Anti-Inflammatory Activity of the Combination of Nobiletin and Docosahexaenoic Acid in Lipopolysaccharide-Stimulated RAW 264.7 Cells: A Potential Synergistic Anti-Inflammatory Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Griess Assay
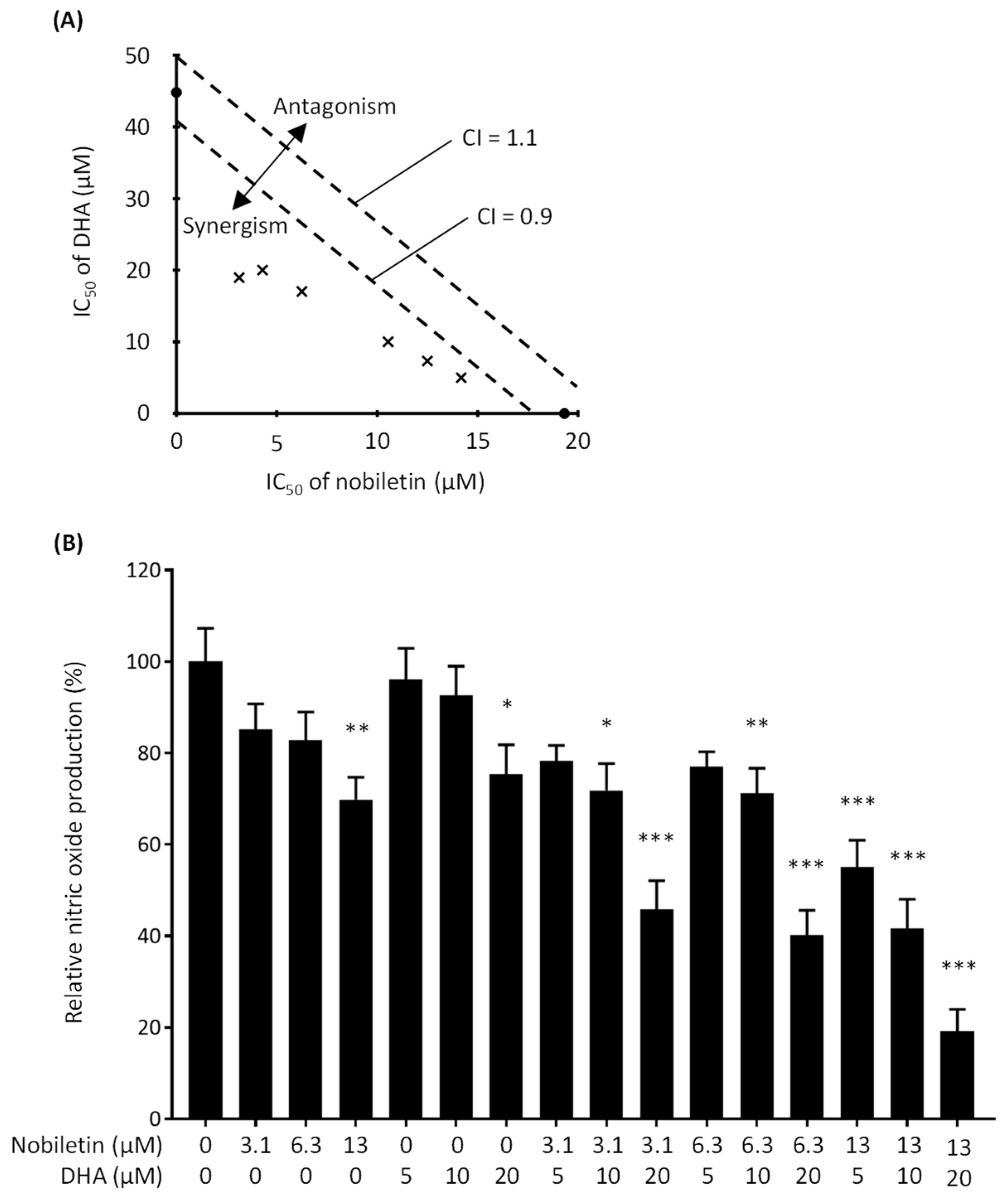
2.4. Combination Index (CI) Calculation
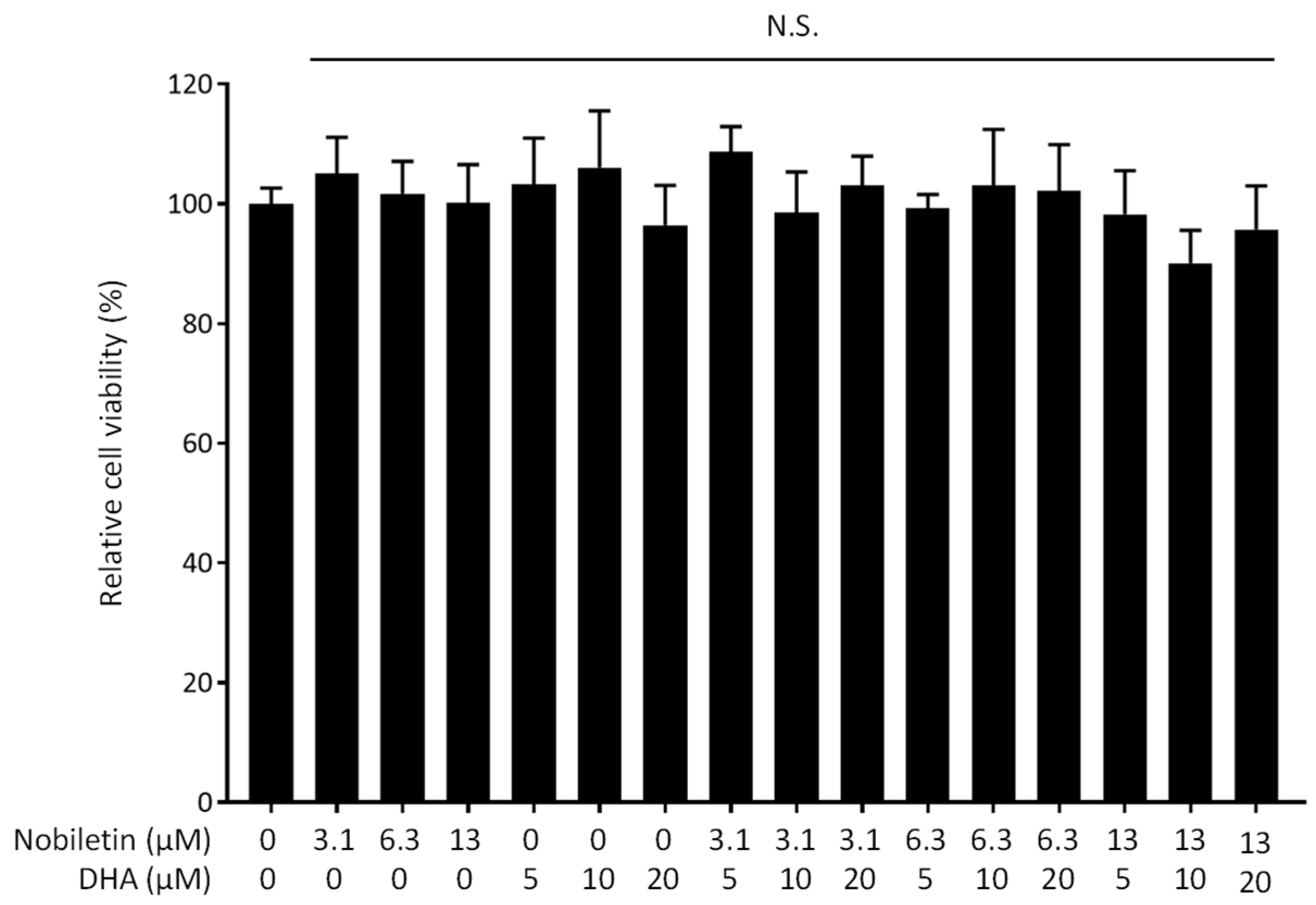
2.5. WST-8 Assay
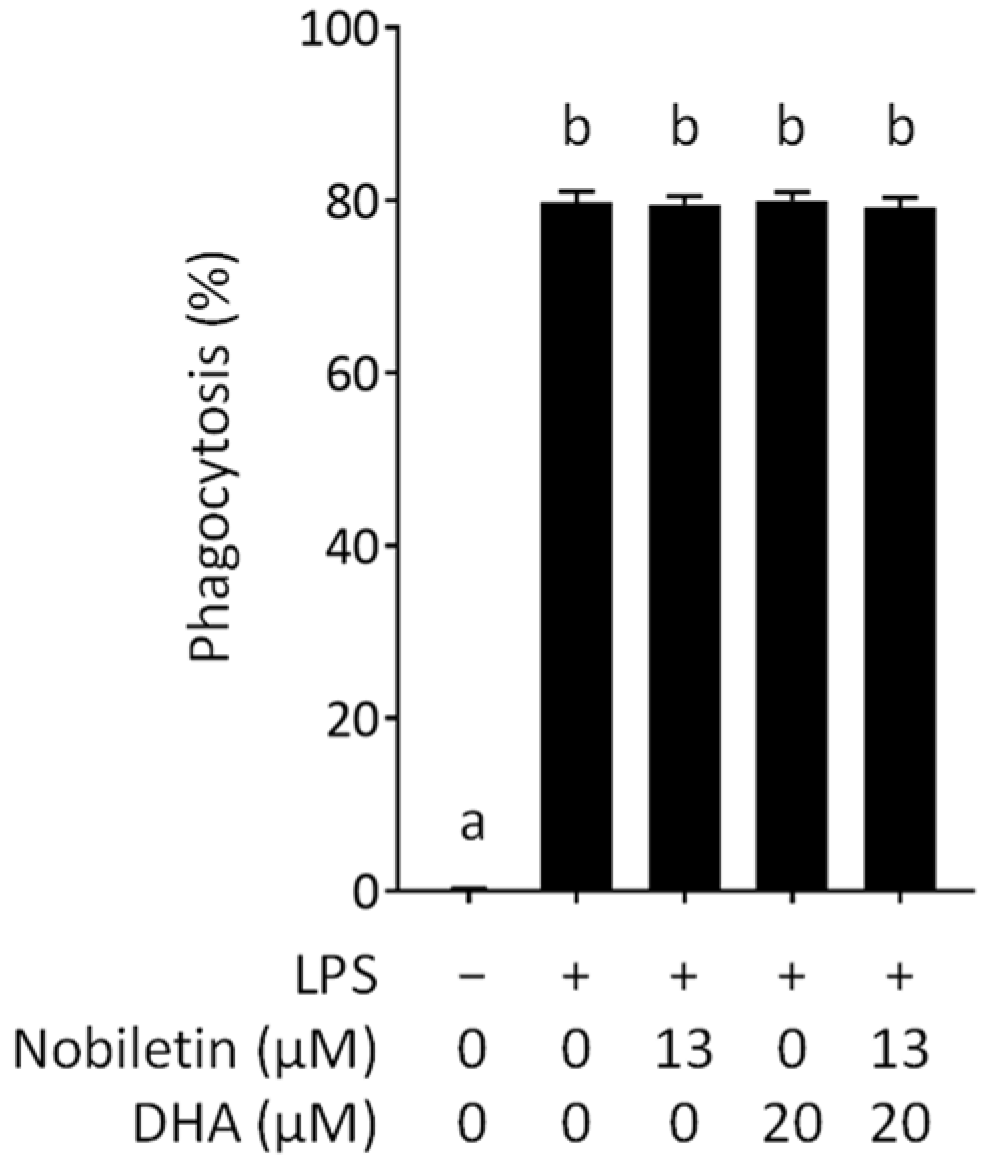
2.6. Phagocytosis Assay
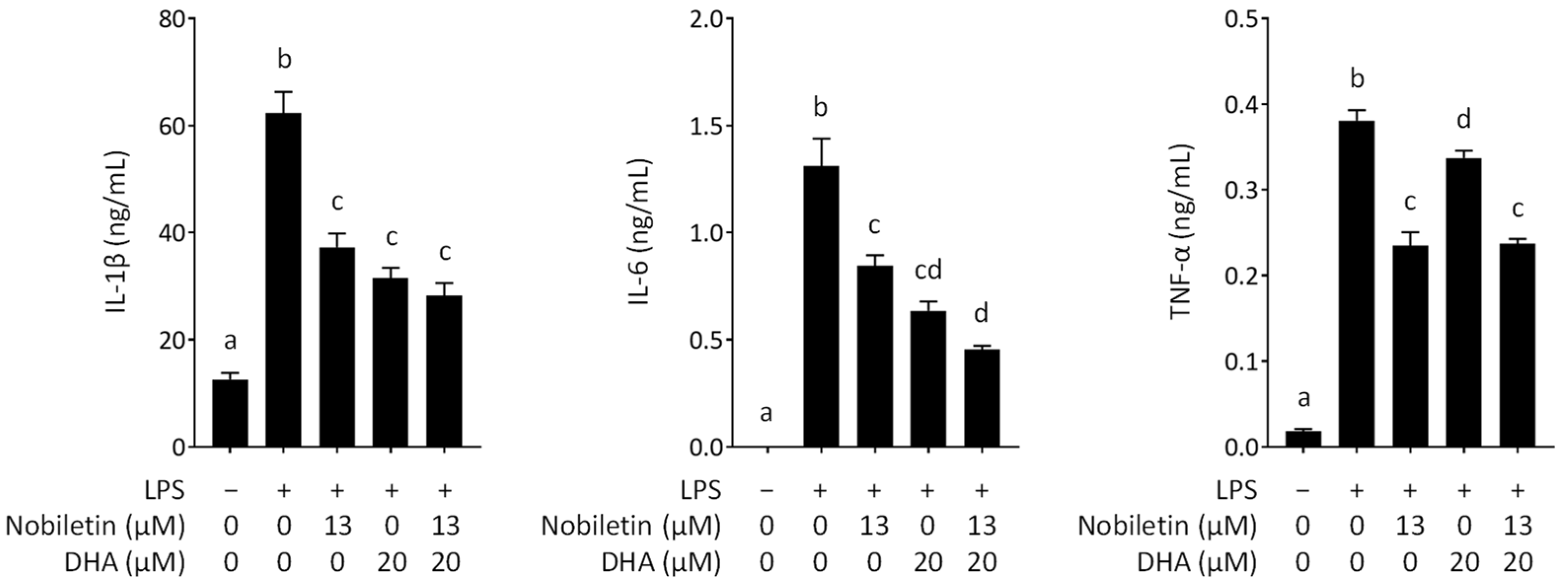
2.7. Cytokine Measurement
2.8. Real-Time RT-PCR
2.9. Immunostaining
2.10. Immunoblot Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Synergistic Inhibitory Effect of Nobiletin and DHA in Combination on Nitric Oxide Production by LPS-Stimulated RAW 264.7 Cells
3.2. Effect of Nobiletin and DHA in Combination on the Phagocytosis of LPS-Stimulated RAW 264.7 Cells
3.3. Inhibitory Effect of Nobiletin and DHA in Combination on the Secretion of Proinflammatory Cytokines from LPS-Stimulated RAW 264.7 Cells
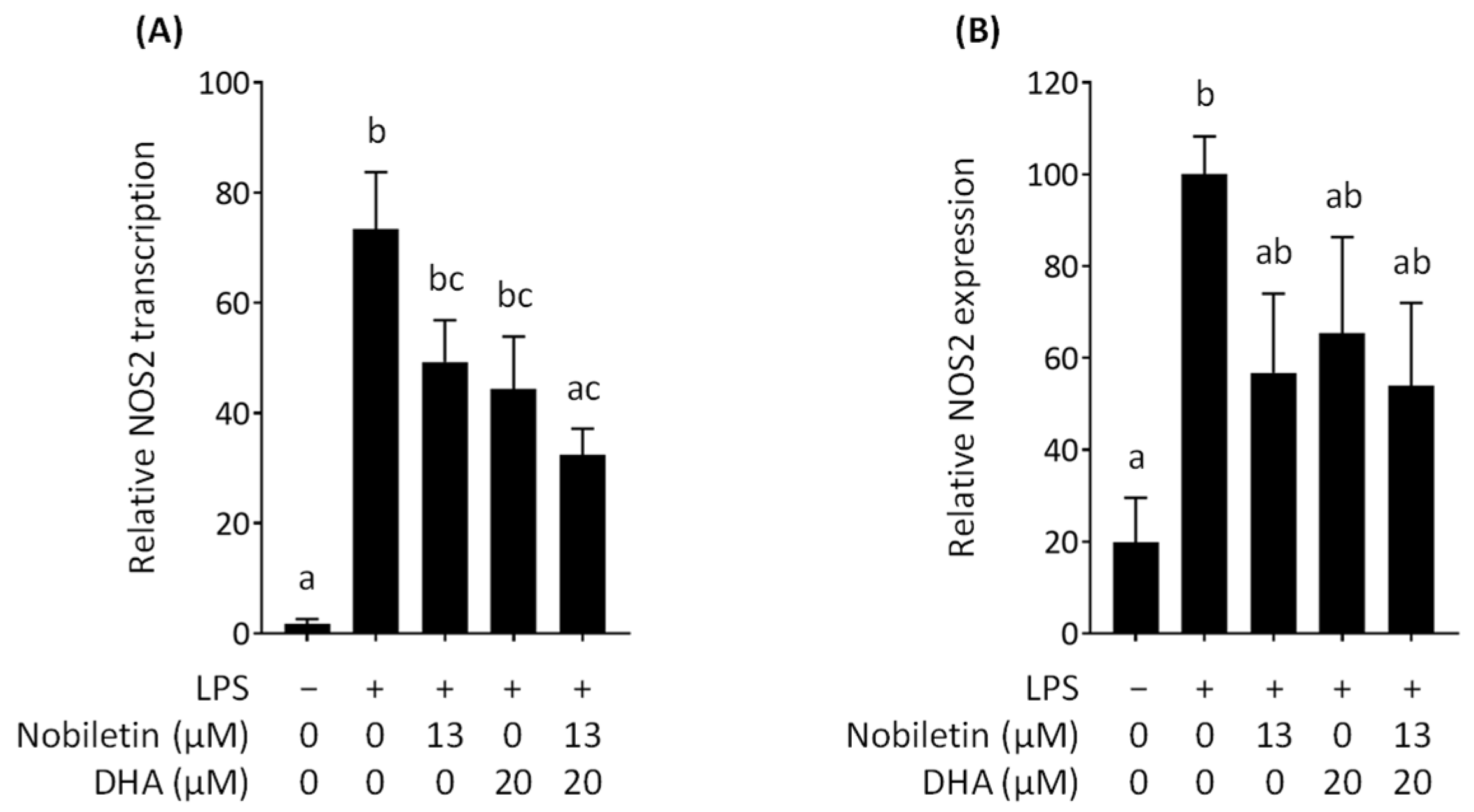
3.4. Effect of Nobiletin and DHA in Combination on NOS2 Expression in LPS-Stimulated RAW 264.7 Cells
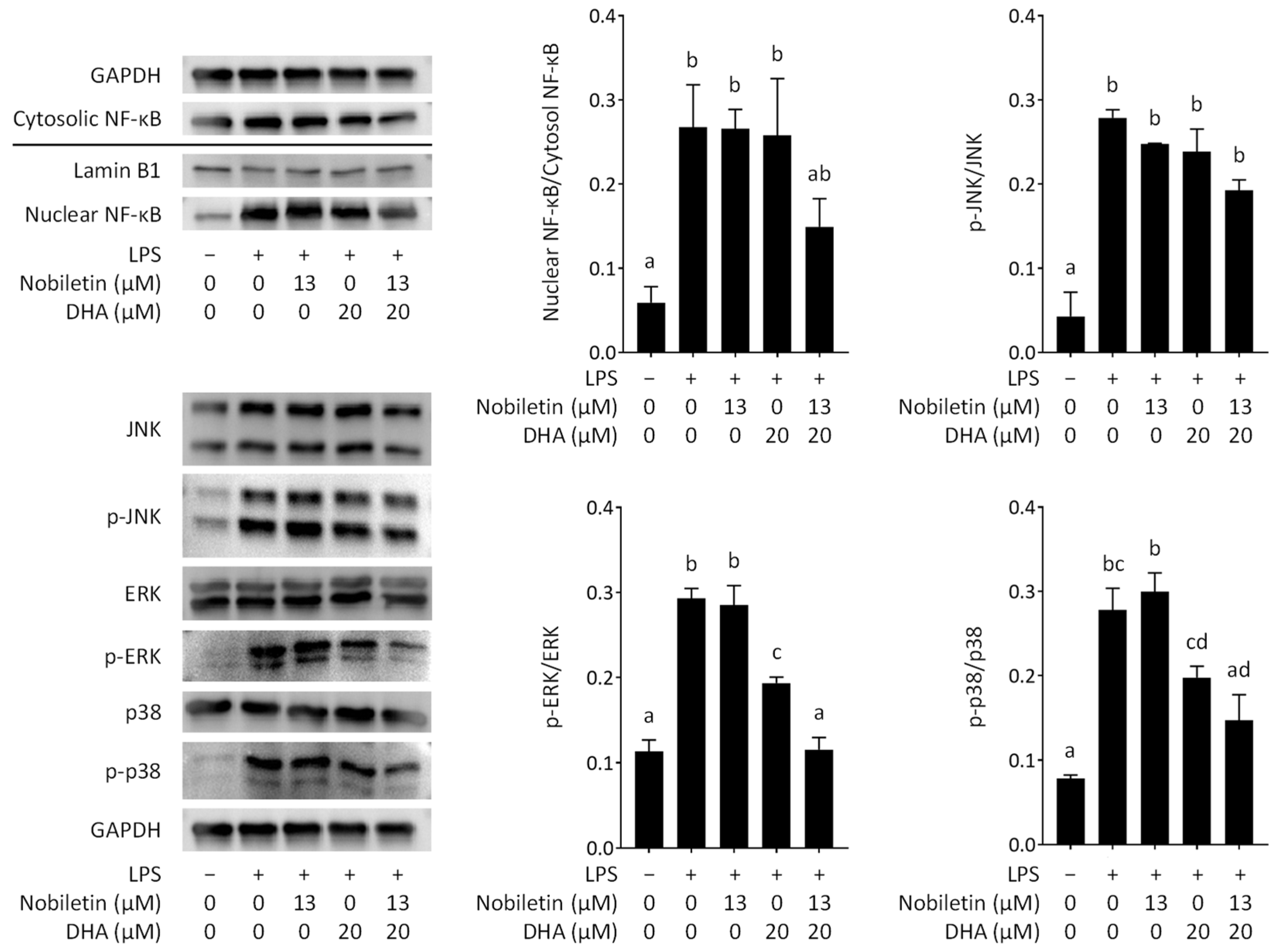
3.5. Effect of Nobiletin and DHA in Combination on Intracellular Signal Transduction in LPS-Stimulated RAW 264.7 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koh, T.J. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Di Liberto, D.; Vecchi, A.; La Manna, M.P.; Buracchi, C.; Caccamo, N.; Salerno, A.; Dieli, F.; Mantovani, A. Damping excessive inflammation and tissue damage in Mycobacterium tuberculosis infection by Toll IL-1 receptor 8/single Ig IL-1-related receptor, a negative regulator of IL-1/TLR signaling. J. Immunol. 2007, 179, 3119–3125. [Google Scholar] [CrossRef] [PubMed]
- Dell’Agli, M.; Di Lorenzo, C.; Badea, M.; Sangiovanni, E.; Dima, L.; Bosisio, E.; Restani, P. Plant food supplements with anti-inflammatory properties: A systematic review (I). Crit. Rev. Food Sci. Nutr. 2013, 53, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.C.; Yen, G.C. Antioxidative and anti-inflammatory activity of functional foods. Curr. Opin. Food Sci. 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I. Functional foods for health: The interrelated antioxidant and anti-inflammatory role of fruits, vegetables, herbs, spices and cocoa in humans. Curr. Pharm. Des. 2016, 22, 6701–6715. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Dell’Agli, M.; Badea, M.; Dima, L.; Colombo, E.; Sangiovanni, E.; Restani, P.; Bosisio, E. Plant food supplements with anti-inflammatory properties: A systematic review (II). Crit. Rev. Food Sci. Nutr. 2013, 53, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Virgous, C.; Si, H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Nakamura, Y.; Ohto, Y.; Yano, M.; Koshiba, T.; Koshimizu, K.; Tokuda, H.; Nishino, H.; Ohigashi, H. Suppressive effects of citrus fruits on free radical generation and nobiletin, an anti-inflammatory polymethoxyflavonoid. Biofactors 2000, 12, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Sato, T.; Takayama, Y.; Mimaki, Y.; Sashida, Y.; Yano, M.; Ito, A. Novel anti-inflammatory actions of nobiletin, a citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages. Biochem. Pharmacol. 2003, 65, 2065–2071. [Google Scholar] [CrossRef]
- Guo, S.; Qiu, P.; Xu, G.; Wu, X.; Dong, P.; Yang, G.; Zheng, J.; McClements, D.J.; Xiao, H. Synergistic anti-inflammatory effects of nobiletin and sulforaphane in lipopolysaccharide-stimulated RAW 264.7 cells. J. Agric. Food Chem. 2012, 60, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Raederstorff, D.; Pantze, M.; Bachmann, H.; Moser, U. Anti-inflammatory properties of docosahexaenoic and eicosapentaenoic acids in phorbol-ester-induced mouse ear inflammation. Int. Arch. Allergy Immunol. 1996, 111, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Weldon, S.M.; Mullen, A.C.; Loscher, C.E.; Hurley, L.A.; Roche, H.M. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. J. Nutr. Biochem. 2007, 18, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.; Huang, Y.; Kong, A.N. Synergistic anti-inflammatory effects of low doses of curcumin in combination with polyunsaturated fatty acids: Docosahexaenoic acid or eicosapentaenoic acid. Biochem. Pharmacol. 2010, 79, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Kondreddy, V.K.; Kamatham, A.N. Celecoxib, a COX-2 inhibitor, synergistically potentiates the anti-inflammatory activity of docosahexaenoic acid in macrophage cell line. Immunopharmacol. Immunotoxicol. 2016, 38, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski-Rebecca, E.S.; Rocha, B.A.; Wiirzler, L.A.; Cuman, R.K.; Velazquez-Martinez, C.A.; Bersani-Amado, C.A. Synergistic effects of anethole and ibuprofen in acute inflammatory response. Chem. Biol. Interact. 2015, 242, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Vissiennon, C.; Hammoud, D.; Goos, K.H.; Nieber, K.; Arnhold, J. Synergistic interactions of chamomile flower, myrrh and coffee charcoal in inhibiting pro-inflammatory chemokine release from activated human macrophages. Synergy 2017, 4, 13–18. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Levis, M.; McIntyre, E.; Griesemer, M.; Small, D. Combinations of the FLT3 inhibitor CEP-701 and chemotherapy synergistically kill infant and childhood MLL-rearranged ALL cells in a sequence-dependent manner. Leukemia 2006, 20, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Tardi, P.; Johnstone, S.; Harasym, N.; Xie, S.; Harasym, T.; Zisman, N.; Harvie, P.; Bermudes, D.; Mayer, L. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk. Res. 2009, 33, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Férir, G.; Palmer, K.E.; Schols, D. Synergistic activity profile of griffithsin in combination with tenofovir, maraviroc and enfuvirtide against HIV-1 clade C. Virology 2011, 417, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Tagashira, A.; Nishi, K.; Matsumoto, S.; Sugahara, T. Anti-inflammatory effect of lysozyme from hen egg white on mouse peritoneal macrophages. Cytotechnology 2018, 70, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, R.; Wang, X.; Liu, F.; Zhao, J.; Yao, Q.; Zhi, W.; He, Z.; Niu, X. Nobiletin-ameliorated lipopolysaccharide-induced inflammation in acute lung injury by suppression of NF-κB pathway in vivo and vitro. Inflammation 2018, 41, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Xu, J.; Jiang, Y.; Li, F.; Chen, Y.; Dou, Q.P.; Li, D. Citrus peel flavonoid nobiletin alleviates lipopolysaccharide-induced inflammation by activating IL-6/STAT3/FOXO3a-mediated autophagy. Food Funct. 2021, 12, 1305–1317. [Google Scholar] [CrossRef]
- Komatsu, W.; Ishihara, K.; Murata, M.; Saito, H.; Shinohara, K. Docosahexaenoic acid suppresses nitric oxide production and inducible nitric oxide synthase expression in interferon-gamma plus lipopolysaccharide-stimulated murine macrophages by inhibiting the oxidative stress. Free Radic. Biol. Med. 2003, 34, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.Y.; Jin, J.Y.; Choi, J.I.; Choi, I.S.; Kim, S.J. DHA suppresses Prevotella intermedia lipopolysaccharide-induced production of proinflammatory mediators in murine macrophages. Br. J. Nutr. 2014, 111, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Aderem, A.; Underhill, D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999, 17, 593–623. [Google Scholar] [CrossRef] [PubMed]
- Bulbul, M.; Tan, R.; Gemici, B.; Hacioglu, G.; Agar, A.; Izgut-Uysal, V.N. Effect of docosahexaenoic acid on macrophage functions of rats. Immunobiology 2007, 212, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Lee, H.N.; Kim, W.; Surh, Y.J. Docosahexaenoic acid induces M2 macrophage polarization through peroxisome proliferator-activated receptor γ activation. Life Sci. 2015, 120, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Lokesh, B.R.; Kinsella, J.E. Modulation of prostaglandin synthesis in mouse peritoneal macrophages by enrichment of lipids with either eicosapentaenoic or docosahexaenoic acids in vitro. Immunobiology 1987, 175, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Bonta, I.L.; Ben-Efraim, S.; Mózes, T.; Fieren, M.W. Tumour necrosis factor in inflammation: Relation to other mediators and to macrophage antitumour defence. Pharmacol. Res. 1991, 24, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Joosten, L.A.; Netea, M.G.; Dinarello, C.A. Interleukin-1β in innate inflammation, autophagy and immunity. Semin. Immunol. 2013, 25, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Garbers, C.; Rose-John, S. Interleukin-6: From basic biology to selective blockade of pro-inflammatory activities. Semin. Immunol. 2014, 26, 2–12. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Yoshigai, E.; Machida, T.; Okuyama, T.; Mori, M.; Murase, H.; Yamanishi, R.; Okumura, T.; Ikeya, Y.; Nishino, H.; Nishizawa, M. Citrus nobiletin suppresses inducible nitric oxide synthase gene expression in interleukin-1β-treated hepatocytes. Biochem. Biophys. Res. Commun. 2013, 439, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xie, H.; Chen, C.; Tao, Z.; Zhang, C.; Cai, L. Inhibiting the PI3K/AKT/NF-κB signal pathway with nobiletin for attenuating the development of osteoarthritis: In vitro and in vivo studies. Food Funct. 2019, 10, 2161–2175. [Google Scholar] [CrossRef] [PubMed]
- Khair-El-Din, T.; Sicher, S.C.; Vazquez, M.A.; Chung, G.W.; Stallworth, K.A.; Kitamura, K.; Miller, R.T.; Lu, C.Y. Transcription of the murine iNOS gene is inhibited by docosahexaenoic acid, a major constituent of fetal and neonatal sera as well as fish oils. J. Exp. Med. 1996, 183, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y.; Penfield, J.G.; Khair-el-Din, T.A.; Sicher, S.C.; Kielar, M.L.; Vazquez, M.A.; Che, L. Docosahexaenoic acid, a constituent of fetal and neonatal serum, inhibits nitric oxide production by murine macrophages stimulated by IFNγ plus LPS, or by IFNγ plus Listeria monocytogenes. J. Reprod. Immunol. 1998, 38, 31–53. [Google Scholar] [CrossRef]
- Choi, S.Y.; Hwang, J.H.; Ko, H.C.; Park, J.G.; Kim, S.J. Nobiletin from citrus fruit peel inhibits the DNA-binding activity of NF-κB and ROS production in LPS-activated RAW 264.7 cells. J. Ethnopharmacol. 2007, 113, 149–155. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, D.; Yu, C.; Lv, B.; Peng, J.; Wang, J.; Lin, Y. Citrus nobiletin ameliorates experimental colitis by reducing inflammation and restoring impaired intestinal barrier function. Mol. Nutr. Food Res. 2015, 59, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Mi, Y.; Fan, R.; Li, R.; Liu, Z.; Liu, X. Nobiletin protects against systemic inflammation-stimulated memory impairment via MAPK and NF-κB signaling pathways. J. Agric. Food Chem. 2019, 67, 5122–5134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, J.; Liang, R.; Liu, C.; Chen, M.; Chen, J. Synergistic anti-inflammatory effects of lipophilic grape seed proanthocyanidin and camellia oil combination in LPS-stimulated RAW264.7 cells. Antioxidants 2022, 11, 289. [Google Scholar] [CrossRef] [PubMed]
- Genito, C.J.; Eckshtain-Levi, M.; Piedra-Quintero, Z.L.; Krovi, S.A.; Kroboth, A.; Stiepel, R.T.; Guerau-de-Arellano, M.; Bachelder, E.M.; Ainslie, K.M. Dexamethasone and fumaric acid ester conjugate synergistically inhibits inflammation and NF-κB in macrophages. Bioconjug. Chem. 2021, 32, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Afzal, S.; Wohlmuth, H.; Münch, G.; Leach, D.; Low, M.; Li, C.G. Synergistic anti-inflammatory activity of ginger and turmeric extracts in inhibiting lipopolysaccharide and interferon-γ-induced proinflammatory mediators. Molecules 2022, 27, 3877. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Lee, M.S.; Shin, T.S.; Hua, H.; Jang, B.C.; Choi, J.S.; Byun, D.S.; Utsuki, T.; Ingram, D.; Kim, H.R. Phlorofucofuroeckol A inhibits the LPS-stimulated iNOS and COX-2 expressions in macrophages via inhibition of NF-κB, Akt, and p38 MAPK. Toxicol. In Vitro 2011, 25, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.A.; Kim, S.Y.; Ye, B.R.; Kim, J.; Ko, S.C.; Lee, W.W.; Kim, K.N.; Choi, I.W.; Jung, W.K.; Heo, S.J. Anti-inflammatory effect of Apo-9’-fucoxanthinone via inhibition of MAPKs and NF-kB signaling pathway in LPS-stimulated RAW 264.7 macrophages and zebrafish model. Int. Immunopharmacol. 2018, 59, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Si, H. Synergistic anti-inflammatory effects and mechanisms of the combination of resveratrol and curcumin in human vascular endothelial cells and rodent aorta. J. Nutr. Biochem. 2022, 108, 109083. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, D.L.; Xie, L.N.; Ma, Y.R.; Wu, P.P.; Li, C.; Liu, W.F.; Zhang, K.; Zhou, R.P.; Xu, X.T.; et al. Synergistic anti-inflammatory effects of silibinin and thymol combination on LPS-induced RAW264.7 cells by inhibition of NF-κB and MAPK activation. Phytomedicine 2020, 78, 153309. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Lee, S.H.; Han, J.Y.; Oh, D.S. Altered TNF-α response by Aconibal® and methotrexate in a lipopolysaccharide-induced setting of inflammatory conditions: Potential on a synergistic combination. J. Ethnopharmacol. 2018, 213, 191–197. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishi, K.; Nakatani, Y.; Ishida, M.; Kadota, A.; Sugahara, T. Anti-Inflammatory Activity of the Combination of Nobiletin and Docosahexaenoic Acid in Lipopolysaccharide-Stimulated RAW 264.7 Cells: A Potential Synergistic Anti-Inflammatory Effect. Nutrients 2024, 16, 2080. https://doi.org/10.3390/nu16132080
Nishi K, Nakatani Y, Ishida M, Kadota A, Sugahara T. Anti-Inflammatory Activity of the Combination of Nobiletin and Docosahexaenoic Acid in Lipopolysaccharide-Stimulated RAW 264.7 Cells: A Potential Synergistic Anti-Inflammatory Effect. Nutrients. 2024; 16(13):2080. https://doi.org/10.3390/nu16132080
Chicago/Turabian StyleNishi, Kosuke, Yuki Nakatani, Momoko Ishida, Ayumu Kadota, and Takuya Sugahara. 2024. "Anti-Inflammatory Activity of the Combination of Nobiletin and Docosahexaenoic Acid in Lipopolysaccharide-Stimulated RAW 264.7 Cells: A Potential Synergistic Anti-Inflammatory Effect" Nutrients 16, no. 13: 2080. https://doi.org/10.3390/nu16132080
APA StyleNishi, K., Nakatani, Y., Ishida, M., Kadota, A., & Sugahara, T. (2024). Anti-Inflammatory Activity of the Combination of Nobiletin and Docosahexaenoic Acid in Lipopolysaccharide-Stimulated RAW 264.7 Cells: A Potential Synergistic Anti-Inflammatory Effect. Nutrients, 16(13), 2080. https://doi.org/10.3390/nu16132080






