Antihypertensive Potential of Pistacia lentiscus var. Chia: Molecular Insights and Therapeutic Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Angiotensin II Model of Hypertension
2.3. DOCA-HS Model of Hypertension
2.4. Chios Mastic Gum Dose Selection
2.5. Chios Mastic Gum Resin Analysis
2.6. Blood Pressure Monitoring
2.7. Rat Plasma Liquid Chromatography—Mass Spectrometry Metabolomic Analysis
2.8. RNA-Sequencing Analysis
2.9. Real-Time PCR
2.10. Western Blot
2.11. Statistical Analysis
3. Results
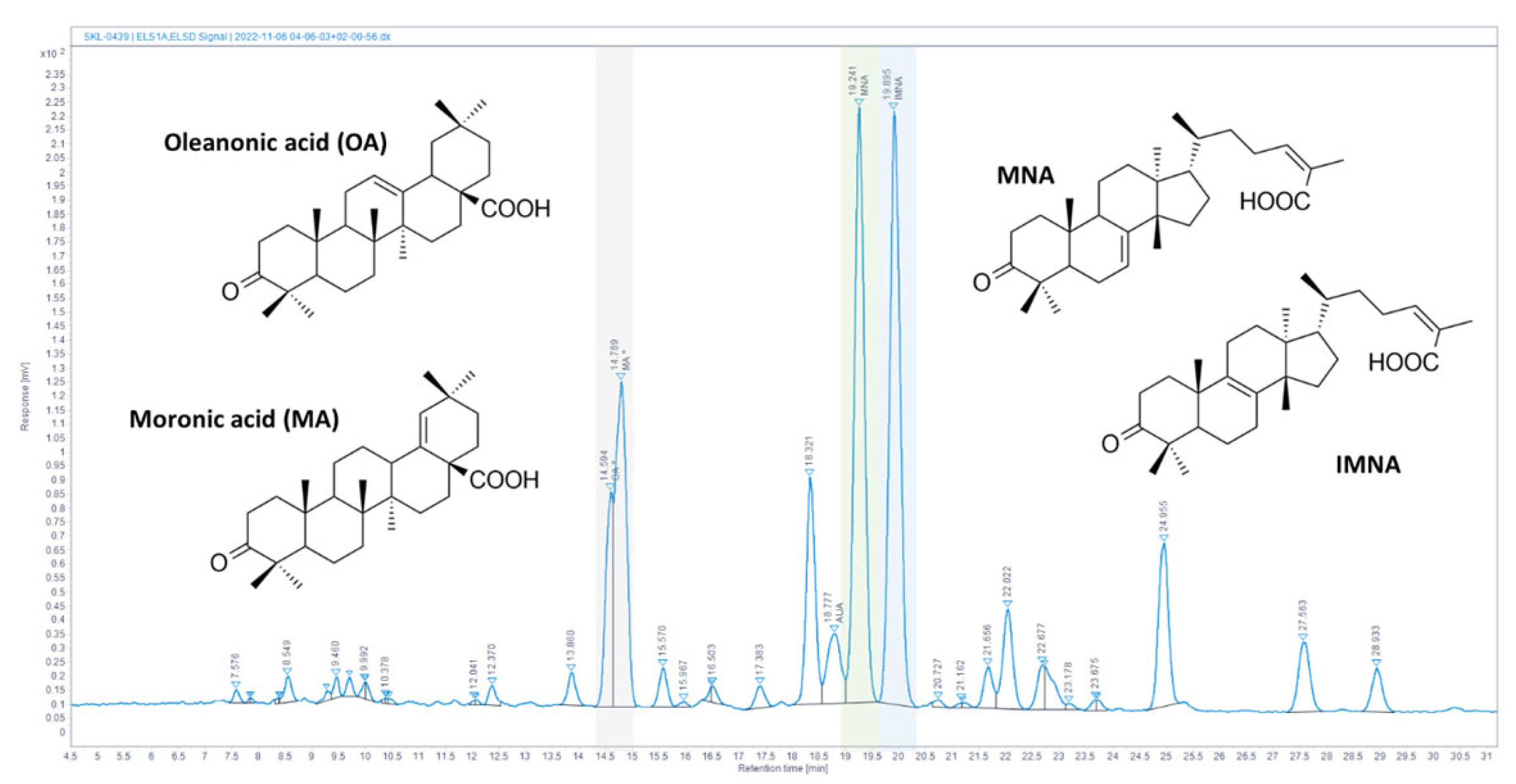
3.1. Quantification of CMG Major Constituents
3.2. CMG Reduced Systolic, Diastolic, and Mean Blood Pressure after Four Weeks of Administration Both in the AngII- and DOCA–HS-Induced In Vivo Models of Hypertension
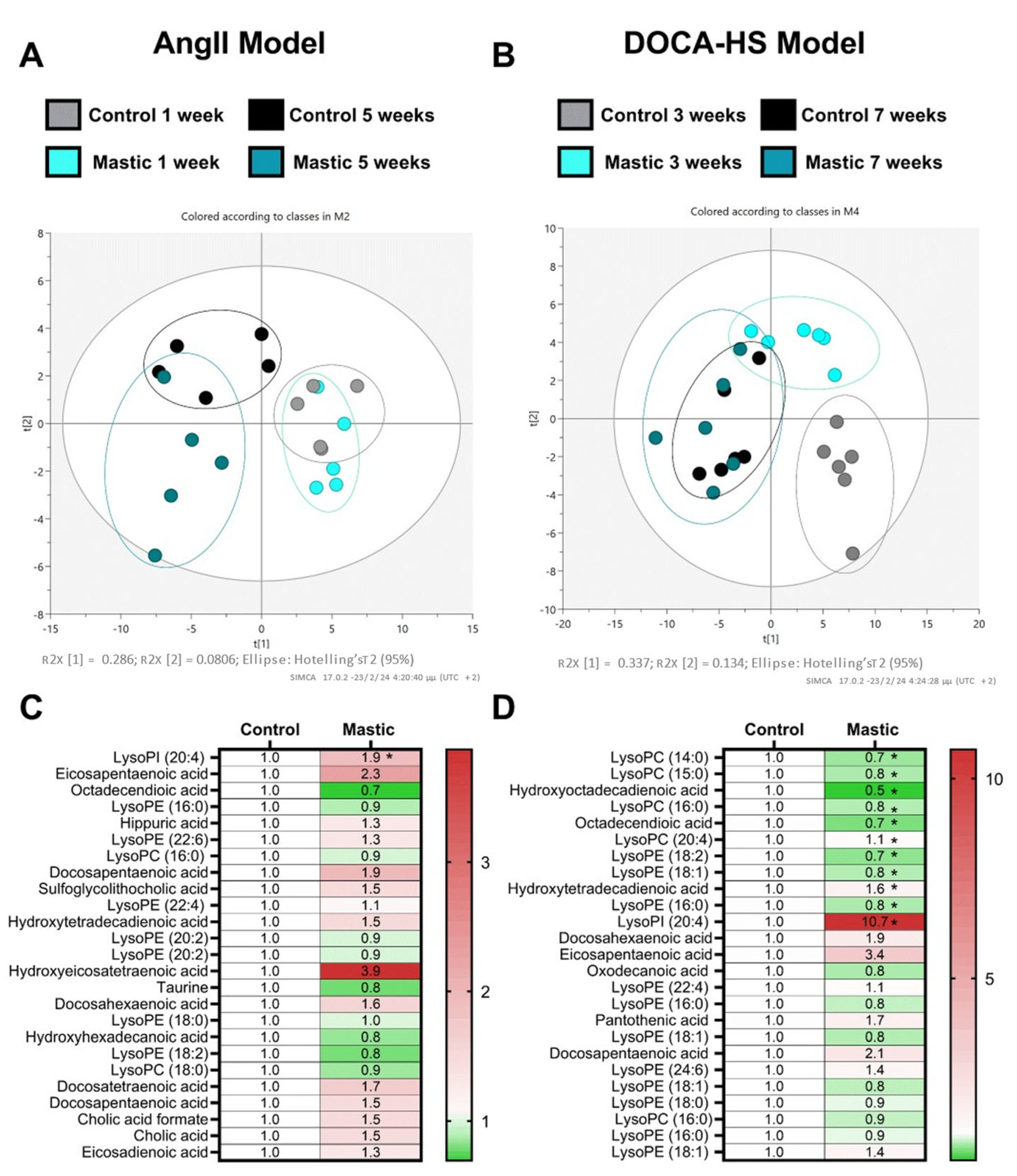
3.3. CMG Led to Distinct Circulatory Gene Expression Profiles in the AngII- and DOCA–HS-Induced In Vivo Models of Hypertension. Emerging Role of Endothelial Homeostasis in CMG Antihypertensive Potential
3.4. CMG Led to an Upregulation of Circulating Lysophosphatidylinositol Both in the AngII- and DOCA–HS-Induced In Vivo Models of Hypertension
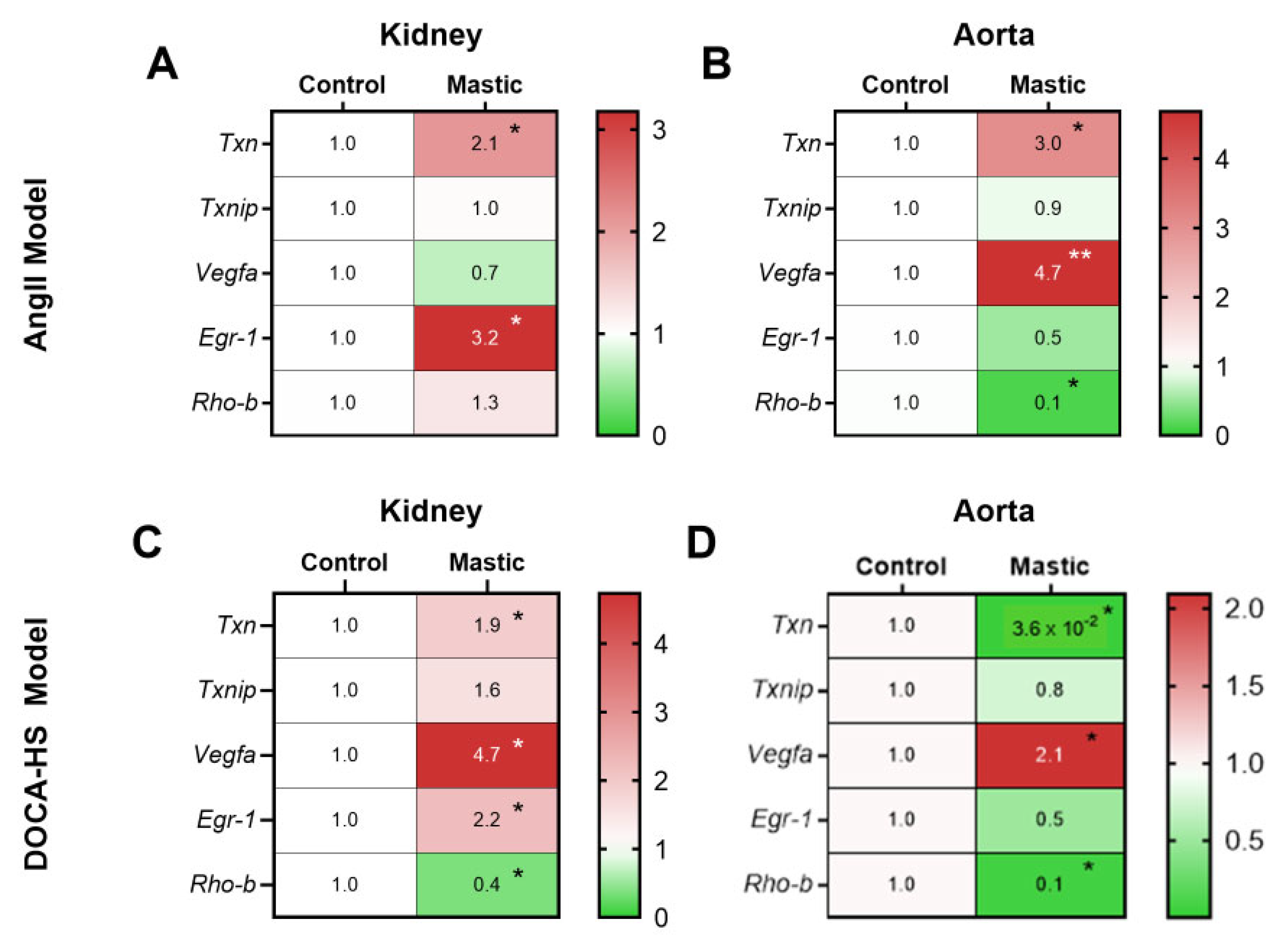
3.5. CMG Exerted an Endothelium-Mediated Protection Effect in the Hypertensive Kidney and Aorta In Vivo
3.6. CMG Increases Renal Endothelial NO Synthase Phosphorylation Both in the AngII- and DOCA-HS-Induced In Vivo Models of Hypertension
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lauder, L.; Mahfoud, F.; Azizi, M.; Bhatt, D.L.; Ewen, S.; Kario, K.; Parati, G.; Rossignol, P.; Schlaich, M.P.; Teo, K.K.; et al. Hypertension management in patients with cardiovascular comorbidities. Eur. Heart J. 2023, 44, 2066–2077. [Google Scholar] [CrossRef] [PubMed]
- Long, A.N.; Dagogo-Jack, S. Comorbidities of diabetes and hypertension: Mechanisms and approach to target organ protection. J. Clin. Hypertens. 2011, 13, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E.; Asmar, R.; Benetos, A.; Blacher, J.; Boutouyrie, P.; Lacolley, P.; Laurent, S.; London, G.; Pannier, B.; Protogerou, A.; et al. Interaction Between Hypertension and Arterial Stiffness. Hypertension 2018, 72, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Gronewold, J.; Kropp, R.; Lehmann, N.; Stang, A.; Mahabadi, A.A.; Kalsch, H.; Weimar, C.; Dichgans, M.; Budde, T.; Moebus, S.; et al. Cardiovascular Risk and Atherosclerosis Progression in Hypertensive Persons Treated to Blood Pressure Targets. Hypertension 2019, 74, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Bidani, A.K.; Griffin, K.A. Pathophysiology of hypertensive renal damage: Implications for therapy. Hypertension 2004, 44, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Mitakou, S.; Halabalaki, M. Traditional uses, phytochemistry and pharmacology of Chios mastic gum (Pistacia lentiscus var. Chia, Anacardiaceae): A review. J. Ethnopharmacol. 2020, 254, 112485. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Mitakou, S.; Paraschos, S.; Efentakis, P.; Magiatis, P.; Kaklamanis, L.; Halabalaki, M.; Skaltsounis, L.; Iliodromitis, E.K. “Pistacia lentiscus L.” reduces the infarct size in normal fed anesthetized rabbits and possess antiatheromatic and hypolipidemic activity in cholesterol fed rabbits. Phytomedicine 2016, 23, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Kumar, P.; Deshmukh, R.R.; Bishayee, A.; Kumar, S. Pentacyclic triterpenes: New tools to fight metabolic syndrome. Phytomedicine 2018, 50, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Monserrat-Mesquida, M.; Pinya, S.; Ferriol, P.; Tejada, S. Hypotensive Effects of the Triterpene Oleanolic Acid for Cardiovascular Prevention. Curr. Mol. Pharmacol. 2021, 14, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Gioxari, A.; Amerikanou, C.; Valsamidou, E.; Kleftaki, S.A.; Tzavara, C.; Kalaitzopoulou, A.; Stergiou, I.; Smyrnioudis, I.; Kaliora, A.C. Chios mastiha essential oil exhibits antihypertensive, hypolipidemic and anti-obesity effects in metabolically unhealthy adults—A randomized controlled trial. Pharmacol. Res. 2023, 194, 106821. [Google Scholar] [CrossRef] [PubMed]
- Gortzi, O.; Rovoli, M.; Katsoulis, K.; Graikou, K.; Karagkini, D.A.; Stagos, D.; Kouretas, D.; Tsaknis, J.; Chinou, I. Study of Stability, Cytotoxic and Antimicrobial Activity of Chios Mastic Gum Fractions (Neutral, Acidic) after Encapsulation in Liposomes. Foods 2022, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Textor, S.C.; Lerman, L. Renovascular hypertension and ischemic nephropathy. Am. J. Hypertens. 2010, 23, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Bruckert, C.; Matsushita, K.; Mroueh, A.; Amissi, S.; Auger, C.; Houngue, U.; Remila, L.; Chaker, A.B.; Park, S.H.; Algara-Suarez, P.; et al. Empagliflozin prevents angiotensin II-induced hypertension related micro and macrovascular endothelial cell activation and diastolic dysfunction in rats despite persistent hypertension: Role of endothelial SGLT1 and 2. Vasc. Pharmacol. 2022, 146, 107095. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Chan, V.; Brown, L. The DOCA-Salt Hypertensive Rat as a Model of Cardiovascular Oxidative and Inflammatory Stress. Curr. Cardiol. Rev. 2010, 6, 291–297. [Google Scholar] [CrossRef]
- Jama, H.A.; Muralitharan, R.R.; Xu, C.; O’Donnell, J.A.; Bertagnolli, M.; Broughton, B.R.S.; Head, G.A.; Marques, F.Z. Rodent models of hypertension. Br. J. Pharmacol. 2022, 179, 918–937. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.C.; Lazartigues, E.; Sharma, R.V.; Davisson, R.L. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ. Res. 2004, 95, 210–216. [Google Scholar] [CrossRef]
- Tzani, A.I.; Doulamis, I.P.; Konstantopoulos, P.S.; Pasiou, E.D.; Daskalopoulou, A.; Iliopoulos, D.C.; Georgiadis, I.V.; Kavantzas, N.; Kourkoulis, S.K.; Perrea, D.N. Chios mastic gum decreases renin levels and ameliorates vascular remodeling in renovascular hypertensive rats. Biomed. Pharmacother. 2018, 105, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G.; Group NCRRGW. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- Olivera, S.; Graham, D. Sex differences in preclinical models of hypertension. J. Hum. Hypertens. 2023, 37, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; McNeill, J.H. To scale or not to scale: The principles of dose extrapolation. Br. J. Pharmacol. 2009, 157, 907–921. [Google Scholar] [CrossRef]
- Svingou, D.; Mikropoulou, E.V.; Pachi, V.K.; Smyrnioudis, I.; Halabalaki, M. Chios mastic gum: A validated method towards authentication. J. Food Compos. Anal. 2023, 115, 104997. [Google Scholar] [CrossRef]
- Efentakis, P.; Molitor, M.; Kossmann, S.; Bochenek, M.L.; Wild, J.; Lagrange, J.; Finger, S.; Jung, R.; Karbach, S.; Schafer, K.; et al. Tubulin-folding cofactor E deficiency promotes vascular dysfunction by increased endoplasmic reticulum stress. Eur. Heart J. 2022, 43, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef] [PubMed]
- Guijas, C.; Montenegro-Burke, J.R.; Domingo-Almenara, X.; Palermo, A.; Warth, B.; Hermann, G.; Koellensperger, G.; Huan, T.; Uritboonthai, W.; Aisporna, A.E.; et al. METLIN: A Technology Platform for Identifying Knowns and Unknowns. Anal. Chem. 2018, 90, 3156–3164. [Google Scholar] [CrossRef] [PubMed]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [PubMed]
- Efentakis, P.; Lamprou, S.; Makridakis, M.; Barla, I.; Nikolaou, P.E.; Christodoulou, A.; Dimitriou, C.; Kostomitsopoulos, N.; Ntanasis-Stathopoulos, I.; Theochari, I.; et al. Mineralocorticoid Receptor Pathway Is a Key Mediator of Carfilzomib-induced Nephrotoxicity: Preventive Role of Eplerenone. Hemasphere 2022, 6, e791. [Google Scholar] [CrossRef] [PubMed]
- Efentakis, P.; Kremastiotis, G.; Varela, A.; Nikolaou, P.E.; Papanagnou, E.D.; Davos, C.H.; Tsoumani, M.; Agrogiannis, G.; Konstantinidou, A.; Kastritis, E.; et al. Molecular mechanisms of carfilzomib-induced cardiotoxicity in mice and the emerging cardioprotective role of metformin. Blood 2019, 133, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Coffman, T.M.; Crowley, S.D. Kidney in hypertension: Guyton redux. Hypertension 2008, 51, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Luo, Y.; Zhang, W.; He, Y.; Dai, S.; Zhang, R.; Huang, Y.; Bernatchez, P.; Giordano, F.J.; Shadel, G.; et al. Endothelial-specific expression of mitochondrial thioredoxin improves endothelial cell function and reduces atherosclerotic lesions. Am. J. Pathol. 2007, 170, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, R.G.; Dass, C.R.; Sun, L.Q.; Chesterman, C.N.; Khachigian, L.M. Transcription factor Egr-1 supports FGF-dependent angiogenesis during neovascularization and tumor growth. Nat. Med. 2003, 9, 1026–1032. [Google Scholar] [CrossRef]
- Le Noble, F.A.C.; Mourad, J.J.; Levy, B.I.; Struijker-Boudier, H.A.J. VEGF (Vascular Endothelial Growth Factor) Inhibition and Hypertension: Does Microvascular Rarefaction Play a Role? Hypertension 2023, 80, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.S.; Wachtell, K.; Papademetriou, V.; Olsen, M.H.; Devereux, R.B.; Fyhrquist, F.; Ibsen, H.; Kjeldsen, S.E.; Dahlof, B. Cardiovascular morbidity and mortality in hypertensive patients with lower versus higher risk: A LIFE substudy. Hypertension 2005, 46, 492–499. [Google Scholar] [CrossRef]
- Kawada, N.; Imai, E.; Karber, A.; Welch, W.J.; Wilcox, C.S. A mouse model of angiotensin II slow pressor response: Role of oxidative stress. J. Am. Soc. Nephrol. 2002, 13, 2860–2868. [Google Scholar] [CrossRef]
- Goel, M.; Varandani, R.S.; Okwuosa, T.M. Resistance to antihypertensive drugs targeting Renin-Angiotensin-Aldosterone-System in cancer patients: A case series. Cardiooncology 2020, 6, 15. [Google Scholar] [CrossRef]
- Basting, T.; Lazartigues, E. DOCA-Salt Hypertension: An Update. Curr. Hypertens. Rep. 2017, 19, 32. [Google Scholar] [CrossRef]
- Yang, R.; Smolders, I.; Dupont, A.G. Blood pressure and renal hemodynamic effects of angiotensin fragments. Hypertens. Res. 2011, 34, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, P. Monocytes as immune targets in arterial hypertension. Br. J. Pharmacol. 2019, 176, 1966–1977. [Google Scholar] [CrossRef]
- Zhang, R.M.; McNerney, K.P.; Riek, A.E.; Bernal-Mizrachi, C. Immunity and Hypertension. Acta Physiol. 2021, 231, e13487. [Google Scholar] [CrossRef] [PubMed]
- DeBerge, M.; Chaudhary, R.; Schroth, S.; Thorp, E.B. Immunometabolism at the Heart of Cardiovascular Disease. JACC Basic. Transl. Sci. 2023, 8, 884–904. [Google Scholar] [CrossRef] [PubMed]
- Arifin, S.A.; Falasca, M. Lysophosphatidylinositol Signalling and Metabolic Diseases. Metabolites 2016, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Lerman, A.; Lerman, L.O. Enhancing Mitochondrial Health to Treat Hypertension. Curr. Hypertens. Rep. 2018, 20, 89. [Google Scholar] [CrossRef] [PubMed]
- Siasos, G.; Tsigkou, V.; Kosmopoulos, M.; Theodosiadis, D.; Simantiris, S.; Tagkou, N.M.; Tsimpiktsioglou, A.; Stampouloglou, P.K.; Oikonomou, E.; Mourouzis, K.; et al. Mitochondria and cardiovascular diseases-from pathophysiology to treatment. Ann. Transl. Med. 2018, 6, 256. [Google Scholar] [CrossRef] [PubMed]
- Eckenstaler, R.; Hauke, M.; Benndorf, R.A. A current overview of RhoA, RhoB, and RhoC functions in vascular biology and pathology. Biochem. Pharmacol. 2022, 206, 115321. [Google Scholar] [CrossRef]
- Wang, R.; Guo, Y.; Li, L.; Luo, M.; Peng, L.; Lv, D.; Cheng, Z.; Xue, Q.; Wang, L.; Huang, J. Role of thioredoxin-interacting protein in mediating endothelial dysfunction in hypertension. Genes Dis. 2022, 9, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Huang, M.J.; Chen, X.N.; Wu, L.L.; Li, Q.G.; Hong, Q.; Wu, J.; Li, F.; Chen, L.M.; Dong, Y.; et al. Transient upregulation of EGR1 signaling enhances kidney repair by activating SOX9(+) renal tubular cells. Theranostics 2022, 12, 5434–5450. [Google Scholar] [CrossRef] [PubMed]
- Van der Feen, D.E.; Dickinson, M.G.; Bartelds, B.; Borgdorff, M.A.; Sietsma, H.; Levy, M.; Berger, R.M. Egr-1 identifies neointimal remodeling and relates to progression in human pulmonary arterial hypertension. J. Heart Lung Transplant. 2016, 35, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Andoh, T.; Chock, P.B.; Chiueh, C.C. The roles of thioredoxin in protection against oxidative stress-induced apoptosis in SH-SY5Y cells. J. Biol. Chem. 2002, 277, 9655–9660. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Efentakis, P.; Frenis, K.; Daiber, A.; Schulz, R. Thiol-based redox-active proteins as cardioprotective therapeutic agents in cardiovascular diseases. Basic Res. Cardiol. 2021, 116, 44. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Guo, H.; Yu, H.; Chen, Y.; Xu, H.; Zhao, G. The Role of the Transcription Factor EGR1 in Cancer. Front. Oncol. 2021, 11, 642547. [Google Scholar] [CrossRef] [PubMed]
- Neves, K.B.; Rios, F.J.; van der Mey, L.; Alves-Lopes, R.; Cameron, A.C.; Volpe, M.; Montezano, A.C.; Savoia, C.; Touyz, R.M. VEGFR (Vascular Endothelial Growth Factor Receptor) Inhibition Induces Cardiovascular Damage via Redox-Sensitive Processes. Hypertension 2018, 71, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Maki-Petaja, K.M.; McGeoch, A.; Yang, L.L.; Hubsch, A.; McEniery, C.M.; Meyer, P.A.R.; Mir, F.; Gajendragadkar, P.; Ramenatte, N.; Anandappa, G.; et al. Mechanisms Underlying Vascular Endothelial Growth Factor Receptor Inhibition-Induced Hypertension: The HYPAZ Trial. Hypertension 2021, 77, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Parikh, V.N.; Jin, R.C.; Rabello, S.; Gulbahce, N.; White, K.; Hale, A.; Cottrill, K.A.; Shaik, R.S.; Waxman, A.B.; Zhang, Y.Y.; et al. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: Results of a network bioinformatics approach. Circulation 2012, 125, 1520–1532. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef]
- Fu, J.; Han, Y.; Wang, J.; Liu, Y.; Zheng, S.; Zhou, L.; Jose, P.A.; Zeng, C. Irisin Lowers Blood Pressure by Improvement of Endothelial Dysfunction via AMPK-Akt-eNOS-NO Pathway in the Spontaneously Hypertensive Rat. J. Am. Heart Assoc. 2016, 5, e003433. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.F.; Liu, L.M.; Pan, C.S.; Wang, C.S.; Gao, Y.S.; Fan, J.Y.; Han, J.Y. Rhynchophylline Ameliorates Endothelial Dysfunction via Src-PI3K/Akt-eNOS Cascade in the Cultured Intrarenal Arteries of Spontaneous Hypertensive Rats. Front. Physiol. 2017, 8, 928. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Su, S.; Xin, M.; Zhang, Z.; Nan, X.; Li, Z.; Lu, D. Luteolin ameliorates hypoxia-induced pulmonary hypertension via regulating HIF-2alpha-Arg-NO axis and PI3K-AKT-eNOS-NO signaling pathway. Phytomedicine 2022, 104, 154329. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Su, S.; Xie, X.; Yang, Z.; Li, Z.; Lu, D. Tsantan Sumtang, a traditional Tibetan medicine, protects pulmonary vascular endothelial function of hypoxia-induced pulmonary hypertension rats through AKT/eNOS signaling pathway. J. Ethnopharmacol. 2024, 320, 117436. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Mita, S.; Yoshida, K.; Honda, T.; Kobayashi, T.; Hara, K.; Nakano, S.; Tsubokou, Y.; Matsuoka, H. Celiprolol activates eNOS through the PI3K-Akt pathway and inhibits VCAM-1 Via NF-kappaB induced by oxidative stress. Hypertension 2003, 42, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, W.; Cai, W.W.; Wang, P.J.; Zhang, N.; Yu, C.P.; Wang, D.L.; Liu, B.C.; Sun, W. Telmisartan attenuates monocrotaline-induced pulmonary artery endothelial dysfunction through a PPAR gamma-dependent PI3K/Akt/eNOS pathway. Pulm. Pharmacol. Ther. 2014, 28, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y. Administration of telmisartan reduced systolic blood pressure and oxidative stress probably through the activation of PI3K/Akt/eNOS pathway and NO release in spontaneously hypertensive rats. Physiol. Res. 2013, 62, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, Y.; Tomida, T.; Matsui, H.; Ito, T.; Okumura, K. Decrease in renal medullary endothelial nitric oxide synthase of fructose-fed, salt-sensitive hypertensive rats. Hypertension 2002, 40, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Castrop, H.; Schweda, F.; Mizel, D.; Huang, Y.; Briggs, J.; Kurtz, A.; Schnermann, J. Permissive role of nitric oxide in macula densa control of renin secretion. Am. J. Physiol. Renal Physiol. 2004, 286, F848–F857. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, E.; Narkiewicz, K. Hypertension and Dyslipidemia: The Two Partners in Endothelium-Related Crime. Curr. Atheroscler. Rep. 2023, 25, 605–612. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efentakis, P.; Symeonidi, L.; Gianniou, D.D.; Mikropoulou, E.V.; Giardoglou, P.; Valakos, D.; Vatsellas, G.; Tsota, M.; Kostomitsopoulos, N.; Smyrnioudis, I.; et al. Antihypertensive Potential of Pistacia lentiscus var. Chia: Molecular Insights and Therapeutic Implications. Nutrients 2024, 16, 2152. https://doi.org/10.3390/nu16132152
Efentakis P, Symeonidi L, Gianniou DD, Mikropoulou EV, Giardoglou P, Valakos D, Vatsellas G, Tsota M, Kostomitsopoulos N, Smyrnioudis I, et al. Antihypertensive Potential of Pistacia lentiscus var. Chia: Molecular Insights and Therapeutic Implications. Nutrients. 2024; 16(13):2152. https://doi.org/10.3390/nu16132152
Chicago/Turabian StyleEfentakis, Panagiotis, Lydia Symeonidi, Despoina D. Gianniou, Eleni V. Mikropoulou, Panagiota Giardoglou, Dimitrios Valakos, Giannis Vatsellas, Maria Tsota, Nikolaos Kostomitsopoulos, Ilias Smyrnioudis, and et al. 2024. "Antihypertensive Potential of Pistacia lentiscus var. Chia: Molecular Insights and Therapeutic Implications" Nutrients 16, no. 13: 2152. https://doi.org/10.3390/nu16132152
APA StyleEfentakis, P., Symeonidi, L., Gianniou, D. D., Mikropoulou, E. V., Giardoglou, P., Valakos, D., Vatsellas, G., Tsota, M., Kostomitsopoulos, N., Smyrnioudis, I., Trougakos, I. P., Halabalaki, M., Dedoussis, G. V., & Andreadou, I. (2024). Antihypertensive Potential of Pistacia lentiscus var. Chia: Molecular Insights and Therapeutic Implications. Nutrients, 16(13), 2152. https://doi.org/10.3390/nu16132152







