Abstract
Trace elements are essential for several physiological processes. To date, various data have suggested that inadequate levels of trace elements may be involved in the pathogenesis of different chronic diseases, including immune-mediated ones, or may develop during their course. Systemic sclerosis (SSc) is a complex autoimmune multisystemic disease, primarily characterized by microvascular dysregulation, the widespread activation of the immune system and tissue fibrosis. According to the latest reports regarding the pathogenesis of SSc, the main pathophysiological processes—inflammation, vasculopathy and fibrosis—may include various trace element derangements. The present literature review aims to update the available data regarding iron, zinc, copper and selenium status in SSc as well as to underline the possible implications of these trace elements in the complexity of the pathogenic process of the disease. We observe that the status of trace elements in SSc plays a crucial role in numerous pathogenic processes, emphasizing the necessity for proper monitoring and supplementation. The reported data are heterogenous and scarce, and future studies are needed in order to draw clearer conclusions about their complete spectrum.
Keywords:
trace elements; systemic sclerosis; scleroderma; iron; selenium; cooper; zinc; malnutrition 1. Introduction
Systemic sclerosis (SSc) is a complex autoimmune multisystemic disease, primarily characterized by microvascular dysregulation, the widespread activation of the immune system and tissue fibrosis [1,2]. Apart from the skin, which is almost constantly affected in various grades of extension (scleroderma), patients can exhibit visceral involvement such as gastro-intestinal (GI), pulmonary, renal or cardiac. Between 70 and 90% of patients have some degree of digestive involvement, but the most affected part is the lower part of the oesophagus and lower oesophageal sphincter that cause further complications with dysmotility and gastroesophageal reflux. The second most frequent SSc-related GI impairment is gut implication and associated malabsorption [3]. Over the last thirty years, once the understanding of the underlying pathological processes improved, an increase in the survival rate in scleroderma patients was registered [4]. Nevertheless, there is still an increased rate of mortality compared to the general population, especially due to interstitial lung disease, pulmonary hypertension and digestive tract involvement [5]. Regarding the latter, it looks like malnutrition, even if not solely, is the main responsible cause that leads to 8 to 18% excesses of mortality due to gastro-intestinal implications [6].
Malnutrition, defined by the European Society of Clinical Nutrition and Metabolism (ESPEN) as “a state resulting from lack of intake or uptake of nutrition that leads to altered body composition”, has proved to be an independent risk factor for severe outcome in SSc [7,8]. The occurrence of malnutrition in this particular group of patients is considered to be multifactorial (see Figure 1), including anorexia, early satiety, sicca symptoms, oral cavity changes, dysmotility, small intestinal bacterial overgrowth (SIBO) and malabsorption [9,10]. One typical aspect found in scleroderma patients is an evolutive pattern with progressive reduction in the oral aperture (microstomia) and interincisal distance that significantly correlates with poor nutrition capacity [11]. Depression is a frequently identified comorbidity compared to the general population and, as described by Türk et al., it correlates with malnutrition risk [11,12]. Considering the complexity of the disease and different specific features of the disease subtypes, the use of general tools for malnutrition risk screening has proved to be unsatisfactory [13]. As a result, special needs for specific macro- and micronutrients are generally overlooked [14].
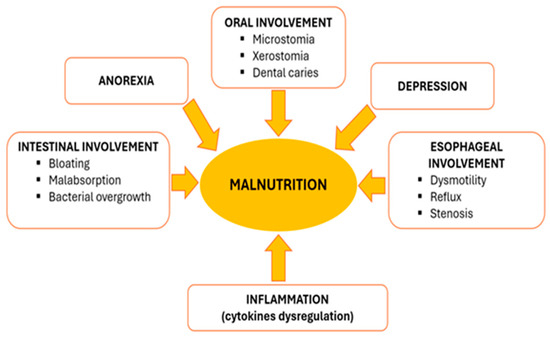
Figure 1.
Schematic representation of multifactorial development of malnutrition in SSc patients.
Trace element abnormalities in patients with SSc may be associated with undernutrition, resulting from decreased food intake or altered intestinal uptake or may be induced by specific pathogenic processes of the disease [7,15]. Although micronutrient deficiencies proved to be more frequent than initially anticipated, conducting a laboratory assessment of micronutrient deficiency is not a routine practice unless it becomes clinically evident [16]. Nonetheless, the obtained laboratory results do not lead to a straightforward decision, considering that micronutrient abnormalities may be influenced by an acute phase response, underlying chronic conditions and related organ dysfunctions [7,17]. Moreover, a clear difference between trace element depletion defined as “concentrations below reference range” and deficiency defined as “concentrations below reference range + clinical or metabolic signs” should be determined [14].
The aim of this review is focused on trace element abnormalities in SSc patients and on the evidence available supporting the rationale of trace element supplementation.
2. Materials and Methods
We performed a systematic literature research based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Studies were selected by searching in the PubMed, Scopus and Cochrane databases, including original articles, randomized control trials and observational studies, respectively, in order to identify relevant full texts in English, published clinical trials that included information about the levels of the trace elements iron (Fe), zinc (Zn), selenium (Se) and copper (Cu) and clinical correlations with different organ involvement in patients with SSc (Figure 2).
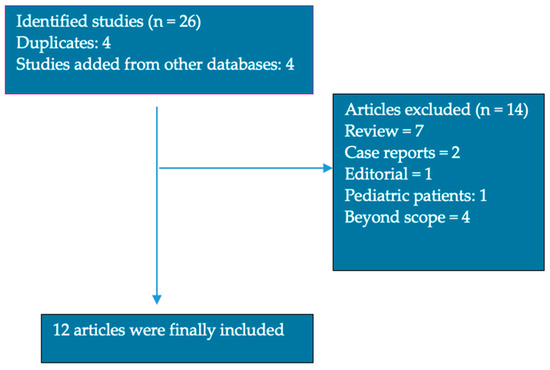
Figure 2.
Article selection for review.
We searched using the following keywords: “(systemic sclerosis OR scleroderma AND trace elements)”. To expand our research, we also manually searched the references in relevant studies on SSc and trace elements. This allowed us to identify additional studies for potential inclusion based on their titles.
Using the PICOS framework (Population/Problem, Intervention, Comparisons, Outcomes and Study design) to formulate research questions and develop search strategies, inclusion and exclusion criteria for the study review were established. The inclusion and exclusion criteria are summarized in Table 1.

Table 1.
Inclusion and exclusion criteria.
The following data were collected from the included studies: the study design, the first author, the year of publication, the sample size, the types of trace elements investigated, the mean values of the investigated trace elements, and trends of the trace elements in the study group.
3. Results
We identified 26 articles written in English (8 in PubMed, 18 in Scopus and, respectively, 0 in Cochrane database); furthermore, two researchers screened the titles and abstracts in order to identify relevant articles, and if any disagreements occurred in the selection process, they were settled by a third reviewer. A total of 12 articles were included Figure 2. The reported data of the include articles is summarized in Table 2.

Table 2.
Overview of the included studies investigating Fe, Zn, Se and Cu in SSc patients.
4. Discussions
4.1. Absolute and/or Functional Iron Deficiency?
Given that iron (Fe) deficiency is considered the most frequent nutritional deficiency worldwide, SSc patients are likewise affected [14,18]. Considering the complex pathogenesis of SSc, patients may develop, over the course of the disease progression, both absolute and functional iron deficiency.
4.1.1. Iron Status in SSc Population
Reports regarding iron deficiency anemia have been published since the late 1960s, when Westerman et al. indicated that 50% of the investigated cases of SSc with anemia had iron depletion [19].
SSc patients may experience an absolute iron deficiency resulting from a negative iron balance caused by frequent gastrointestinal blood loss as well as due to extended malabsorption or hemolysis [9,20,21]. It has been reported that iron deficiency is more prevalent in SSc patients with pulmonary hypertension (46.1% vs. 16.4%) and is associated with decreased exercise capacity and worse survival rates [22].
Iron deficiency anemia in patients with SSc is usually related to gastrointestinal microhemorrhages or the presence of gastric antral vascular ectasia (GAVE) [23]. A study investigating hematological abnormalities in 180 SSc patients reported that one-third of patients registered iron deficiency anemia, with the main reported cause being gastrointestinal bleeding [23].
Telangiectatic gastrointestinal bleeding is considered one of the main causes of iron store depletion in SSc patients [24,25]. Although the European League Against Rheumatism Scleroderma Trials and Research (EUSTAR) network study conducted by Hughes et al. reported that telangiectasias may be encountered in almost 68% of SSc patients, at the moment, there are no available data regarding the prevalence of telangiectasias affecting the gastrointestinal tract [26].
Another EUSTAR network study, which included the largest SSc cohort, reported a prevalence of symptomatic GAVE of only 1% (n = 49); however, not all included patients were systematically assessed using an endoscopy [27]. In a prospective study (n = 103) which included SSc patients considered for the Scleroderma: Cyclophosphamide Or Transplant (SCOT) trial, who underwent a screening process with an upper endoscopy, indicated a GAVE prevalence of 22.3% [28]. An analysis of the largest Australian cohort of SSc patients (n = 2039) also revealed a higher prevalence of GAVE, reaching 10.6% [29]. More than a half of patients (53.8%) diagnosed with GAVE had already experienced a drop in hemoglobin to 10 g before the moment of the endoscopy [29]. As a result, GAVE is considered one of the leading causes for iron deficiency anemia in SSc patients. According to a prospective comparative study conducted by El-Hawary, who aimed to evaluate different types of therapies for GAVE, iron levels as well as iron metabolism markers were significantly diminished before the intervention [30]. Although a progressive improvement in iron status was reported after cyclophosphamide administration and argon plasma coagulation, no data regarding iron supplementation were reported [30].
Considering the central pathogenic processes of the disease, it is suggested that every segment of the gastrointestinal tract may be affected by collagenous fibrosis, causing decreased intestinal permeability and malabsorption [2,31]. Decreased iron intestinal absorption in SSc patients may also be induced by small intestinal bacterial overgrowth (SIBO) [32]. Marie et al. reported that patients with SIBO and increased levels of fecal calprotectin may also be associated with decreased levels of ferritin [33].
The extensive use of proton pump inhibitors (PPIs) recommended for the treatment of SSc-related GI reflux contributes to impaired iron absorption [34].
Numerous studies have reported that SSc patients may also exhibit decreased vitamin C levels without a clear underlying cause. However, it can be assumed that it is most probably multifactorial, potentially involving factors such as inadequate intake, gastrointestinal involvement or PPI use [35]. Vitamin C deficit in SSc patients is particularly important given that its role extends beyond the iron absorption process, such as ferritin synthesis and degradation, the modulation of cellular iron efflux or transferrin–iron uptake mechanisms [36].
SSc patients with severe gastrointestinal involvement may often require blood transfusions which may be associated with further iron dysregulation, as free iron provided during transfusions could potentially be used to catalyze inflammation [37].
4.1.2. Potential Impact of Iron on SSc Pathogenesis
Functional iron deficiency may also be encountered in SSc patients and may be directly related to the underlying chronic activation of the immune system [20,38].
Since interleukin-6 (IL-6) has a key role in SSc pathogenesis, it is accountable for the increased levels of hepcidin [37,39]. Hepcidin regulates absorption and iron circulation in the body and increases intracellular iron levels by promoting the degradation and internalization of ferroportin [40]. Increased hepcidin values were associated with functional iron deficiency and falsely elevated ferritin [22,37,41]. Ruiter et al. reported both increased hepcidin and IL-6 levels in SSc patients with iron deficiency; however, no association between these two was found [22]. Experimental studies revealed a crosstalk between IL-6 and the bone morphogenetic protein BMP/SMAD pathway, and mutations of BMPR2 were associated with pulmonary hypertension and iron deficiency [40]. However, it has already been demonstrated that SSc patients, even those with associated pulmonary hypertension, do not exhibit BMPR-2 mutations [42]. These findings support the hypothesis that hepcidin expression may be induced by a more complex mechanism than only under the action of IL-6.
According to recent preclinical data regarding the pathogenesis of SSc, iron plays a central role in a distinctive process of programmed cell death called “ferroptosis” [43]. It is considered that excessive ferroptosis cell death is characterized by increased intracellular free ferrous iron accumulation, lipid peroxidation and oxidative stress [43].
The dietary reference intake (DRI) of iron should be adapted based on the patient’s gender and age. Currently, the DRI is 8 mg/day, but it should be increased in young pre-menopausal women. In line with the latest ESPEN guideline, treatment for iron deficiency should be initiated based on a comprehensive evaluation of iron status including plasma iron, transferrin, transferrin saturation, ferritin, C-reactive proteins, hepcidin, and red blood cell morphology [14].
4.2. Zinc Deficiency—A Potential Risk Factor for Disease Progression?
Zinc (Zn) is a key trace element involved in a variety of cellular processes, such as structural, catalytic, extracellular and intracellular signaling, cell proliferation and apoptosis [44]. Although less studied, zinc deficiency has a high incidence, especially among patients with chronic inflammatory diseases, and is associated with compromised immune systems and increased inflammation [45].
4.2.1. Zinc Status in SSc Population
Among SSc patients, one observational, single-center, longitudinal cohort study (n = 176 patients) and one retrospective cross-sectional study (n = 82 patients) indicated reduced Zn levels ranging from 48% to 10.9% [35,46]. Sun et al. reported, in a case–control study, that total zinc serum levels were lower in SSc patients with associated pulmonary hypertension [47]. Conversely, two case–control studies revealed no significant differences between SSc patients and the control group [48,49]. Risk factors of zinc deficiency in SSc patients may include inadequate intake, malnutrition, gastrointestinal involvement and secondary malabsorption [9,13,14,50].
4.2.2. Potential Impact of Zinc on SSc Pathogenesis
As the intricate pathogenesis of SSc includes inflammatory processes that engage T-cells, macrophages and B-lymphocytes, an associated zinc deficiency which has been linked with various immune disarrangements, especially increased inflammatory mediator release, may impact disease progression [51,52]. An experimental study conducted by Wong et al. demonstrated that zinc deficiency induced a macrophage-like phenotype in a human monocytic cell line THP-1 culture, as well as increased cell adherence capacity and cytokine release [51].
The pathogenesis of SSc is predominantly marked by dysfunctional tissue repair following autoimmune aggression and microvascular injury [2,52]. Transforming growth factor beta (TGF-β) is one of the most extensively documented factors involved in the fibrotic tissue of SSc patients [2,52]. TGF-β proved to exhibit various functions, including the stimulation of fibroblast activity, resulting in increased extracellular matrix production [53]. In the early 2000s, Massague et al. reported that the main mediators of TGF-β signaling activity were SMADs (mother against decapentaplegic proteins) [54]. Moreover, the dysregulated SMAD pathway was proved to play a central role in the fibrotic process in SSc patients [52]. While there are no specific data available concerning the role of zinc in the fibrotic process in SSc patients, it should be noted that zinc modulates the activity of the MG53 protein, a factor involved in wound healing and tissue repair through the inhibition of TGF-β/SMAD signaling [55]. Therefore, it can be assumed that zinc deficiency may be linked to disease progression and severity; however, substantial research data are further required.
Although there are many recommendations regarding the dietary reference intake of zinc, the latest ESPEN guideline proposes 8–15 mg/day of zinc. Decisions for deficiency treatment should be based on zinc plasma levels in conjunction with albumin and C-reactive protein levels [14].
4.3. Copper Inadequacy—A Key Element of Exacerbated Oxidative Stress?
Copper (Cu) is an essential trace element functioning as an enzymatic cofactor and interacting with several proteins known as cuproproteins [56]. Various types of copper proteins have been described and classified based on their chemical and geometrical properties (cupredoxins vs. oxidoreductases), as well as on their roles in transporting copper or using it as a cofactor [57].
4.3.1. Copper Status SSc Population
Limited evidence regarding copper levels in SSc patients is available. Sun et al. reported no difference between controls and SSc patients with pulmonary arterial hypertension regarding copper levels [47]. Qayoom et al. found increased levels of both copper and ceruloplasmin in a cross-sectional study which included only twelve patients with SSc and morphea [58]. Similar data were also published by Li et al. who reported a decreased level of copper in SSc patients, especially in those with associated pulmonary fibrosis [15].
The dietary reference intake of copper for healthy adults ranges between 1.1 and 2 mg/day. In SSc patients, both copper deficiency and toxicity are possible and detrimental. Therefore, copper plasmatic levels, ceruloplasmin levels and C-reactive protein levels should be determined before any treatment is initiated [14].
4.3.2. Potential Impact of Copper on SSc Pathogenesis
Copper oxidation–reduction properties (Cu2+-Cu+) promote electron transfer reactions causing oxidative stress and reactive oxygen species; therefore, the strict control of copper homeostasis is mandatory [56,59].
Decreased Cu-Zn superoxide dismutase (SOD3) activity, which is part of the endogenous defense mechanism against superoxide overproduction, has been shown to be responsible for various diseases characterized by extensive fibrosis. Experimental data from Sun et al. indicated that SOD3 deficiency induces and exacerbates liver fibrogenesis through epithelial–mesenchymal transitions [60]. Moreover, Frank et al. demonstrated that Cu-Zn SOD expression may be mediated by the NO activity [61]. Nevertheless, experimental evidence has revealed the potential antifibrotic effects of Cu-Zn SOD supplementation targeting TGF-β1 overexpression [62]. Very few data reported the use of Cu-Zn SOD supplementation as antifibrotic treatment, especially in patients under radiotherapy [62].
4.4. Selenium Deficiency—A Promising Central Piece in the SSc Pathogenic Puzzle?
Selenium (Se) is an essential trace element that has a crucial role in thyroid hormone production, the immune system, antioxidant defense, fertility and skeletal development [63]. After intestinal absorption, Selenoprotein P (SELENOP) provides selenium to the other organs. Indeed, the diverse functions of selenium are facilitated through the action of diverse selenoproteins such as glutathione peroxidase, iodothyronine deiodinase or thioredoxin reductase [64].
4.4.1. Selenium Status in SSc Population
One observational longitudinal study which included 176 patients at different stages of SSc reported that selenium deficiency affected 15.6% of the participants, with severity increasing in late stages of the disease [46]. In a retrospective cross-sectional study involving 82 SSc patients, it was indicated that 35% of patients had lower selenium levels which were associated with myocardial dysfunction [35]. These results align with previously published clinical and experimental data, according to which selenium deficiency is associated with decreased exercise capacity and an increased risk of heart failure [65].
Because of its presence in various selenoproteins, selenium is involved in redox reactions and the antioxidant system. Several experimental studies confirmed that Se deficiency causes increased levels of reactive oxygen species and decreased glutathione peroxidase activity in mice [66,67]. Moreover, it was reported that selenium deficiency may increase gastrointestinal oxidative stress, DNA damage and apoptosis [68]. In a case–control study conducted by Tikly et al., a reduced selenium level along with decreased global antioxidant activity in SSc patients was reported [48]. Sun et al. conducted a more comprehensive assessment of selenium status by measuring total selenium levels, SELENOP, as well as glutathione peroxidase 3. All three biomarkers were significantly low in patients with severe skin involvement as well as in patients that developed pulmonary arterial hypertension [47].
4.4.2. Potential Impact of Selenium on SSc Pathogenesis
The typical vascular changes encountered in SSc patients, affecting both micro- and macro-circulation, are mainly caused by an unbalanced production of vasoconstricting and vasodilating agents at the endothelial level [53]. At the cellular level, it has been demonstrated that nitric oxide (NO) activity and metabolism is profoundly disrupted, such as NO overproduction, concomitant with diminished NO production by endothelial NO synthase (eNOS) [65]. Although data regarding the role of selenium in SSc pathogenesis are currently limited, the available results regarding vascular pathological changes induced by selenium deficiency, such as decreased NO in its reduced form due to reduced levels of glutathione peroxidase, warrant further research consideration [69,70].
The dietary reference intake of selenium varies between 20 μg/day and 90 μg/day and supplementation should be considered after measuring selenium plasma levels, GPX3, albumin levels and C-reactive protein levels [14].
5. Conclusions
The role of trace elements in the pathogenesis of different chronic diseases have already been documented. However, due to the lack of high-quality trials, clear procedures for their determination and the subsequent recommendation for their supplementation are not yet established.
Further investigation is warranted for all four analyzed trace elements in order to determine the exact role and the necessity for supplementation in SSc patients. Figure 3 summarizes the potential role of all four studied trace elements in the pathogenesis of SSc. Nevertheless, the roles of other trace elements such as chromium, cobalt, manganese or molybdenum should also be investigated, considering that the physiologic role of trace elements is usually convergent and synergistic.
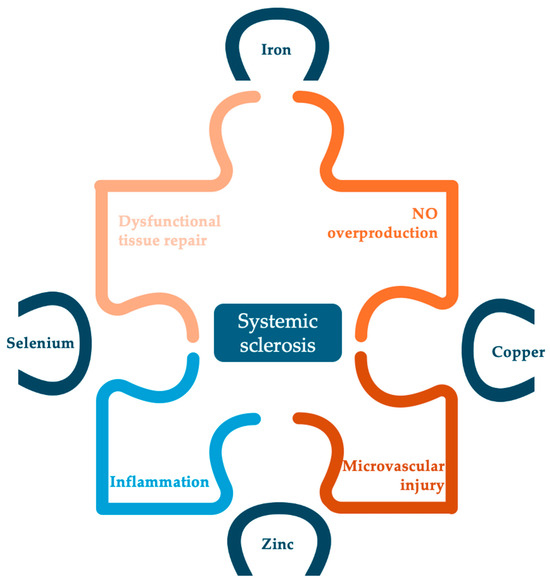
Figure 3.
Potential implications of studied trace elements in the pathogenesis of SSc.
Taking into account that neither the European Alliance of Associations for Rheumatology (EULAR) nor the EUSTAR network provide any adapted guidance for the management of trace element determination and supplementation, we consider that the ESPEN recommendations should be followed until further data are available.
Author Contributions
Conceptualization, C.O.C. and D.O.-B.; methodology, A.C.A.; software, A.C.A.; validation, I.M.G., S.C. and R.U.; formal analysis, A.-M.C.; investigation, C.O.C.; resources, C.C.; data curation, R.Ț.; writing—original draft preparation, C.C.; writing—review and editing, L.M.; visualization, R.Ț.; supervision, R.E.; project administration, C.O.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hughes, M.; Herrick, A.L. Systemic sclerosis. Br. J. Hosp. Med. 2019, 80, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Muangchan, C.; Markland, J.; Robinson, D.; Jones, N.; Khalidi, N.; Docherty, P.; Kaminska, E.; Masetto, A.; Sutton, E.; Mathieu, J.P.; et al. The 15% Rule in Scleroderma: The Frequency of Severe Organ Complications in Systemic Sclerosis. A Systematic Review. J. Rheumatol. 2013, 40, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Steen, V.D.; Medsger, T.A. Changes in causes of death in systemic sclerosis, 1972–2002. Ann. Rheum. Dis. 2007, 66, 940–944. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Chaves, S.; Porel, T.; Mounié, M.; Alric, L.; Astudillo, L.; Huart, A.; Lairez, O.; Michaud, M.; Prévot, G.; Ribes, D.; et al. Sine scleroderma, limited cutaneous, and diffused cutaneous systemic sclerosis survival and predictors of mortality. Arthritis Res. Ther. 2021, 23, 295. [Google Scholar] [CrossRef] [PubMed]
- Pope, J.E.; Denton, C.P.; Johnson, S.R.; Fernandez-Codina, A.; Hudson, M.; Nevskaya, T. State-of-the-art evidence in the treatment of systemic sclerosis. Nat. Rev. Rheumatol. 2023, 19, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Roque, L.C.S.C.; Gonçalves, R.S.G.; Duarte, A.L.B.P.; Dantas, A.T. Nutritional risk in patients with systemic sclerosis. Clin. Rheumatol. 2020, 39, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Frech, T. Gastrointestinal and Hepatic Disease in Systemic Sclerosis Tracy. Rheum. Dis. Clin. N. Am. 2018, 44, 15–28. [Google Scholar] [CrossRef]
- Smirani, R.; Poursac, N.; Naveau, A.; Schaeverbeke, T.; Devillard, R.; Truchetet, M.E. Orofacial consequences of systemic sclerosis: A systematic review. J. Scleroderma Relat. Disord. 2018, 3, 81–90. [Google Scholar] [CrossRef]
- Türk, İ.; Cüzdan, N.; Çiftçi, V.; Arslan, D.; Doğan, M.C.; Unal, İ. Malnutrition, associated clinical factors, and depression in systemic sclerosis: A cross-sectional study. Clin. Rheumatol. 2020, 39, 57–67. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Watad, A.; Gizunterman, A.; McGonagle, D.; Mahagna, H.; Comaneshter, D.; Amital, H.; Cohen, A.D.; Amital, D. The burden of depression in systemic sclerosis patients: A nationwide population-based study. J. Affect. Disord. 2019, 243, 427–431. [Google Scholar] [CrossRef]
- Bagnato, G.; Pigatto, E.; Bitto, A.; Pizzino, G.; Irrera, N.; Abignano, G.; Ferrera, A.; Sciortino, D.; Wilson, M.; Squadrito, F.; et al. The PREdictor of MAlnutrition in Systemic Sclerosis (PREMASS) Score: A Combined Index to Predict 12 Months Onset of Malnutrition in Systemic Sclerosis. Front. Med. 2021, 8, 651748. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Li, J.; Liu, S.; Liu, Z. Serum Concentrations of Trace Elements/Minerals in Patients with Diffuse Systemic Sclerosis. Biol. Trace Elem. Res. 2021, 199, 2440–2443. [Google Scholar] [CrossRef]
- Berger, M.M.; Pantet, O.; Schneider, A.; Ben-Hamouda, N. Micronutrient deficiencies in medical and surgical inpatients. J. Clin. Med. 2019, 8, 931. [Google Scholar] [CrossRef]
- Berger, M.M.; Talwar, D.; Shenkin, A. Pitfalls in the interpretation of blood tests used to assess and monitor micronutrient nutrition status. Nutr. Clin. Pract. 2023, 38, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Chandra, J. Iron deficiency: Beyond anemia. Indian J. Pediatr. 2011, 78, 65–72. [Google Scholar] [CrossRef]
- Westerman, M.P.; Martinez, R.C.; Medsger, T.A.; Totten, R.S.; Rodnan, G.P. Anemia and Scleroderma Frequency, Causes, and Marrow Findings Hematologic evaluation of 164 pa. Arch. Intern. Med. 1968, 122, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, S.R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- Xanthouli, P.; Gordjani, O.; Benjamin, N.; Harutyunova, S.; Egenlauf, B.; Marra, A.M.; Haas, S.; Milde, N.; Blank, N.; Lorenz, H.M.; et al. Hypochromic red cells as a prognostic indicator of survival among patients with systemic sclerosis screened for pulmonary hypertension. Arthritis Res. Ther. 2023, 25, 38. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, G.; Lanser, I.J.; De Man, F.S.; Van der Laarse, W.J.; Wharton, J.; Wilkins, M.R.; Howard, L.S.; Vonk-Noordegraaf, A.; Voskuyl, A.E. Iron deficiency in systemic sclerosis patients with and without pulmonary hypertension. Rheumatology 2014, 53, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Frayha, R.A.; Shulman, L.E.; Stevens, M.B. Hematological abnormalities in scleroderma: A study of 180 cases. Acta Haematol. 1980, 64, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Khanlou, H.; Malhotra, A.; Friedenberg, F.; Rothstein, K. Jejunal telangiectasias as a cause of massive bleeding in a patient with scleroderma. Rev. Rhum. 1999, 66, 119–121. [Google Scholar]
- Jharap, B.; Koudstaal, L.G.; Neefjes-Borst, E.A.; Van Weyenberg, S.J.B. Colonic telangiectasias in progressive systemic sclerosis. Endoscopy 2012, 44 (Suppl. S2), E42–E43. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.; Heal, C.; Henes, J.; Balbir-Gurman, A.; Distler, J.H.W.; Airò, P.; Müller-Ladner, U.; Hunzelmann, N.; Kerzberg, E.; Rudnicka, L.; et al. Digital pitting scars are associated with a severe disease course and death in systemic sclerosis: A study from the EUSTAR cohort. Rheumatology 2022, 61, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Ghrénassia, E.; Avouac, J.; Khanna, D.; Derk, C.T.; Distler, O.; Suliman, Y.A.; Airo, P.; Carreira, P.E.; Foti, R.; Granel, B.; et al. Prevalence, correlates and outcomes of gastric antral vascular ectasia in systemic sclerosis: A eustar case-control study. J. Rheumatol. 2014, 41, 99–105. [Google Scholar] [CrossRef]
- Hung, E.W.; Mayes, M.D.; Sharif, R.; Assassi, S.; Machicao, V.I.; Hosing, C.; St Clair, E.W.; Furst, D.E.; Khanna, D.; Forman, S.; et al. Gastric antral vascular ectasia and its clinical correlates in patients with early diffuse systemic sclerosis in the SCOT trial. J. Rheumatol. 2013, 40, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Morrisroe, K.; Hansen, D.; Stevens, W.; Sahhar, J.; Ngian, G.S.; Hill, C.; Roddy, J.; Walker, J.; Proudman, S.; Nikpour, M. Gastric antral vascular ectasia in systemic sclerosis: A study of its epidemiology, disease characteristics and impact on survival. Arthritis Res. Ther. 2022, 24, 103. [Google Scholar] [CrossRef]
- El-Hawary, A.T.; Mostafa, E.F.; Mohamed, S.Y.; Kotb, L.I. Improvement of iron-deficiency anemia resulting from gastric antral vascular ectasia in patients with systemic sclerosis: Cyclophosphamide versus argon plasma coagulation. Egypt. J. Intern. Med. 2018, 30, 175–181. [Google Scholar] [CrossRef]
- Marie, I.; Leroi, A.M.; Gourcerol, G.; Levesque, H.; Ménard, J.F.; Ducrotte, P. Fructose malabsorption in systemic sclerosis. Medicine 2015, 94, e1601. [Google Scholar] [CrossRef]
- Marie, I.; Ducrotté, P.; Denis, P.; Menard, J.F.; Levesque, H. Small intestinal bacterial overgrowth in systemic sclerosis. Rheumatology 2009, 48, 1314–1319. [Google Scholar] [CrossRef]
- Marie, I.; Leroi, A.M.; Menard, J.F.; Levesque, H.; Quillard, M.; Ducrotte, P. Fecal calprotectin in systemic sclerosis and review of the literature. Autoimmun. Rev. 2015, 14, 547–554. [Google Scholar] [CrossRef]
- Kowal-Bielecka, O.; Fransen, J.; Avouac, J.; Becker, M.; Kulak, A.; Allanore, Y.; Distler, O.; Clements, P.; Cutolo, M.; Czirjak, L.; et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann. Rheum. Dis. 2017, 76, 1327–1339. [Google Scholar] [CrossRef]
- Dupont, R.; Longué, M.; Galinier, A.; Cinq Frais, C.; Ingueneau, C.; Astudillo, L.; Arlet, P.; Adoue, D.; Alric, L.; Prévot, G.; et al. Impact of micronutrient deficiency & malnutrition in systemic sclerosis: Cohort study and literature review. Autoimmun. Rev. 2018, 17, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J.R.; Richardson, D.R. The active role of vitamin C in mammalian iron metabolism: Much more than just enhanced iron absorption! Free Radic. Biol. Med. 2014, 75, 69–83. [Google Scholar] [CrossRef]
- Goubran, H.; Ragab, G.; Seghatchian, J.; Burnouf, T. Blood transfusion in autoimmune rheumatic diseases. Transfus. Apher. Sci. 2022, 61, 103596. [Google Scholar] [CrossRef] [PubMed]
- Fuschiotti, P. Current perspectives on the immunopathogenesis of systemic sclerosis. ImmunoTargets Ther. 2016, 5, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Cardoneanu, A.; Burlui, A.M.; Macovei, L.A.; Bratoiu, I.; Richter, P.; Rezus, E. Targeting Systemic Sclerosis from Pathogenic Mechanisms to Clinical Manifestations: Why IL-6? Biomedicines 2022, 10, 318. [Google Scholar] [CrossRef]
- Varga, E.; Pap, R.; Jánosa, G.; Sipos, K.; Pandur, E. IL-6 Regulates Hepcidin Expression Via the BMP/SMAD Pathway by Altering BMP6, TMPRSS6 and TfR2 Expressions at Normal and Inflammatory Conditions in BV2 Microglia. Neurochem. Res. 2021, 46, 1224–1238. [Google Scholar] [CrossRef]
- Srole, D.N.; Ganz, T.; Pharmacology, M.; Angeles, L.; Angeles, L.; Angeles, L. Erythroferrone structure, function, and physiology: Iron homeostasis and beyond. J. Cell. Physiol. 2021, 236, 4888–4901. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.; Barst, R.; Horn, E.; Cuervo, N.; Deng, Z.; Knowles, J. Pulmonary hypertension in scleroderma spectrum of disease: Lack of bone morphogenetic protein receptor 2 mutations. J. Rheumatol. 2002, 29, 2379–2381. [Google Scholar] [PubMed]
- Hu, W.; Liang, K.; Zhu, H.; Zhao, C.; Hu, H.; Yin, S. Ferroptosis and Its Role in Chronic Diseases. Cells 2022, 11, 2040. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.I.; Sarmento-Ribeiro, A.B.; Gonçalves, A.C. Zinc: From Biological Functions to Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 4822. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]
- Läubli, J.; Dobrota, R.; Maurer, B.; Jordan, S.; Misselwitz, B.; Fox, M.; Distler, O. Impaired micronutrients and prealbumin in patients with established and very early systemic sclerosis. Clin. Exp. Rheumatol. 2020, 38, S120–S126. [Google Scholar]
- Sun, Q.; Hackler, J.; Hilger, J.; Gluschke, H.; Muric, A.; Simmons, S.; Schomburg, L.; Siegert, E. Selenium and copper as biomarkers for pulmonary arterial hypertension in systemic sclerosis. Nutrients 2020, 12, 1894. [Google Scholar] [CrossRef]
- Tikly, M.; Channa, K.; Theodorou, P.; Gulumian, M. Lipid peroxidation and trace elements in systemic sclerosis. Clin. Rheumatol. 2006, 25, 320–324. [Google Scholar] [CrossRef]
- Lundberg, A.; Akesson, A.; Akesson, B. Dietary intake and nutritional status in patients with systemic sclerosis. Ann. Rheum. Dis. 1992, 51, 1143–1148. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, J.; Rattan, S.; DiMarino, A.J.; Cohen, S.; Jimenez, S.A. Review article: Pathogenesis and clinical manifestations of gastrointestinal involvement in systemic sclerosis. Aliment. Pharmacol. Ther. 2017, 45, 883–898. [Google Scholar] [CrossRef]
- Wong, C.; Rinaldi, N.; Ho, E. Zinc deficiency enhanced inflammatory response by increasing immune cell activation and inducing IL6 promoter demethylation. Mol. Nutr. Food Res. 2015, 59, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Colletti, M.; Galardi, A.; De Santis, M.; Guidelli, G.M.; Di Giannatale, A.; Di Luigi, L.; Antinozzi, C. Exosomes in systemic sclerosis: Messengers between immune, vascular and fibrotic components? Int. J. Mol. Sci. 2019, 20, 4337. [Google Scholar] [CrossRef] [PubMed]
- Truchetet, M.E.; Brembilla, N.C.; Chizzolini, C. Current Concepts on the Pathogenesis of Systemic Sclerosis. Clin. Rev. Allergy Immunol. 2023, 64, 262–283. [Google Scholar] [CrossRef] [PubMed]
- Massague, J.; Chen, Y. Controlling TGF-beta signaling. Genes Dev. 2000, 14, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in wound healing modulation. Nutrients 2018, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.F. Copper; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780323661621. [Google Scholar]
- Rubino, J.T.; Franz, K.J. Coordination chemistry of copper proteins: How nature handles a toxic cargo for essential function. J. Inorg. Biochem. 2012, 107, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Qayoom, S.; Sultan, J.; Khan, A.R.; Manzoor, S.; Khan, K. Serum copper and ceruloplasmin levels in systemic sclerosis and various types of morphea in the Kashmir Valley. J. Pak. Assoc. Dermatol. 2015, 25, 9–11. [Google Scholar]
- Abdullah, K.M.; Kaushal, J.B.; Takkar, S.; Sharma, G.; Alsafwani, Z.W.; Pothuraju, R.; Batra, S.K.; Siddiqui, J.A. Copper metabolism and cuproptosis in human malignancies: Unraveling the complex interplay for therapeutic insights. Heliyon 2024, 10, e27496. [Google Scholar] [CrossRef]
- Sun, Y.L.; Bai, T.; Zhou, L.; Zhu, R.T.; Wang, W.J.; Liang, R.P.; Li, J.; Zhang, C.X.; Gou, J.J. SOD3 deficiency induces liver fibrosis by promoting hepatic stellate cell activation and epithelial–mesenchymal transition. J. Cell. Physiol. 2021, 236, 4313–4329. [Google Scholar] [CrossRef]
- Frank, S.; Zacharowski, K.; Wray, G.M.; Thiemermann, C.; Pfeilschifter, J. Identification of copper/zinc superoxide dismutase as a novel nitric oxide-regulated gene in rat glomerular mesangial cells and kidneys of endotoxemic rats. FASEB J. 1999, 13, 869–882. [Google Scholar] [CrossRef]
- Emerit, J.; Samuel, D.; Pavio, N. Cu-Zn super oxide dismutase as a potential antifibrotic drug for hepatitis C related fibrosis. Biomed. Pharmacother. 2006, 60, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, X.; Wei, Y. Selenium and Selenoproteins in Health. Biomolecules 2023, 13, 799. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Sukhorukov, V.N.; Melnichenko, A.A.; Khotina, V.A.; Orekhov, A.N. The Role of Selenium in Atherosclerosis Development, Progression, Prevention and Treatment. Biomedicines 2023, 11, 2010. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Zhang, F.; Huang, J.; Yang, X.; Zhou, X. Selenium deficiency causes hypertension by increasing renal AT 1 receptor expression via GPx1/H2O2/NF-κB pathway. Free Radic. Biol. Med. 2023, 200, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Nikolic-Paterson, D.J.; Li, K.; Li, Y.; Wang, Y.; Chen, X.; Duan, Z.; Zhang, Y.; Liu, P.; Lu, S.; et al. Selenium binding protein 1 protects renal tubular epithelial cells from ferroptosis by upregulating glutathione peroxidase 4: SBP1 protects HK-2 cells from ferroptosis by upregulating GPX4. Chem.-Biol. Interact. 2024, 393, 110944. [Google Scholar] [CrossRef]
- Luo, D.; Tang, X.; Wang, Y.; Ying, S.; He, Y.; Lin, H.; Khoso, P.A.; Li, S. Selenium deficiency exacerbated Bisphenol A-induced intestinal toxicity in chickens: Apoptosis and cell cycle arrest mediated by ROS/P53. Sci. Total Environ. 2024, 913, 169730. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Liu, T.; Yang, J.; Sun, X.; Gao, X. The mechanism of selenium regulating the permeability of vascular endothelial cells through selenoprotein O. Redox Biol. 2024, 70, 103063. [Google Scholar] [CrossRef]
- Dooley, A.; Bruckdorfer, K.R.; Abraham, D.J. Modulation of fibrosis in systemic sclerosis by nitric oxide and antioxidants. Cardiol. Res. Pract. 2012, 2012, 521958. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).