to TB and UCB levels. | TB > 1.0 mg/dL | Saracino et al. [37] |
| 3 | Children, n = 29 Age: 8–17 years; Sex: 10 girls, 19 boys Ethnicity: no data Country: Serbia | GS (n = 29) | 9 months | 24 h of fasting and after 24 h of hypercaloric intake | Questionnaire related to urinary tract Blood: glucose, TB, CB Urine: ketone bodies uroflowmetric test | There was a normal uroflowmetric pattern in 69% of children and after 24 h of hypercaloric diet in all children. Children with an abnormal uroflowmetric pattern had higher CB concentrations after 24 h of fasting than children with a normal pattern. There were no differences in TB and UCB concentrations. In children with normal and abnormal UFC, pre-fasting CB concentration was lower than post-fasting, and glucose concentration was higher. VC in children was normal after 24 after 24 h fasting and after 24 h of hypercaloric intake. | TB > 1.3 mg/dL | Stojanović and Vukavić [38] |
| 3 | Adults, n = 30 Age: 18–71 years Sex:12 women, 18 men Ethnicity: no data Country: Germany | GS (n = 30) | No data | After 2 days of abstinence participants drank 0.1 L of sparkling wine (9 g ethanol) | Anthropometric measurements; Lifestyle and Eating Habits Questionnaire Urine: EtG, EtS, creatinine Venous blood: morphology, ethanol, TB, CB, liver enzymes | In 69% of the children, the uroflowmetric pattern was expected, and after 24 h on a hypercaloric diet, it was typical in all children. Children with an abnormal uroflowmetric pattern had higher CB levels after 24 h of fasting than children with a regular pattern. There were no differences in TB and UCB levels. CB levels were lower pre-fasting than post-fasting in children with normal and abnormal UFC, while glucose levels were higher. Bladder capacity (VC) in children was average after 24 h of fasting and after 24 h of hypercaloric intake. | TB > 0.67 mg/dL | Huppertz et al. [39] |
| 2 | Obese adults n = 10; Age: 58.4 years Sex: 7 women, 3 men; Ethnicity: no data Country: USA | GS (n = 10) with cirrhosis and nonalcoholic steatohepatitis (NASH). | >4 year (8.7 ± 1.4 year) | Bariatric surgery (laparoscopic sleeve gastrectomy or Roux-en-Y gastric bypass) | Body mass, weight; Venous blood: HbAC1, Plasma: albumin, TB, Indices: TBWL, BMI, INR, MELD-Na | The patient with GS experienced a TBWL of 23.5% and a reduction in BMI of 7.7 kg/m2. Despite a decrease in HbAC1 concentration of 1.3%, insulin treatment was necessary. An increase in TB concentration of 1.4 mg/dL of blood was observed. The MELDNa Score for End-Stage Liver Disease in the patient with GS was 14, indicating a 6% mortality risk. | TB > 0.6 mg/dL | Izzy et al. [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goluch, Z.; Wierzbicka-Rucińska, A.; Książek, E. Nutrition in Gilbert’s Syndrome—A Systematic Review of Clinical Trials According to the PRISMA Statement. Nutrients 2024, 16, 2247. https://doi.org/10.3390/nu16142247
Goluch Z, Wierzbicka-Rucińska A, Książek E. Nutrition in Gilbert’s Syndrome—A Systematic Review of Clinical Trials According to the PRISMA Statement. Nutrients. 2024; 16(14):2247. https://doi.org/10.3390/nu16142247
Chicago/Turabian StyleGoluch, Zuzanna, Aldona Wierzbicka-Rucińska, and Ewelina Książek. 2024. "Nutrition in Gilbert’s Syndrome—A Systematic Review of Clinical Trials According to the PRISMA Statement" Nutrients 16, no. 14: 2247. https://doi.org/10.3390/nu16142247
APA StyleGoluch, Z., Wierzbicka-Rucińska, A., & Książek, E. (2024). Nutrition in Gilbert’s Syndrome—A Systematic Review of Clinical Trials According to the PRISMA Statement. Nutrients, 16(14), 2247. https://doi.org/10.3390/nu16142247








