The Use of Polysaccharide AOP30 from the Rhizome of Alpinia officinarum Hance to Alleviate Lipopolysaccharide-Induced Intestinal Epithelial Barrier Dysfunction and Inflammation via the TLR4/NfκB Signaling Pathway in Caco-2 Cell Monolayers
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. Cell Viability
2.4. Determination of Transepithelial Electrical Resistance
2.5. Determination of FITC–Dextran Paracellular Flux Analysis
2.6. Reverse Transcription-Quantitative Polymerase Chain Reaction Analysis
2.7. Western Blotting Analysis
2.8. ELISA Analysis
2.9. Statistical Analysis
3. Results
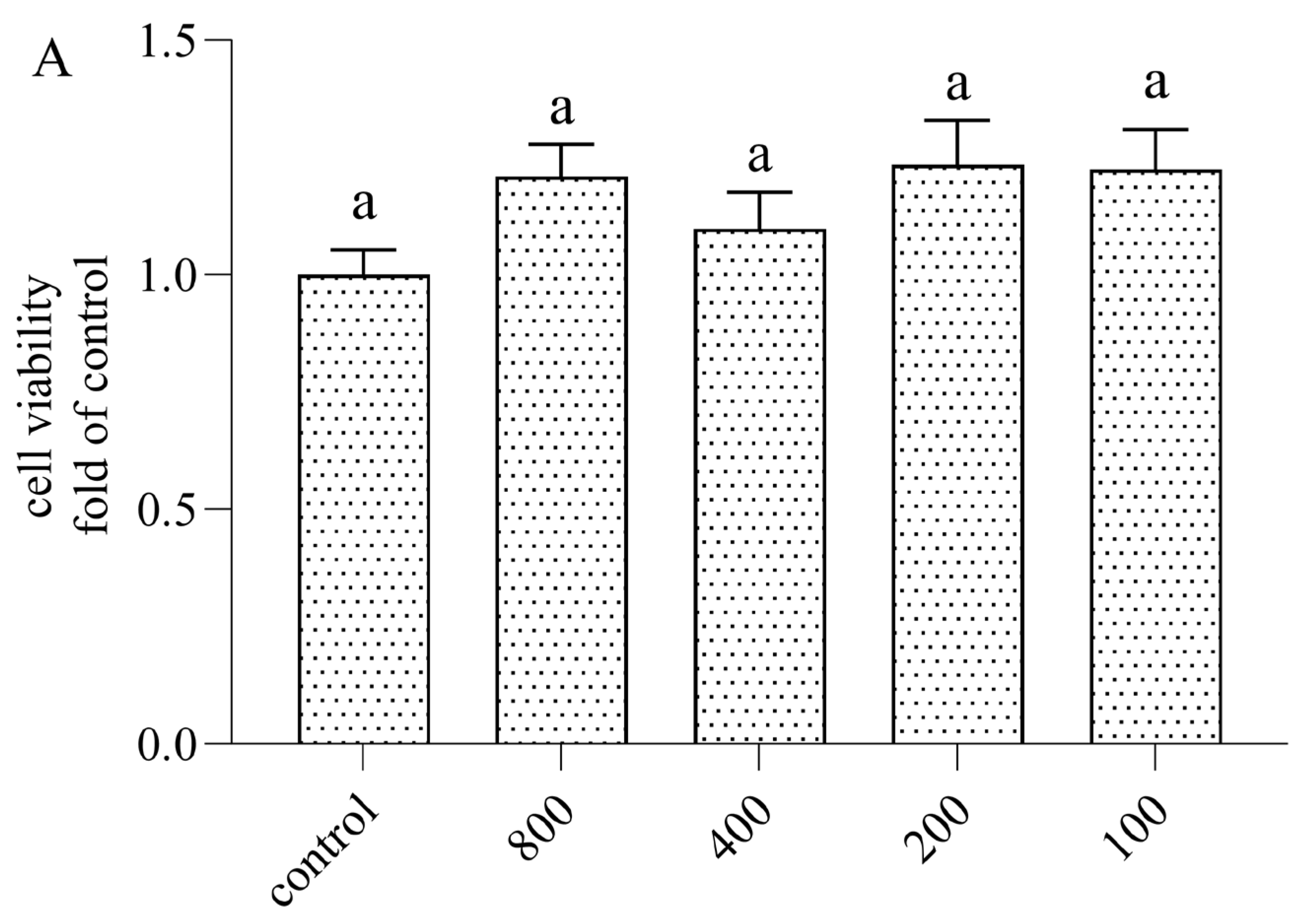
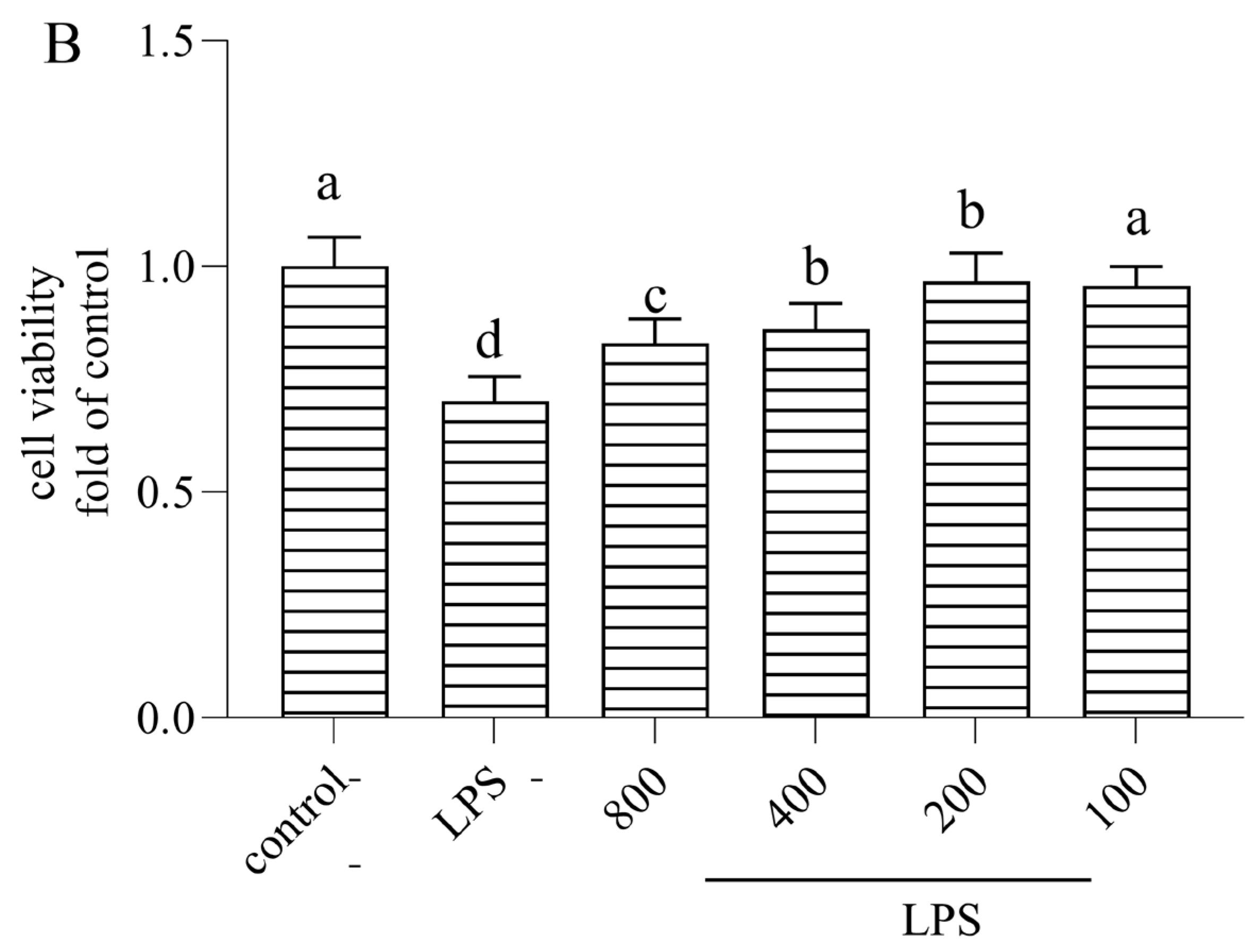
3.1. Cytotoxicity of AOP30 on Caco-2 Cells
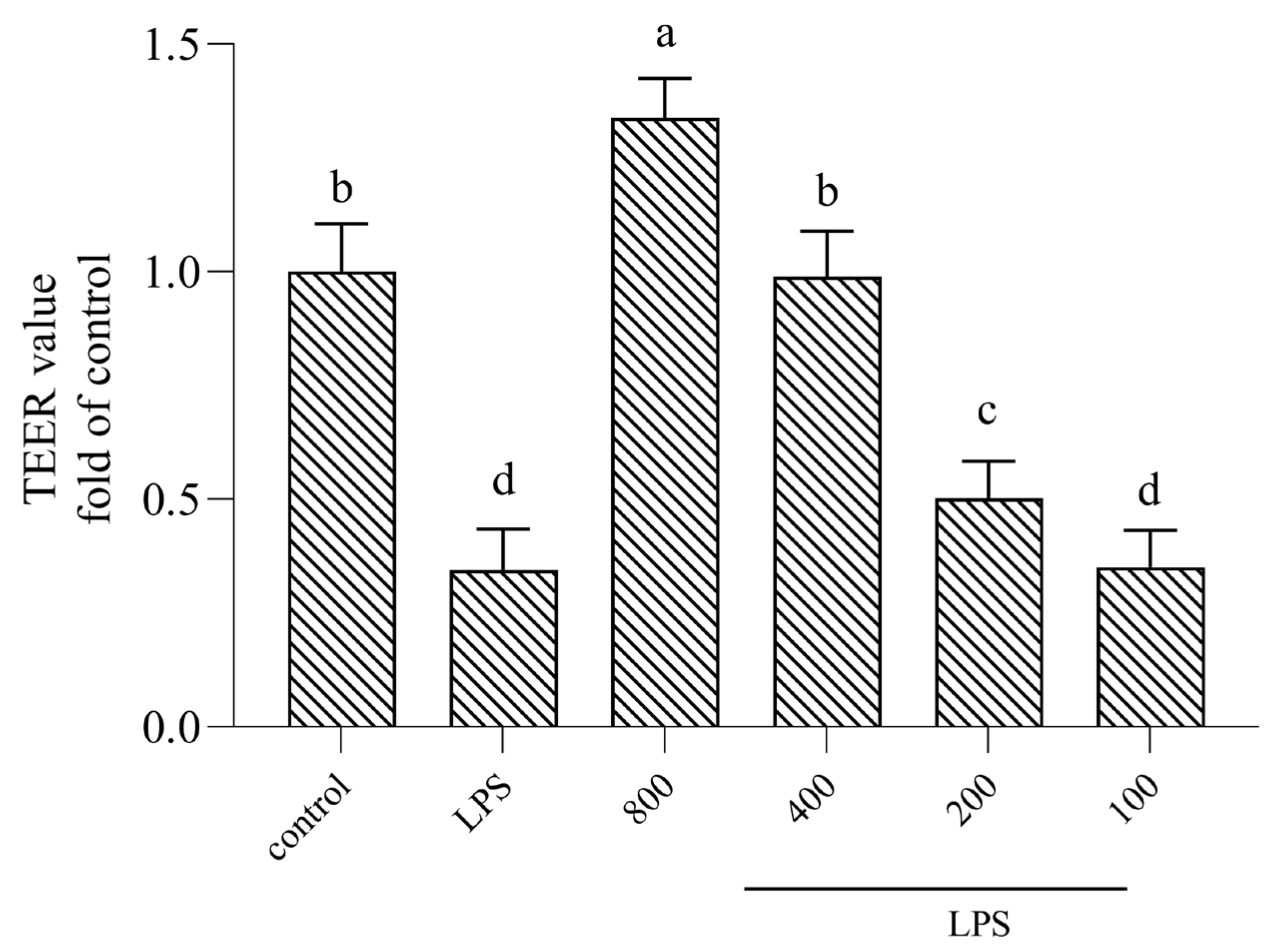
3.2. AOP30 Attenuates LPS-Induced Decrease in TEER Value in Caco-2 Cell Monolayer
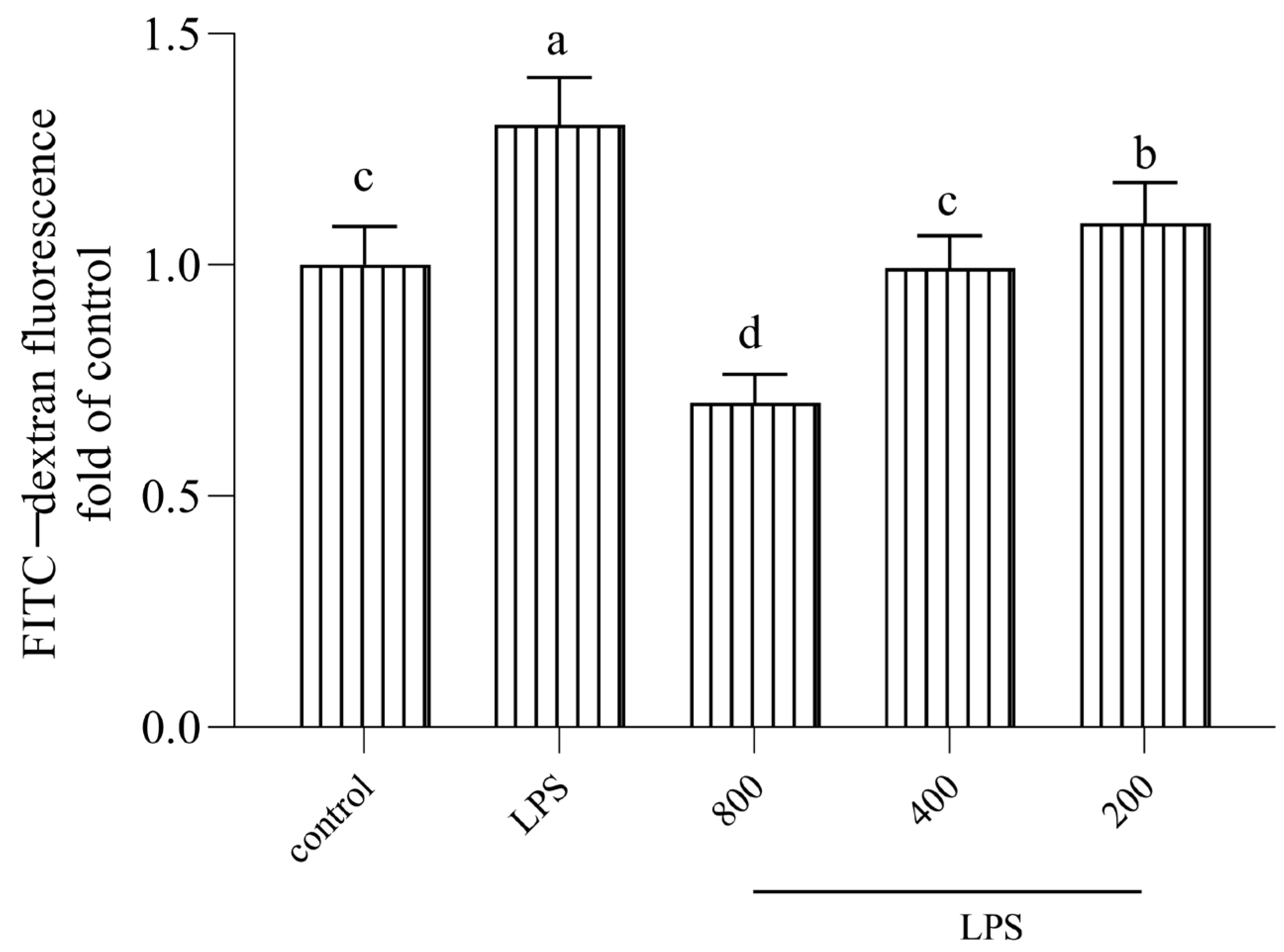
3.3. AOP30 Alleviates LPS-Induced Alterations in FITC–Dextran Paracellular Transport in Caco-2 Cell Monolayer
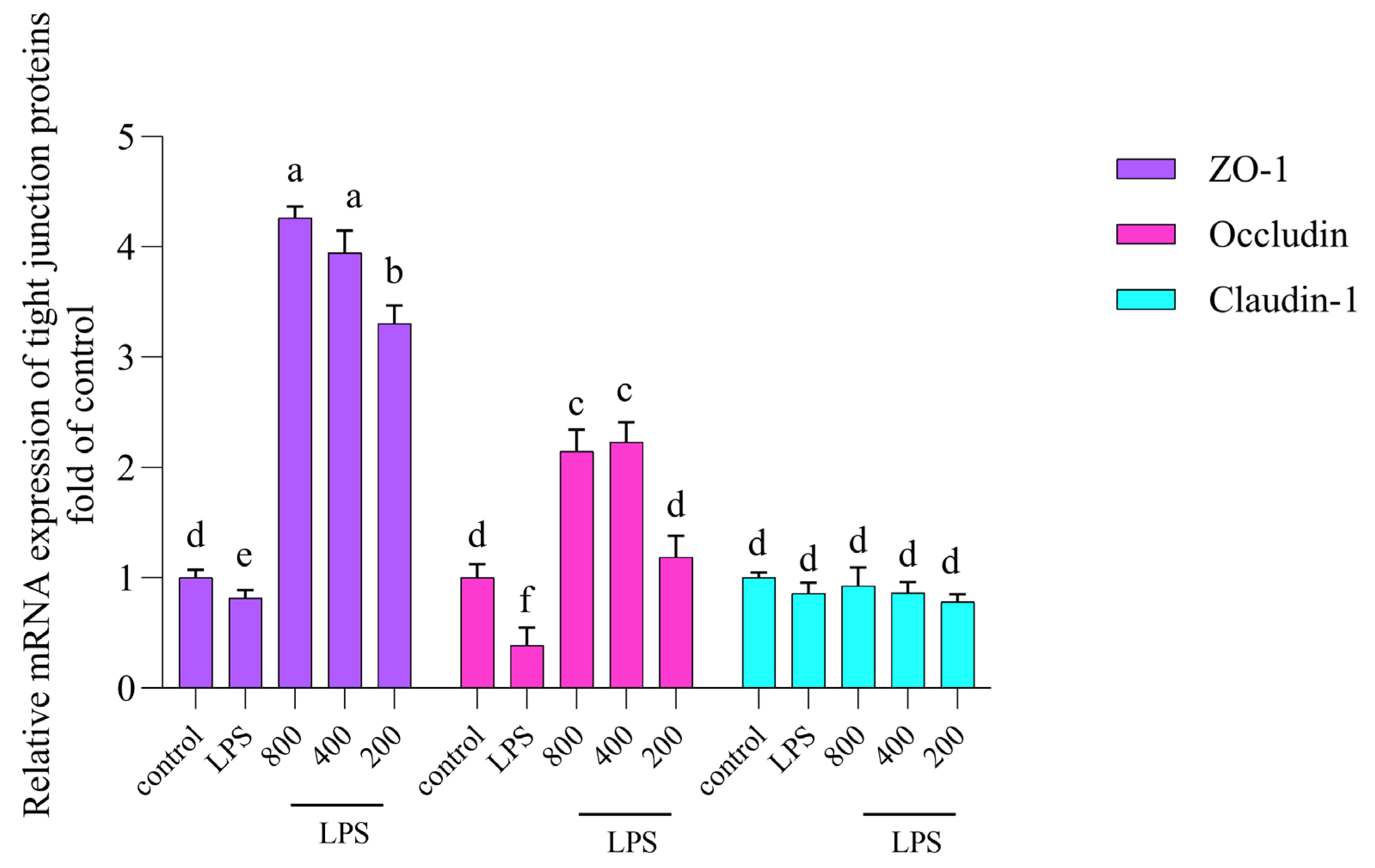
3.4. AOP30 Alleviates LPS-Induced Alterations in mRNA Expressions of Tight Junction Protein in Caco-2 Cells
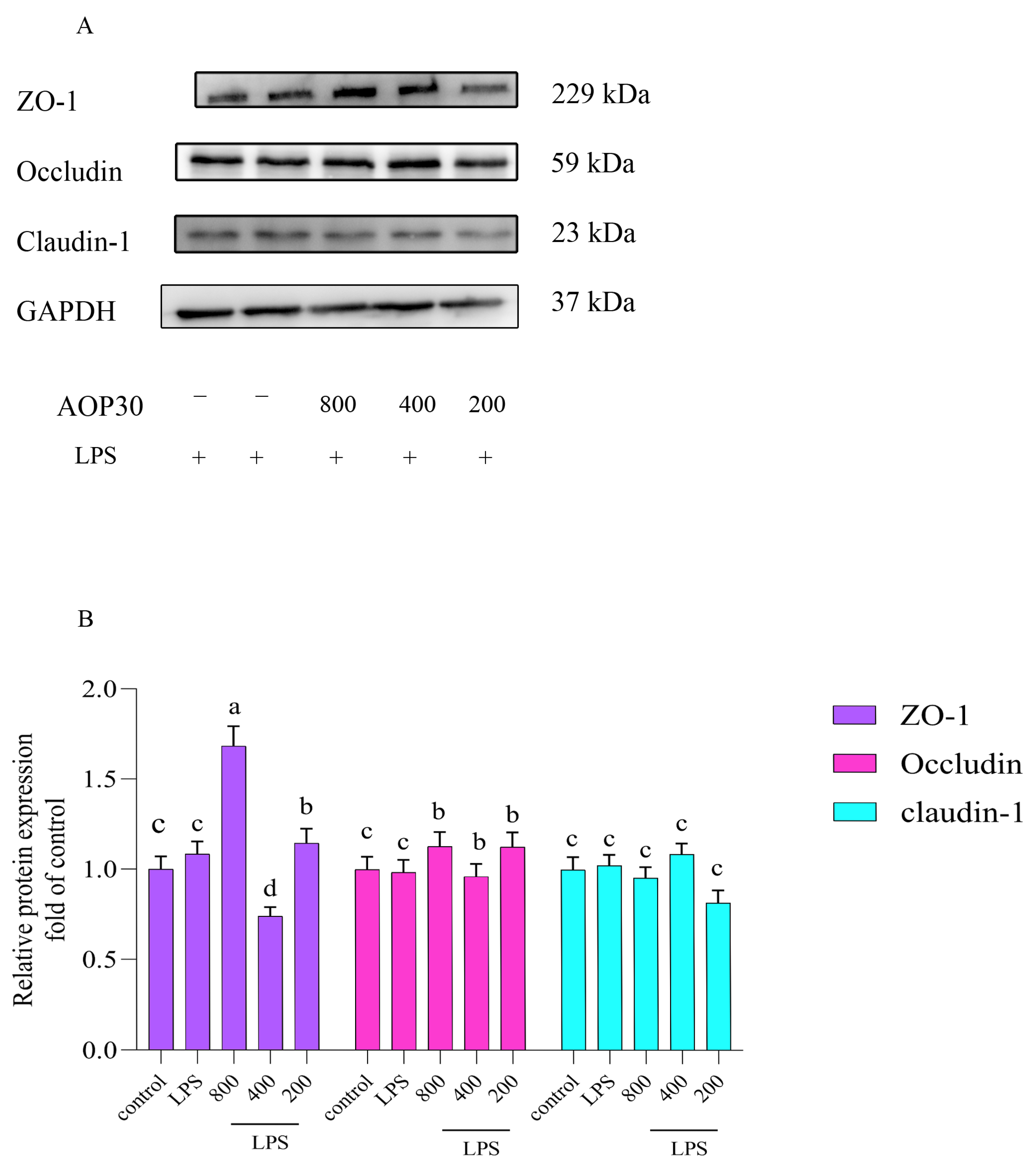
3.5. AOP30 Alleviates LPS-Induced Alterations of Tight Junction Protein Expressions in Caco-2 Cells
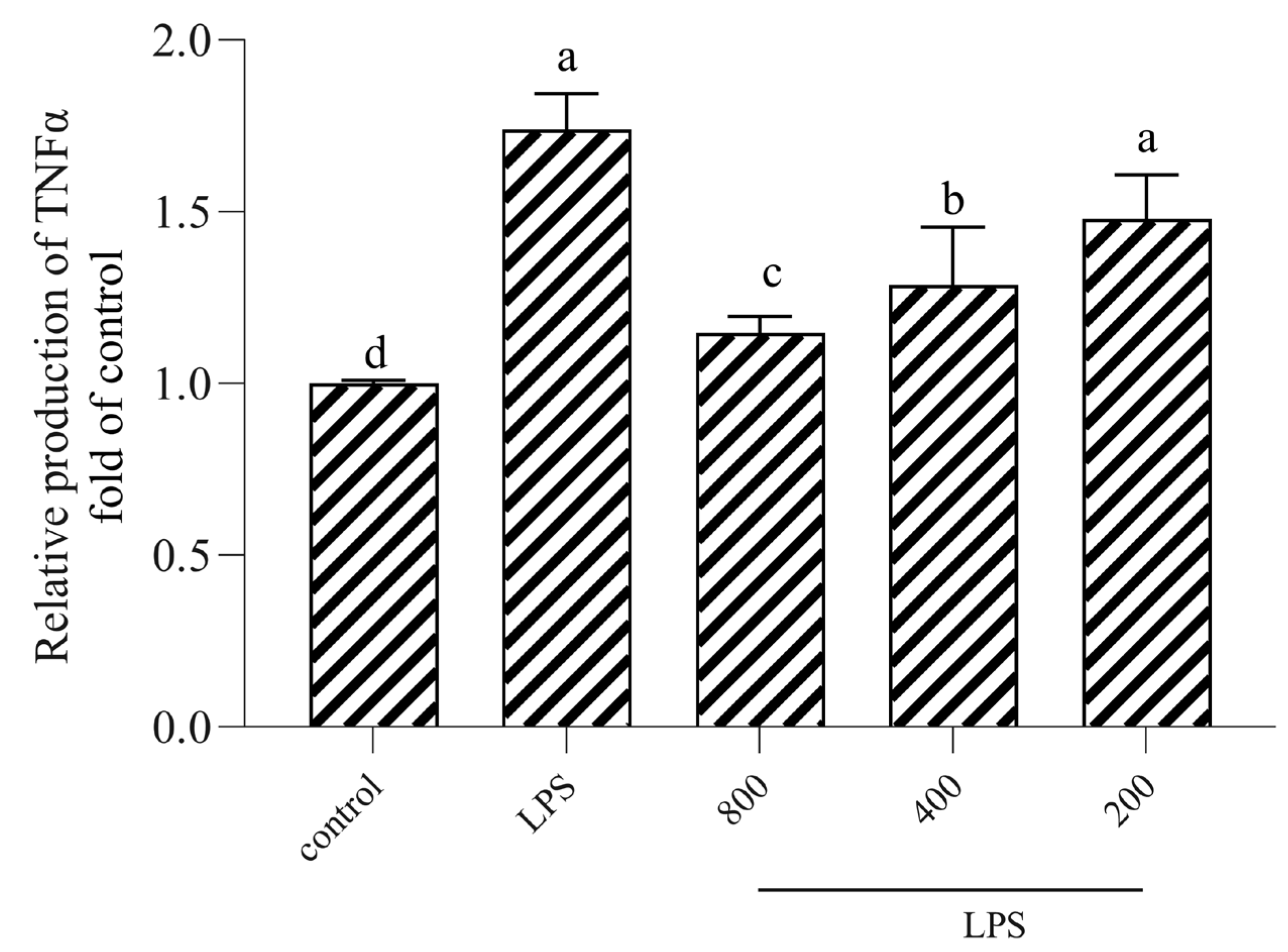
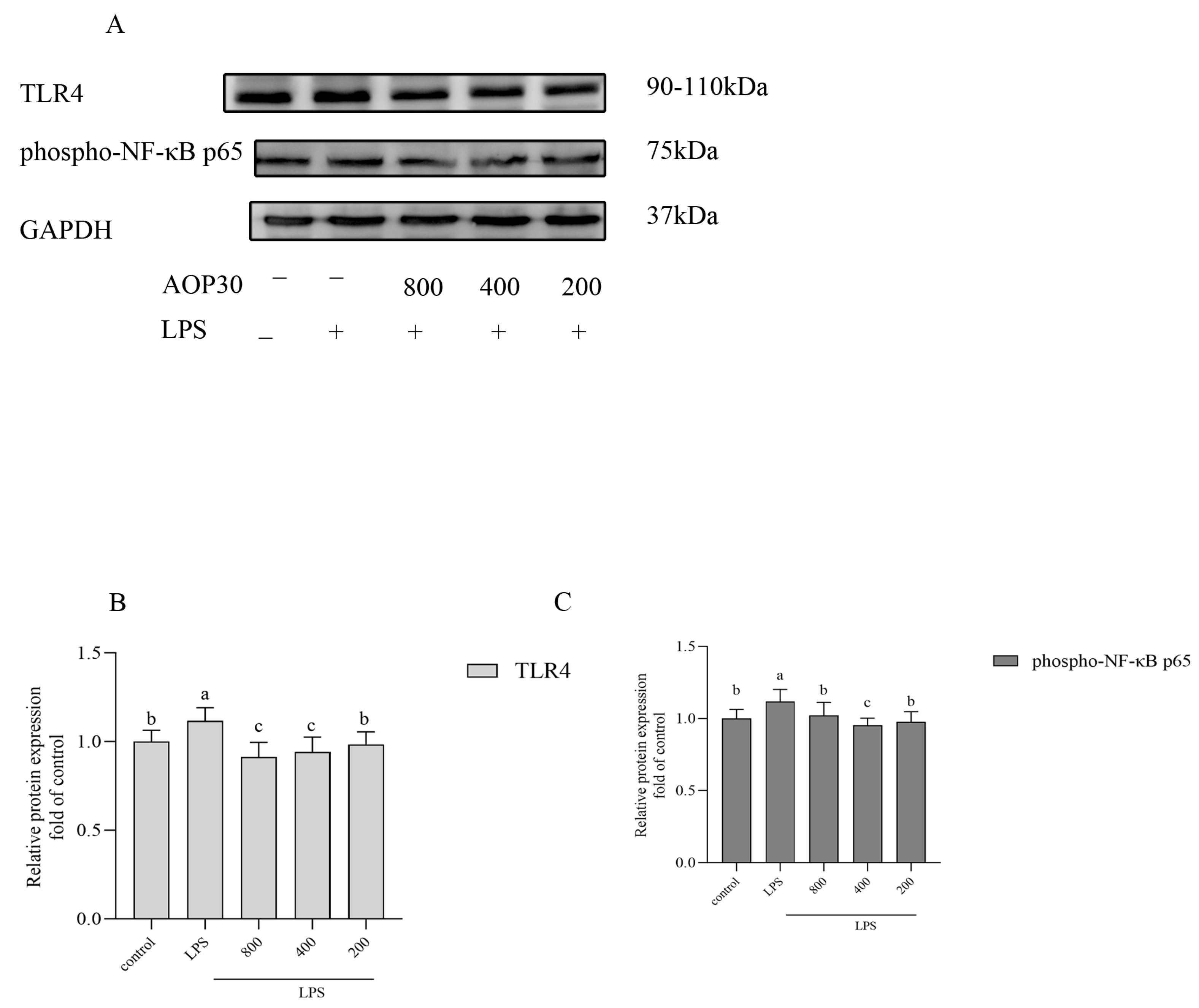
3.6. AOP30 Downregulates the Production of TNFα via TLR4/NFκB Signaling Pathway
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Upadhaya, S.D.; Kim, I.H. Importance of micronutrients in bone health of monogastric animals and techniques to improve the bioavailability of micronutrient supplements—A review. Asian-Australas J. Anim. Sci. 2020, 33, 1885. [Google Scholar] [CrossRef] [PubMed]
- Gieryńska, M.; Szulc-Dąbrowska, L.; Struzik, J.; Mielcarska, M.B.; Gregorczyk-Zboroch, K.P. Integrity of the intestinal barrier: The involvement of epithelial cells and microbiota—A mutual relationship. Animals 2022, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Nourse, J.L.; Pathak, M.M. How cells channel their stress: Interplay between piezo1 and the cytoskeleton. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–12. [Google Scholar]
- Zhang, B.; Guo, Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (zo-1) expression in weaning piglets. Br. J. Nutr. 2009, 102, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhang, C.; Qiu, J.; Wang, L.; Bao, J.; Wang, K.; Zhang, Y.; Chen, M.; Wan, J.; Su, H.; et al. Purification, structural characterization and anticancer activity of the novel polysaccharides from Rhynchosia minima root. Carbohydr. Polym. 2015, 132, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhang, C.; Hu, J.; He, M.; Bao, J.; Wang, K.; Li, P.; Chen, M.; Wan, J.; Su, H.; et al. Ultrasound-assisted extraction, antioxidant and anticancer activities of the polysaccharides from Rhynchosia minima root. Molecules 2015, 20, 20901–20911. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Hu, J.; He, M.; Zhang, Q.; Li, P.; Wan, J.; He, C. A-glucosidase inhibitory activity and structural characterization of polysaccharide fraction from Rhynchosia minima root. J. Funct. Foods 2017, 28, 76–82. [Google Scholar] [CrossRef]
- Yuan, J.; Che, S.; Ruan, Z.; Song, L.; Tang, R.; Zhang, L. Regulatory effects of flavonoids luteolin on bde-209-induced intestinal epithelial barrier damage in caco-2 cell monolayer model. Food Chem. Toxicol. 2021, 150, 112098. [Google Scholar] [CrossRef]
- Gao, G.; Zhou, J.; Jin, Y.; Wang, H.; Ding, Y.; Zhou, J.; Ke, L.; Rao, P.; Chong, P.H.; Wang, Q.; et al. Nanoparticles derived from porcine bone soup attenuate oxidative stress-induced intestinal barrier injury in caco-2 cell monolayer model. J. Funct. Foods 2021, 83, 104573. [Google Scholar] [CrossRef]
- Gong, S.; Zheng, J.; Zhang, J.; Wang, Y.; Xie, Z.; Wang, Y.; Han, J. Taxifolin ameliorates lipopolysaccharide-induced intestinal epithelial barrier dysfunction via attenuating nf-kappa b/mlck pathway in a caco-2 cell monolayer model. Food Res. Int. 2022, 158, 111502. [Google Scholar] [CrossRef]
- Alasmary, F.A.; Assirey, E.A.; El-Meligy, R.M.; Awaad, A.S.; El-Sawaf, L.A.; Allah, M.M.; Alqasoumi, S.I. Analysis of Alpinia officinarum hance, chemically and biologically. Saudi Pharm. J. 2019, 27, 1107–1112. [Google Scholar] [CrossRef]
- Jia, X.; Liu, G.; Huang, Y.; Li, Z.; Liu, X.; Wang, Z.; Li, R.; Song, B.; Zhong, S.J.F. Ultrasonic-assisted extraction, structural characteristics, and antioxidant activities of polysaccharides from Alpinia officinarum hance. Foods 2024, 13, 333. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Y.; Xiong, S.; Wu, M.; Li, B.; Ruan, Z.; Hu, X. Correction: Dietary l-tryptophan alleviated lps-induced intestinal barrier injury by regulating tight junctions in a caco-2 cell monolayer model. Food Funct. 2023, 14, 8031–8032. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhong, Y.; Wang, D.; Lu, Z. A simple colorimetric method for viable bacteria detection based on cell counting kit-8. Anal. Methods 2021, 13, 5211–5215. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Einspanier, R.; Schoen, J. Transepithelial electrical resistance (teer): A functional parameter to monitor the quality of oviduct epithelial cells cultured on filter supports. Histochem. Cell Biol. 2015, 144, 509–515. [Google Scholar] [CrossRef]
- Buccitelli, C.; Selbach, M. Mrnas, proteins and the emerging principles of gene expression control. Nat. Rev. Genet. 2020, 21, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Huang, F.; Zhang, R.; Dong, L.; Jia, X.; Liu, L.; Yi, Y.; Zhang, M. Longan pulp polysaccharides relieve intestinal injury in vivo and in vitro by promoting tight junction expression. Carbohydr. Polym. 2020, 229, 115475. [Google Scholar] [CrossRef]
- Li, F.; Du, P.; Yang, W.; Huang, D.; Nie, S.; Xie, M. Polysaccharide from the seeds of Plantago asiatica L. Alleviates nonylphenol induced intestinal barrier injury by regulating tight junctions in human caco-2 cell line. Int. J. Biol. Macromol. 2020, 164, 2134–2140. [Google Scholar]
- Stepanova, M.; Aherne, C.M. Adenosine in intestinal epithelial barrier function. Cells 2024, 13, 381. [Google Scholar] [CrossRef]
- Sugawara, T.; Furuse, K.; Otani, T.; Wakayama, T.; Furuse, M. Angulin-1 seals tricellular contacts independently of tricellulin and claudins. J. Cell Biol. 2021, 220, e202005062. [Google Scholar] [CrossRef]
- Bhat, A.A.; Syed, N.; Therachiyil, L.; Nisar, S.; Hashem, S.; Macha, M.A.; Yadav, S.K.; Krishnankutty, R.; Muralitharan, S.; Al-Naemi, H.; et al. Claudin-1, a double-edged sword in cancer. Int. J. Mol. Sci. 2020, 21, 569. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and zo-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef] [PubMed]
- González-Mariscal, L.; Betanzos, A.; Nava, P.; Jaramillo, B.E. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003, 81, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Krug, S.M.; Schulzke, J.D.; Fromm, M. Tight junction, selective permeability, and related diseases. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 166–176. [Google Scholar]
- Li, W.; Gao, M.; Han, T. Lycium barbarum polysaccharides ameliorate intestinal barrier dysfunction and inflammation through the mlck-mlc signaling pathway in caco-2 cells. Food Funct. 2020, 11, 3741–3748. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Li, S.; Chang, L.; Sun, P.; Lu, Y.; Yu, X.; Chen, S.; Wu, Z.; Xu, Z. Total polysaccharides of adlay bran (coix lachryma-jobi l.) improve tnf-α induced epithelial barrier dysfunction in caco-2 cells via inhibition of the inflammatory response. Food Funct. 2019, 10, 2906–2913. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.; Song, J.; Tan, X.; Sun, E.; Ning, Q.; et al. Scutellaria baicalensis georgi polysaccharide ameliorates dss-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, X.; Sun-Waterhouse, D.; Zhu, B.; You, L.; Hileuskaya, K. Polysaccharides from sargassum fusiforme after UV/H2O2 degradation effectively ameliorate dextran sulfate sodium-induced colitis. Food Funct. 2021, 12, 11747–11759. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Wang, Z.; Geng, Y. Pine pollen polysaccharides’ and sulfated polysaccharides’ effects on uc mice through modulation of cell tight junctions and ripk3-dependent necroptosis pathways. Molecules 2022, 27, 7682. [Google Scholar] [CrossRef]
- Zuo, T.; Cao, L.; Li, X.; Zhang, Q.; Xue, C.; Tang, Q. The squid ink polysaccharides protect tight junctions and adherens junctions from chemotherapeutic injury in the small intestinal epithelium of mice. Nutr. Cancer 2015, 67, 364–371. [Google Scholar] [CrossRef]
- Tian, B.; Wang, P.; Xu, T.; Cai, M.; Mao, R.; Huang, L.; Sun, P.; Yang, K. Ameliorating effects of hericium erinaceus polysaccharides on intestinal barrier injury in immunocompromised mice induced by cyclophosphamide. Food Funct. 2023, 14, 2921–2932. [Google Scholar] [CrossRef]
- Duan, Y.; Huang, J.; Sun, M.; Jiang, Y.; Wang, S.; Wang, L.; Yu, N.; Peng, D.; Wang, Y.; Chen, W.; et al. Poria cocos polysaccharide improves intestinal barrier function and maintains intestinal homeostasis in mice. Int. J. Biol. Macromol. 2023, 249, 125953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. Nf-κb signaling in inflammation and cancer. MedComm 2021, 2, 618–653. [Google Scholar] [CrossRef] [PubMed]
- Kocot, A.M.; Jarocka-Cyrta, E.; Drabińska, N. Overview of the importance of biotics in gut barrier integrity. Int. J. Mol. Sci. 2022, 23, 2896. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Furuse, M. Tight junction structure and function revisited. Trends Cell Biol. 2020, 30, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Khatib, K.; Guo, S.; Ye, D.; Youssef, M.; Ma, T.; Grothaus, J.S.; Ares, G.; Yuan, C.; Wood, D.R.; et al. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am. J. Physiol. Liver Physiol. 2011, 300, G1054–G1064. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Q.; Rogers, C.J.; Du, M.; Zhu, M.-J. Ampk improves gut epithelial differentiation and barrier function via regulating cdx2 expression. Cell Death Differ. 2017, 24, 819–831. [Google Scholar] [CrossRef]
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| ZO-1 | GAGGTAGAACGAGGCATCATCCC | CTCCAGAAGTCAGCACGGTCTC |
| Occludin | ACTTCGCCTGTGGATGACTTCAG | TTCTCTTTGACCTTCCTGCTCTTCC |
| Claudin-1 | AGGTACGAATTTGGTCAGGCTCTC | GGGACAGGAACAGCAAAGTAGGG |
| GAPDH | CACCCACTCCTCCACCTTTGAC | GTCCACCACCCTGTTGCTGTAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, X.; Huang, Y.; Liu, G.; Li, Z.; Tan, Q.; Zhong, S. The Use of Polysaccharide AOP30 from the Rhizome of Alpinia officinarum Hance to Alleviate Lipopolysaccharide-Induced Intestinal Epithelial Barrier Dysfunction and Inflammation via the TLR4/NfκB Signaling Pathway in Caco-2 Cell Monolayers. Nutrients 2024, 16, 2151. https://doi.org/10.3390/nu16132151
Jia X, Huang Y, Liu G, Li Z, Tan Q, Zhong S. The Use of Polysaccharide AOP30 from the Rhizome of Alpinia officinarum Hance to Alleviate Lipopolysaccharide-Induced Intestinal Epithelial Barrier Dysfunction and Inflammation via the TLR4/NfκB Signaling Pathway in Caco-2 Cell Monolayers. Nutrients. 2024; 16(13):2151. https://doi.org/10.3390/nu16132151
Chicago/Turabian StyleJia, Xuejing, Yun Huang, Guanghuo Liu, Zipeng Li, Qiwei Tan, and Saiyi Zhong. 2024. "The Use of Polysaccharide AOP30 from the Rhizome of Alpinia officinarum Hance to Alleviate Lipopolysaccharide-Induced Intestinal Epithelial Barrier Dysfunction and Inflammation via the TLR4/NfκB Signaling Pathway in Caco-2 Cell Monolayers" Nutrients 16, no. 13: 2151. https://doi.org/10.3390/nu16132151
APA StyleJia, X., Huang, Y., Liu, G., Li, Z., Tan, Q., & Zhong, S. (2024). The Use of Polysaccharide AOP30 from the Rhizome of Alpinia officinarum Hance to Alleviate Lipopolysaccharide-Induced Intestinal Epithelial Barrier Dysfunction and Inflammation via the TLR4/NfκB Signaling Pathway in Caco-2 Cell Monolayers. Nutrients, 16(13), 2151. https://doi.org/10.3390/nu16132151







