Association between Diet Quality and Eating Behavior in Type 2 Diabetes Adults: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.2.1. Sociodemographic Characteristics and Cardiovascular Risk Factors
2.2.2. Diet Characteristics
2.2.3. Physical Activity Level
2.3. Statistical Analysis
3. Results
3.1. Socio-Demographic, Anthropometric, and Biochemical Characteristics of the Study Population
3.2. Diet Quality of the Study Population
3.3. Eating Behavior of the Study Population
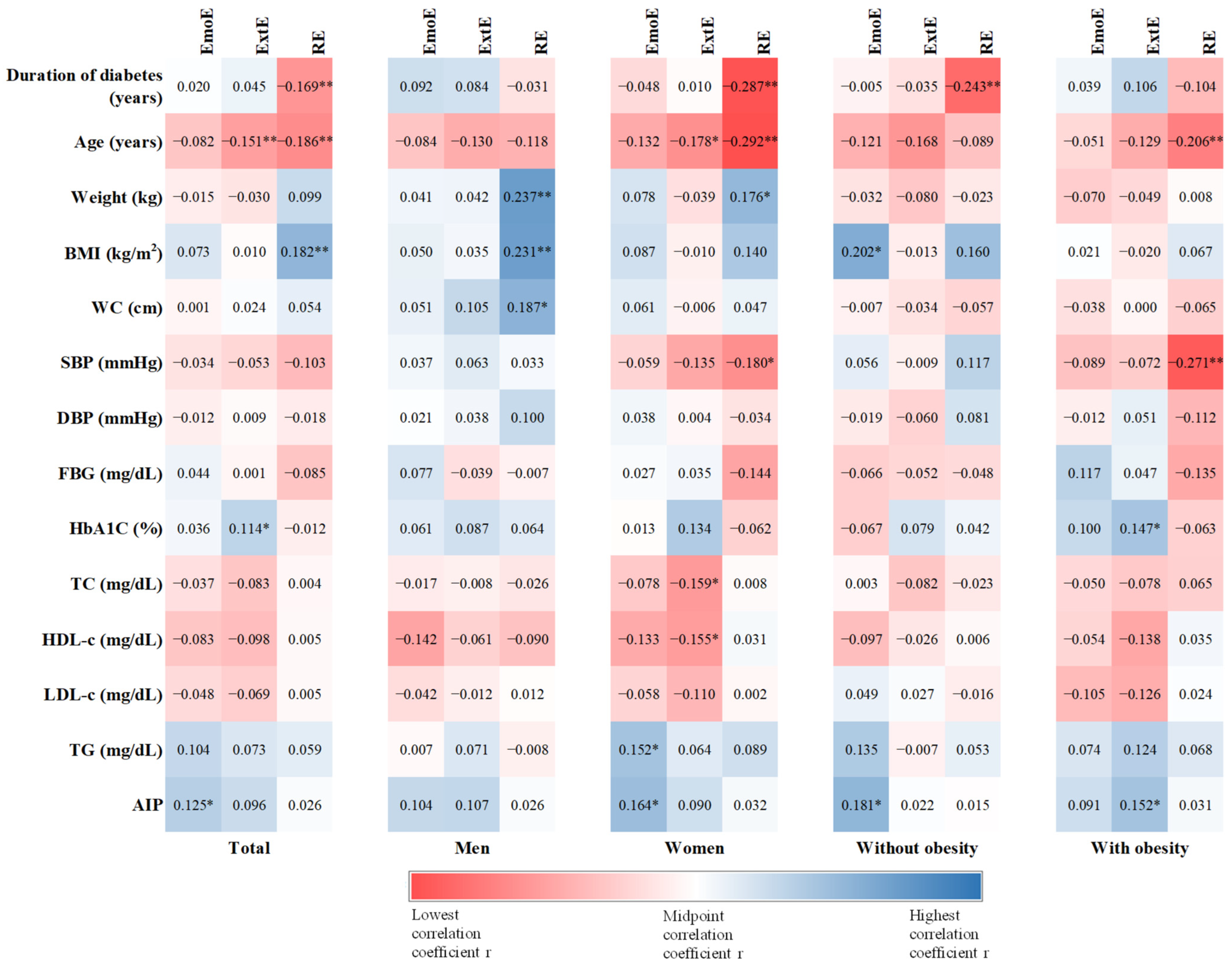
3.4. Associations between Eating Behavior and Anthropometric and Biochemical Data
3.5. Associations between Eating Behavior and Diet Quality
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.; Popa, S.G.; Mota, E.; Mitrea, A.; Catrinoiu, D.; Cheta, D.M.; Guja, C.; Hancu, N.; Ionescu-Tirgoviste, C.; Lichiardopol, R.; et al. Prevalence of Diabetes Mellitus and Prediabetes in the Adult Romanian Population: PREDATORR Study. J. Diabetes 2016, 8, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Gherbon, A.; Frandes, M.; Dîrpeş, D.; Timar, R.; Timar, B. Impact of SGLT-2 Inhibitors on Modifiable Cardiovascular Risk Factors in Romanian Patients with Type 2 Diabetes Mellitus. Diabetol. Metab. Syndr. 2024, 16, 85. [Google Scholar] [CrossRef]
- Morgovan, C.; Cosma, S.A.; Valeanu, M.; Juncan, A.M.; Rus, L.L.; Gligor, F.G.; Butuca, A.; Tit, D.M.; Bungau, S.; Ghibu, S. An Exploratory Research of 18 Years on the Economic Burden of Diabetes for the Romanian National Health Insurance System. Int. J. Environ. Res. Public Health 2020, 17, 4456. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.L.; Morais, C.; Pimenta, R.; Ribeiro, I.; Amorim, I.; Alves, S.M.; Santiago, L. Knowledge about Type 2 Diabetes: Its Impact for Future Management. Front. Public Health 2024, 12, 1328001. [Google Scholar] [CrossRef] [PubMed]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and Type 2 Diabetes Mellitus: Connections in Epidemiology, Pathogenesis, and Treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Beverly, E.A.; Bruemmer, D.; Collins, B.S.; Darville, A.; Ekhlaspour, L.; Hassanein, M.; et al. 5. Facilitating Positive Health Behaviors and Well-Being to Improve Health Outcomes: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, S77–S110. [Google Scholar] [CrossRef]
- Lee, S.H.; Cho, M.H.; Kim, Y.H.; Chung, S. Two Cases of Successful Type 2 Diabetes Control with Lifestyle Modification in Children and Adolescents. J. Obes. Metab. Syndr. 2017, 26, 71–75. [Google Scholar] [CrossRef]
- Bonekamp, N.E.; Visseren, F.L.J.; Cramer, M.J.; Dorresteijn, J.A.N.; Van Der Meer, M.G.; Ruigrok, Y.M.; Van Sloten, T.T.; Teraa, M.; Geleijnse, J.M.; Koopal, C. Long-Term Lifestyle Change and Risk of Mortality and Type 2 Diabetes in Patients with Cardiovascular Disease. Eur. J. Prev. Cardiol. 2024, 31, 205–213. [Google Scholar] [CrossRef]
- Benbaibeche, H.; Saidi, H.; Bounihi, A.; Koceir, E.A. Emotional and External Eating Styles Associated with Obesity. J. Eat. Disord. 2023, 11, 67. [Google Scholar] [CrossRef]
- Onofrei, L.-M.; Puiu, M.; Chirita-Emandi, A.; Serban, C.L. A Comprehensive Analysis Concerning Eating Behavior Associated with Chronic Diseases among Romanian Community Nurses. Front. Public Health 2024, 12, 1368069. [Google Scholar] [CrossRef]
- Mostafazadeh, P.; Jafari, M.J.; Mojebi, M.R.; Nemati-Vakilabad, R.; Shafaghat, A.; Abbasi, A.S.; Mirzaei, A. Assessing the Relationship between Nutrition Literacy and Eating Behaviors among Nursing Students: A Cross-Sectional Study. 2023. Available online: https://www.researchsquare.com/article/rs-3087158/v1 (accessed on 28 March 2024).
- Salvia, M.G.; Ritholz, M.D.; Craigen, K.L.E.; Quatromoni, P.A. Managing Type 2 Diabetes or Prediabetes and Binge Eating Disorder: A Qualitative Study of Patients’ Perceptions and Lived Experiences. J. Eat. Disord. 2022, 10, 148. [Google Scholar] [CrossRef]
- Fung, T.T.; Li, Y.; Bhupathiraju, S.N.; Bromage, S.; Batis, C.; Holmes, M.D.; Stampfer, M.; Hu, F.B.; Deitchler, M.; Willett, W.C. Higher Global Diet Quality Score Is Inversely Associated with Risk of Type 2 Diabetes in US Women. J. Nutr. 2021, 151, 168S–175S. [Google Scholar] [CrossRef]
- Antonio, J.P.; Sarmento, R.A.; De Almeida, J.C. Diet Quality and Glycemic Control in Patients with Type 2 Diabetes. J. Acad. Nutr. Diet. 2019, 119, 652–658. [Google Scholar] [CrossRef]
- Sanjeevi, N.; Freeland-Graves, J.H. Low Diet Quality Is Associated with Adverse Levels of Metabolic Health Markers and Clustering of Risk Factors in Adults with Type 2 Diabetes. J. Hum. Nutr. Diet. 2023, 36, 31–39. [Google Scholar] [CrossRef]
- Kauffman, S.A.E.; Averill, M.M.; Delaney, J.A.C.; Lemaitre, R.N.; Howard, B.V.; Fretts, A.M. Associations of Diet Quality and Blood Serum Lipoprotein Levels in a Population at High Risk for Diabetes: The Strong Heart Family Study. Eur. J. Clin. Nutr. 2020, 74, 1084–1090. [Google Scholar] [CrossRef]
- Shams-White, M.M.; Pannucci, T.E.; Lerman, J.L.; Herrick, K.A.; Zimmer, M.; Meyers Mathieu, K.; Stoody, E.E.; Reedy, J. Healthy Eating Index-2020: Review and Update Process to Reflect the Dietary Guidelines for Americans, 2020–2025. J. Acad. Nutr. Diet. 2023, 123, 1280–1288. [Google Scholar] [CrossRef]
- Kim, S.; Haines, P.S.; Siega-Riz, A.M.; Popkin, B.M. The Diet Quality Index-International (DQI-I) Provides an Effective Tool for Cross-National Comparison of Diet Quality as Illustrated by China and the United States. J. Nutr. 2003, 133, 3476–3484. [Google Scholar] [CrossRef]
- Montagnese, C.; Santarpia, L.; Buonifacio, M.; Nardelli, A.; Caldara, A.R.; Silvestri, E.; Contaldo, F.; Pasanisi, F. European Food-Based Dietary Guidelines: A Comparison and Update. Nutrition 2015, 31, 908–915. [Google Scholar] [CrossRef]
- Gal, A.M.; Iatcu, C.O.; Popa, A.D.; Arhire, L.I.; Mihalache, L.; Gherasim, A.; Nita, O.; Soimaru, R.M.; Gheorghita, R.; Graur, M.; et al. Understanding the Interplay of Dietary Intake and Eating Behavior in Type 2 Diabetes. Nutrients 2024, 16, 771. [Google Scholar] [CrossRef]
- World Health Organization. HEARTS D: Diagnosis and Management of Type 2 Diabetes; World Health Organization: Geneva, Switzerland, 2020.
- Division of Noncommunicable Diseases, World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation on Obesity, Geneva, 3–5 June 1997; World Health Organization: Geneva, Switzerland, 1998.
- Dobiásová, M. AIP—Atherogenic index of plasma as a significant predictor of cardiovascular risk: From research to practice. Vnitr. Lek. 2006, 52, 64–71. [Google Scholar] [PubMed]
- Mulligan, A.A.; Luben, R.N.; Bhaniani, A.; Parry-Smith, D.J.; O’Connor, L.; Khawaja, A.P.; Forouhi, N.G.; Khaw, K.-T. A New Tool for Converting Food Frequency Questionnaire Data into Nutrient and Food Group Values: FETA Research Methods and Availability. BMJ Open 2014, 4, e004503. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, A.; Arhire, L.I.; Niță, O.; Strateanu, R.; Oprescu, A.C.; Graur, M.; Mihalache, L. Can the Epic Food Frequency Questionnaire Be Applied to the Population in Romania. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2015, 119, 856–863. [Google Scholar]
- Norbitt, C.F.; Kimita, W.; Bharmal, S.H.; Ko, J.; Petrov, M.S. Relationship between Habitual Intake of Vitamins and New-Onset Prediabetes/Diabetes after Acute Pancreatitis. Nutrients 2022, 14, 1480. [Google Scholar] [CrossRef]
- Kimita, W.; Li, X.; Ko, J.; Bharmal, S.H.; Cameron-Smith, D.; Petrov, M.S. Association between Habitual Dietary Iron Intake and Glucose Metabolism in Individuals after Acute Pancreatitis. Nutrients 2020, 12, 3579. [Google Scholar] [CrossRef]
- van Strien, T.; Frijters, J.E.; Bergers, G.P.; Defares, P.B. The Dutch Eating Behavior Questionnaire (DEBQ) for Assessment of Restrained, Emotional, and External Eating Behavior. Int. J. Eat. Disord. 1986, 5, 295–315. [Google Scholar] [CrossRef]
- Arhire, L.I.; Niță, O.; Popa, A.D.; Gal, A.-M.; Dumitrașcu, O.; Gherasim, A.; Mihalache, L.; Graur, M. Validation of the Dutch Eating Behavior Questionnaire in a Romanian Adult Population. Nutrients 2021, 13, 3890. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Manta, A.; Cojocaru, E.; Leon-Constantin, M.M.; Maștaleru, A.; Roca, M.; Rusu, C.; Cojocariu, S.A.; Mitu, F. IPAQ-L and CPET Usefulness in a North-Eastern Romanian Population Undergoing Cardiac Rehabilitation. Appl. Sci. 2021, 11, 5483. [Google Scholar] [CrossRef]
- Petrescu, D.C.; Petrescu-Mag, R.M.; Burny, P. Resilience to Environmental Pressure through Quality Food Demand: Meat Consumption in Romania. Environ. Eng. Manag. J. 2017, 16, 2391–2400. [Google Scholar] [CrossRef]
- De Rijk, M.G.; Slotegraaf, A.I.; Brouwer-Brolsma, E.M.; Perenboom, C.W.M.; Feskens, E.J.M.; De Vries, J.H.M. Development and Evaluation of a Diet Quality Screener to Assess Adherence to the Dutch Food-Based Dietary Guidelines. Br. J. Nutr. 2022, 128, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Abassi, M.M.; Sassi, S.; El Ati, J.; Ben Gharbia, H.; Delpeuch, F.; Traissac, P. Gender Inequalities in Diet Quality and Their Socioeconomic Patterning in a Nutrition Transition Context in the Middle East and North Africa: A Cross-Sectional Study in Tunisia. Nutr. J. 2019, 18, 18. [Google Scholar] [CrossRef]
- Spinelli, S.; Dinnella, C.; Tesini, F.; Bendini, A.; Braghieri, A.; Proserpio, C.; Torri, L.; Miele, N.A.; Aprea, E.; Mazzaglia, A.; et al. Gender Differences in Fat-Rich Meat Choice: Influence of Personality and Attitudes. Nutrients 2020, 12, 1374. [Google Scholar] [CrossRef]
- Bärebring, L.; Palmqvist, M.; Winkvist, A.; Augustin, H. Gender Differences in Perceived Food Healthiness and Food Avoidance in a Swedish Population-Based Survey: A Cross Sectional Study. Nutr. J. 2020, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Ritzel, C.; Mann, S. The Old Man and the Meat: On Gender Differences in Meat Consumption across Stages of Human Life. Foods 2021, 10, 2809. [Google Scholar] [CrossRef]
- Egele, V.S.; Stark, R. Specific Health Beliefs Mediate Sex Differences in Food Choice. Front. Nutr. 2023, 10, 1159809. [Google Scholar] [CrossRef] [PubMed]
- Sibhatu, K.T.; Qaim, M. Farm Production Diversity and Dietary Quality: Linkages and Measurement Issues. Food Secur. 2018, 10, 47–59. [Google Scholar] [CrossRef]
- Curi-Quinto, K.; Unar-Munguía, M.; Rodríguez-Ramírez, S.; Röös, E.; Willett, W.C.; Rivera, J.A. Diet Cost and Quality Using the Healthy Eating Index-2015 in Adults from Urban and Rural Areas of Mexico. Public Health Nutr. 2022, 25, 2554–2565. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.J.; Kim, K. Do Where The Elderly Live Matter? Factors Associated with Diet Quality among Korean Elderly Population Living in Urban versus Rural Areas. Nutrients 2020, 12, 1314. [Google Scholar] [CrossRef]
- Helldan, A.; Lallukka, T.; Rahkonen, O.; Lahelma, E. Changes in Healthy Food Habits after Transition to Old Age Retirement. Eur. J. Public Health 2012, 22, 582–586. [Google Scholar] [CrossRef]
- Kosti, R.I.; Di Lorenzo, C.; Panagiotakos, D.B.; Sandeman, G.; Frittella, N.; Iasiello, B.; Teissedre, P.-L.; Restani, P. Dietary and Lifestyle Habits of Drinkers with Preference for Alcoholic Beverage: Does It Really Matter for Public Health? A Review of the Evidence. OENO One 2021, 55. [Google Scholar] [CrossRef]
- Guerrero-Hreins, E.; Stammers, L.; Wong, L.; Brown, R.M.; Sumithran, P. A Comparison of Emotional Triggers for Eating in Men and Women with Obesity. Nutrients 2022, 14, 4144. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.; Harris, J.; Gillespie, S. Changing Diets: Urbanization and the Nutrition Transition. In 2017 Global Food Policy Report; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2017; pp. 34–41. [Google Scholar]
- Casari, S.; Di Paola, M.; Banci, E.; Diallo, S.; Scarallo, L.; Renzo, S.; Gori, A.; Renzi, S.; Paci, M.; De Mast, Q.; et al. Changing Dietary Habits: The Impact of Urbanization and Rising Socio-Economic Status in Families from Burkina Faso in Sub-Saharan Africa. Nutrients 2022, 14, 1782. [Google Scholar] [CrossRef]
- Mela, D.J. Determinants of Food Choice: Relationships with Obesity and Weight Control. Obes. Res. 2001, 9, 249S–255S. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R.E.; Schriever, S.C.; Pfluger, P.T. Physiological and Epigenetic Features of Yoyo Dieting and Weight Control. Front. Genet. 2019, 10, 1015. [Google Scholar] [CrossRef]
- Jacquet, P.; Schutz, Y.; Montani, J.-P.; Dulloo, A. How Dieting Might Make Some Fatter: Modeling Weight Cycling toward Obesity from a Perspective of Body Composition Autoregulation. Int. J. Obes. 2020, 44, 1243–1253. [Google Scholar] [CrossRef]
- Parr, E.B.; Devlin, B.L.; Lim, K.H.C.; Moresi, L.N.Z.; Geils, C.; Brennan, L.; Hawley, J.A. Time-Restricted Eating as a Nutrition Strategy for Individuals with Type 2 Diabetes: A Feasibility Study. Nutrients 2020, 12, 3228. [Google Scholar] [CrossRef] [PubMed]
- Sami, W.; Ansari, T.; Butt, N.S.; Hamid, M.R.A. Effect of Diet on Type 2 Diabetes Mellitus: A Review. Int. J. Health Sci. 2017, 11, 65–71. [Google Scholar]
- Erbakan, A.N.; Arslan Bahadir, M.; Gonen, O.; Kaya, F.N. Mindful Eating and Current Glycemic Control in Patients with Type 2 Diabetes. Cureus 2024, 16, e57198. [Google Scholar] [CrossRef]
- Mensorio, M.S.; Cebolla, A.; Lisón, J.F.; Rodilla, E.; Palomar, G.; Miragall, M.; Baños, R.M. Emotional Eating as a Mediator between Anxiety and Cholesterol in Population with Overweight and Hypertension. Psychol. Health Med. 2017, 22, 911–918. [Google Scholar] [CrossRef]
- Snoek, H.M.; Van Strien, T.; Janssens, J.M.A.M.; Engels, R.C.M.E. Restrained Eating and BMI: A Longitudinal Study among Adolescents. Health Psychol. 2008, 27, 753–759. [Google Scholar] [CrossRef]
- Yong, C.; Liu, H.; Yang, Q.; Luo, J.; Ouyang, Y.; Sun, M.; Xi, Y.; Xiang, C.; Lin, Q. The Relationship between Restrained Eating, Body Image, and Dietary Intake among University Students in China: A Cross-Sectional Study. Nutrients 2021, 13, 990. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, A.; Federbusch, M.; Grellmann, C.; Villringer, A.; Horstmann, A. Body Weight Status, Eating Behavior, Sensitivity to Reward/Punishment, and Gender: Relationships and Interdependencies. Front. Psychol. 2014, 5, 1073. [Google Scholar] [CrossRef]
- Di Daniele, N.; Marrone, G.; Di Lauro, M.; Di Daniele, F.; Palazzetti, D.; Guerriero, C.; Noce, A. Effects of Caloric Restriction Diet on Arterial Hypertension and Endothelial Dysfunction. Nutrients 2021, 13, 274. [Google Scholar] [CrossRef]
- Diab, A.; Dastmalchi, L.N.; Gulati, M.; Michos, E.D. A Heart-Healthy Diet for Cardiovascular Disease Prevention: Where Are We Now? Vasc. Health Risk Manag. 2023, 19, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-K.; Pacchioni, T.G.; Gehi, A.K.; Fitzgerald, K.E.; Tailor, D.V. Emotional Eating and Cardiovascular Risk Factors in the Police Force: The Carolina Blue Project. Int. J. Environ. Res. Public Health 2024, 21, 332. [Google Scholar] [CrossRef] [PubMed]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Andrianasolo, R.M.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultra-Processed Food Intake and Risk of Cardiovascular Disease: Prospective Cohort Study (NutriNet-Santé). BMJ 2019, 365, l1451. [Google Scholar] [CrossRef]
- Liu, D.T.; Prem, B.; Sharma, G.; Kaiser, J.; Besser, G.; Mueller, C.A. Eating Behavior in Patients with Smell Loss. Front. Nutr. 2022, 9, 993639. [Google Scholar] [CrossRef]
- Nagl, M.; Hilbert, A.; de Zwaan, M.; Braehler, E.; Kersting, A. The German Version of the Dutch Eating Behavior Questionnaire: Psychometric Properties, Measurement Invariance, and Population-Based Norms. PLoS ONE 2016, 11, e0162510. [Google Scholar] [CrossRef]
- Betancourt-Núñez, A.; Torres-Castillo, N.; Martínez-López, E.; De Loera-Rodríguez, C.O.; Durán-Barajas, E.; Márquez-Sandoval, F.; Bernal-Orozco, M.F.; Garaulet, M.; Vizmanos, B. Emotional Eating and Dietary Patterns: Reflecting Food Choices in People with and without Abdominal Obesity. Nutrients 2022, 14, 1371. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef] [PubMed]
- Fuente González, C.E.; Chávez-Servín, J.L.; De La Torre-Carbot, K.; Ronquillo González, D.; Aguilera Barreiro, M.D.L.Á.; Ojeda Navarro, L.R. Relationship between Emotional Eating, Consumption of Hyperpalatable Energy-Dense Foods, and Indicators of Nutritional Status: A Systematic Review. J. Obes. 2022, 2022, 4243868. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77.e3. [Google Scholar] [CrossRef]
- Desmet, P.M.A.; Schifferstein, H.N.J. Sources of Positive and Negative Emotions in Food Experience. Appetite 2008, 50, 290–301. [Google Scholar] [CrossRef]
- Mastinu, M.; Melis, M.; Yousaf, N.Y.; Barbarossa, I.T.; Tepper, B.J. Emotional Responses to Taste and Smell Stimuli: Self-reports, Physiological Measures, and a Potential Role for Individual and Genetic Factors. J. Food Sci. 2023, 88, A65–A90. [Google Scholar] [CrossRef]
- Nguyen-Michel, S.T.; Unger, J.B.; Spruijt-Metz, D. Dietary Correlates of Emotional Eating in Adolescence. Appetite 2007, 49, 494–499. [Google Scholar] [CrossRef]
- Wei, X.; Yang, W.; Wang, J.; Zhang, Y.; Wang, Y.; Long, Y.; Tan, B.; Wan, X. Health Effects of Whole Grains: A Bibliometric Analysis. Foods 2022, 11, 4094. [Google Scholar] [CrossRef] [PubMed]
- Elfhag, K.; Tholin, S.; Rasmussen, F. Consumption of Fruit, Vegetables, Sweets and Soft Drinks Are Associated with Psychological Dimensions of Eating Behaviour in Parents and Their 12-Year-Old Children. Public Health Nutr. 2008, 11, 914–923. [Google Scholar] [CrossRef]
- Shukri, M.; Jones, F.; Conner, M. Relationship between Work-Family Conflict and Unhealthy Eating: Does Eating Style Matter? Appetite 2018, 123, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Jalo, E.; Fogelholm, M.; Westerterp-Plantenga, M.; Adam, T.C.; Drummen, M.; Huttunen-Lenz, M.; Kjølbæk, L.; Martinez, J.A.; Handjieva-Darlenska, T.; Taylor, M.A.; et al. Role of Eating Behavior and Stress in Maintenance of Dietary Changes during the PREVIEW Intervention. J. Nutr. Educ. Behav. 2024, 56, 276–286. [Google Scholar] [CrossRef]
- Paans, N.P.G.; Gibson-Smith, D.; Bot, M.; van Strien, T.; Brouwer, I.A.; Visser, M.; Penninx, B.W.J.H. Depression and Eating Styles Are Independently Associated with Dietary Intake. Appetite 2019, 134, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Ljubičić, M.; Matek Sarić, M.; Klarin, I.; Rumbak, I.; Colić Barić, I.; Ranilović, J.; Dželalija, B.; Sarić, A.; Nakić, D.; Djekic, I.; et al. Emotions and Food Consumption: Emotional Eating Behavior in a European Population. Foods 2023, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Ha, O.-R.; Lim, S.-L. The Role of Emotion in Eating Behavior and Decisions. Front. Psychol. 2023, 14, 1265074. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Walker, D.; Laczniak, R. Attention Mediates Restrained Eaters’ Food Consumption Intentions. Food Qual. Prefer. 2022, 96, 104382. [Google Scholar] [CrossRef]
- Lawless, M.; Shriver, L.H.; Wideman, L.; Dollar, J.M.; Calkins, S.D.; Keane, S.P.; Shanahan, L. Associations between Eating Behaviors, Diet Quality and Body Mass Index among Adolescents. Eat. Behav. 2020, 36, 101339. [Google Scholar] [CrossRef]
| Total | Men | Women | p-Value | ||
|---|---|---|---|---|---|
| Duration of diabetes (years) | 7.45 ± 5.70 | 7.20 ± 5.29 | 7.64 ± 6.01 | 0.490 | |
| Age (years) | 61.89 ± 9.69 | 60.58 ± 10.61 | 62.91 ± 8.81 | 0.035 | |
| Duration of diabetes | 7.45 ± 5.70 | 7.20 ± 5.28 | 7.64 ± 6.01 | 0.497 | |
| Weight (kg) | 87.34 ± 18.25 | 95.20 ± 18.42 | 81.25 ± 15.64 | <0.001 | |
| BMI (kg/m2) | 31.79 ± 5.52 | 31.45 ± 5.43 | 32.05 ± 5.59 | 0.343 | |
| WC (cm) | 107.27 ± 14.12 | 111.26 ± 12.88 | 104.23 ± 14.29 | <0.001 | |
| SBP (mmHg) | 141.06 ± 20.34 | 142.49 ± 21.68 | 139.99 ± 19.27 | 0.287 | |
| DBP (mmHg) | 83.71 ± 12.38 | 86.71 ± 12.12 | 81.47 ± 12.13 | <0.001 | |
| FBG | 149.31 ± 46.47 | 149.15 ± 53.08 | 149.44 ± 40.78 | 0.956 | |
| HbA1C | 7.29 ± 1.58 | 7.29 ± 1.71 | 7.29 ± 1.49 | 0.977 | |
| TC (mg/dL) | 194.89 ± 47.09 | 187.82 ± 38.59 | 200.37 ± 52.18 | 0.019 | |
| HDL-c (mg/dL) | 48.93 ± 14.26 | 45.51 ± 12.53 | 51.59 ± 14.97 | <0.001 | |
| LDL-c (mg/dL) | 120.28 ± 39.69 | 118.89 ± 34.70 | 121.35 ± 43.20 | 0.592 | |
| TG (mg/dL) | 165.99 ± 87.35 | 156.72 ± 73.76 | 173.16 ± 96.16 | 0.098 | |
| AIP | 0.49 ± 0.27 | 0.50 ± 0.26 | 0.49 ± 0.28 | 0.652 | |
| Daily energy intake (kcal) | 1581.71 ± 619.83 | 1678.71 ± 500.94 | 1506.62 ± 690.18 | 0.014 | |
| Physical activity level (MET) | 3369.68 ± 3351.62 | 4235.99 ± 3970.90 | 2699.15 ± 2599.38 | <0.001 | |
| Area of residence | Urban | 64.00 (201) | 67.9 (93) | 61.0 (108) | 0.209 |
| Rural | 36 (113) | 32.1 (44) | 39.0 (69) | ||
| Employee-status | Employee | 25.2 (79) | 38.7 (53) | 14.7 (26) | <0.001 |
| Retired | 65.6 (206) | 54.7 (75) | 74.0 (131) | ||
| Unemployed | 9.2 (29) | 6.6 (9) | 11.3 (20) | ||
| Smoking status | Non-smoker | 90.4 (284) | 84.7 (116) | 94.9 (168) | 0.005 |
| Smoker | 7.6 (24) | 13.1 (18) | 3.4 (6) | ||
| Undeclared | 1.9 (6) | 2.2 (3) | 1.7 (3) | ||
| Alcohol consumption | No | 51.6 (162) | 27.00 (37) | 70.6 (125) | <0.001 |
| Yes | 48.4 (152) | 73.00 (100) | 29.4 (52) | ||
| BMI status | Without obesity | 40.8 (128) | 43.8 (60) | 38.4 (68) | 0.356 |
| With obesity | 59.2 (186) | 56.2 (77) | 61.6 (109) | ||
| Component | Score | Total | Men | Women | p-Value |
|---|---|---|---|---|---|
| Variety | 0–20 | 18.31 ± 2.29 | 18.28 ± 2.36 | 18.33 ± 2.24 | 0.831 |
| Food groups variety | 0–15 | 13.71 ± 1.72 | 13.64 ± 1.81 | 13.76 ± 1.64 | 0.540 |
| Protein sources variety | 0–5 | 4.60 ± 0.98 | 4.64 ± 0.91 | 4.57 ± 1.04 | 0.567 |
| Adequacy | 0–40 | 28.57 ± 4.94 | 28.84 ± 4.98 | 28.36 ± 4.92 | 0.392 |
| Vegetable group | 0–5 | 4.33 ± 1.12 | 4.15 ± 1.19 | 4.46 ± 1.06 | 0.017 |
| Fruit group | 0–5 | 4.45 ± 1.23 | 4.28 ± 1.38 | 4.58 ± 1.08 | 0.035 |
| Grain group | 0–5 | 0.67 ± 1.27 | 0.80 ± 1.36 | 0.57 ± 1.20 | 0.116 |
| Fiber | 0–5 | 3.56 ± 1.19 | 3.64 ± 1.09 | 3.51 ± 1.27 | 0.354 |
| Protein | 0–5 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 | - |
| Iron | 0–5 | 4.45 ± 1.02 | 4.78 ± 0.62 | 4.19 ± 1.18 | <0.001 |
| Calcium | 0–5 | 2.05 ± 1.58 | 2.49 ± 1.49 | 1.72 ± 1.58 | <0.001 |
| Vitamin C | 0–5 | 4.05 ±1.32 | 3.70 ± 1.42 | 4.32 ± 1.18 | <0.001 |
| Moderation | 0–30 | 12.59 ± 6.54 | 10.77 ± 6.84 | 14.00 ± 5.94 | <0.001 |
| Total fat | 0–6 | 1.34 ± 1.60 | 1.12 ± 1.58 | 1.51 ± 1.60 | 0.032 |
| Saturated fat | 0–6 | 1.67 ± 1.91 | 1.45 ± 1.92 | 1.85 ± 1.89 | 0.065 |
| Cholesterol | 0–6 | 4.11 ± 2.23 | 3.50 ± 2.25 | 4.58 ± 2.09 | <0.001 |
| Sodium | 0–6 | 3.21 ± 2.16 | 2.56 ± 2.13 | 3.71 ± 2.04 | <0.001 |
| Empty-calorie foods | 0–6 | 2.26 ± 1.99 | 2.15 ± 1.99 | 2.36 ± 1.99 | 0.355 |
| Overall balance | 0–10 | 0.75 ± 1.31 | 0.79 ± 1.35 | 0.71 ± 1.28 | 0.613 |
| Macronutrient ratio | 0–6 | 0.13 ± 0.65 | 0.04 ± 0.38 | 0.20 ± 0.80 | 0.020 |
| Fatty-acid ratio | 0–4 | 0.61 ± 1.14 | 0.74 ± 1.23 | 0.51 ± 1.06 | 0.076 |
| Total DQI-I score | 0–100 | 60.21 ± 7.72 | 58.68 ± 8.24 | 61.40 ± 7.09 | 0.002 |
| Variables | Total DQI | Variety | Adequacy | Moderation | Overall Diet | |
|---|---|---|---|---|---|---|
| Area of residence | Urban | 60.31 ± 7.96 | 18.37 ± 2.32 | 28.55 ± 5.06 a | 2.61 ± 6.52 | 0.78 ± 1.32 |
| Rural | 60.04 ± 7.29 | 18.19 ± 2.25 | 28.60 ± 4.75 b | 12.56 ± 6.60 | 0.69 ± 1.30 | |
| Employee status | Employee | 58.87 ± 7.36 | 18.15 ± 2.36 | 29.00 ± 4.96 | 10.86 ± 6.10 a | 0.86 ± 1.38 |
| Retired | 60.63 ± 7.81 | 18.31 ± 2.33 | 28.50 ± 4.90 | 13.15 ± 6.59 b | 0.67 ± 1.20 | |
| Unemployed | 60.93 ± 7.82 | 18.76 ± 1.82 | 18.76 ± 1.82 | 13.34 ± 6.68 | 0.97 ± 1.82 | |
| Smoking status | Smoker | 60.25 ± 7.62 | 18.29 ± 2.30 | 28.47 ± 4.96 | 12.76 ± 6.42 | 0.73 ± 1.25 |
| Non-smoker | 59.54 ± 7.91 | 18.29 ± 2.27 | 30.42 ± 3.94 | 10.00 ± 7.48 | 0.83 ± 1.85 | |
| Undeclared | 61.00 ± 12.37 | 19.17 ± 2.04 | 25.83 ± 6.24 | 15.00 ± 6.57 | 1.00 ± 1.67 | |
| Alcohol consumption | No | 61.81 ± 7.67 a | 18.34 ± 2.22 | 28.12 ± 4.63 | 14.50 ± 5.99 a | 0.85 ± 1.33 |
| Yes | 58.51 ± 7.42 b | 18.28 ± 2.38 | 29.04 ± 5.24 | 10.56 ± 6.50 b | 0.63 ± 1.29 | |
| BMI Status | Without obesity | 60.52 ± 8.09 | 18.25 ± 2.31 | 28.58 ± 4.98 | 12.98 ± 6.47 | 0.70 ± 1.11 |
| With obesity | 60.01 ± 7.47 | 18.35 ± 2.27 | 28.56 ± 4.93 | 12.32 ± 6.58 | 0.77 ± 1.44 | |
| Gender | Area of Residence | BMI Status | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Men | Women | p-Value | Urban | Rural | p-Value | Without Obesity | With Obesity | p-Value | |
| EmoE | 1.53 ± 0.81 | 2.00 ± 1.18 | <0.001 | 1.89 ± 1.04 | 1.63 ± 1.06 | 0.034 | 1.72 ± 1.00 | 1.85 ± 1.10 | 0.277 |
| ExtE | 2.30 ± 0.96 | 2.45 ± 1.02 | 0.175 | 2.44 ± 1.00 | 2.28 ± 0.98 | 0.179 | 2.36 ± 1.03 | 2.40 ± 0.98 | 0.734 |
| RE | 2.05 ± 0.89 | 2.39 ± 0.99 | 0.002 | 2.29 ± 0.94 | 2.15 ± 0.99 | 0.228 | 2.04 ± 0.85 | 2.38 ± 1.01 | 0.002 |
| EmoE | ExtE | RE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DQI-I Component | β | 95%CI | Partial R2 | p-Value | β | 95%CI | Partial R2 | p-Value | β | 95%CI | Partial R2 | p-Value |
| Variety | −0.004 | −0.262–0.243 | 0.016 | 0.942 | −0.095 | −0.485–0.051 | 0.024 | 0.112 | −0.015 | −0.312–0.240 | 0.016 | 0.800 |
| Food groups | −0.013 | −0.210–0.168 | 0.018 | 0.825 | −0.111 | −0.391–0.009 | 0.029 | 0.061 | −0.032 | −0.264–0.149 | 0.019 | 0.586 |
| Protein sources | 0.013 | −0.097–0.121 | 0.010 | 0.829 | −0.026 | −0.142–0.090 | 0.010 | 0.664 | 0.021 | −0.098–0.141 | 0.010 | 0.722 |
| Adequacy | 0.040 | −0.306–0.683 | 0.186 | 0.454 | 0.092 | −0.070–0.980 | 0.192 | 0.089 | 0.055 | −0.258–0.823 | 0.187 | 0.305 |
| Vegetable group | 0.056 | −0.064–0.183 | 0.026 | 0.342 | 0.084 | −0.037–0.226 | 0.030 | 0.157 | 0.075 | −0.047–0.223 | 0.028 | 0.202 |
| Fruit group | 0.026 | −0.105–0.164 | 0.027 | 0.665 | −0.026 | −0.176–0.111 | 0.027 | 0.655 | 0.125 | 0.013–0.306 | 0.041 | 0.033 |
| Grain group | 0.090 | −0.024–0.241 | 0.128 | 0.107 | 0.208 | 0.126–0.403 | 0.159 | <0.001 | −0.040 | −0.198–0.093 | 0.122 | 0.476 |
| Fiber | 0.051 | −0.064–0.179 | 0.160 | 0.353 | 0.087 | −0.026–0.233 | 0.165 | 0.116 | 0.081 | −0.032–0.234 | 0.164 | 0.136 |
| Iron | −0.078 | −0.178–0.028 | 0.175 | 0.152 | 0.002 | −0.108–0.111 | 0.169 | 0.977 | −0.044 | −0.159–0.066 | 0.171 | 0.414 |
| Calcium | −0.093 | −0.288–0.010 | 0.284 | 0.068 | −0.041 | −0.224–0.095 | 0.278 | 0.425 | −0.095 | −0.319–0.007 | 0.285 | 0.060 |
| Vitamin C | 0.117 | 0.003–0.289 | 0.055 | 0.045 | 0.066 | −0.065–0.241 | 0.047 | 0.259 | 0.138 | 0.034–0.346 | 0.060 | 0.017 |
| Moderation | 0.059 | −0.195–0.925 | 0.403 | 0.200 | −0.036 | −0.832–0.363 | 0.401 | 0.440 | 0.048 | −0.288–0.938 | 0.402 | 0.298 |
| Total fat | −0.015 | −0.192–0.147 | 0.093 | 0.797 | 0.023 | −0.143–0.217 | 0.093 | 0.686 | −0.048 | −0.264–0.106 | 0.095 | 0.401 |
| Saturated fat | 0.082 | −0.050–0.347 | 0.126 | 0.141 | 0.017 | −0.179–0.245 | 0.120 | 0.759 | −0.007 | −0.231–0.204 | 0.120 | 0.902 |
| Cholesterol | 0.087 | −0.018–0.383 | 0.342 | 0.074 | 0.019 | −0.171–0.258 | 0.336 | 0.692 | 0.059 | −0.084–0.356 | 0.339 | 0.225 |
| Sodium | 0.103 | 0.026–0.395 | 0.404 | 0.026 | −0.046 | −0.298–0.098 | 0.396 | 0.321 | 0.058 | −0.073–0.334 | 0.398 | 0.209 |
| Empty-calorie foods | −0.082 | −0.368–0.059 | 0.068 | 0.155 | −0.125 | −0.474–−0.022 | 0.076 | 0.032 | 0.073 | −0.082–0.385 | 0.067 | 0.202 |
| Overall balance | 0.033 | −0.103–0.185 | 0.020 | 0.577 | −0.086 | −0.267–0.040 | 0.026 | 0.146 | 0.012 | −0.142–0.174 | 0.019 | 0.843 |
| Macronutrient ratio | 0.053 | −0.039–0.105 | 0.017 | 0.374 | 0.091 | −0.017–0.136 | 0.022 | 0.127 | −0.020 | −0.092–0.065 | 0.015 | 0.739 |
| Fatty-acid ratio | 0.008 | −0.116–0.133 | 0.033 | 0.895 | −0.151 | −0.305–0.042 | 0.054 | 0.010 | 0.025 | −0.107–0.166 | 0.034 | 0.673 |
| Total DQI-I score | 0.080 | −0.228–1.399 | 0.096 | 0.158 | −0.014 | −0.979–0.760 | 0.091 | 0.804 | 0.073 | −0.303–1.478 | 0.095 | 0.195 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gal, A.M.; Arhire, L.I.; Gherasim, A.; Graur, M.; Nita, O.; Dumitrascu, O.; Soimaru, R.M.; Popa, A.D.; Mihalache, L. Association between Diet Quality and Eating Behavior in Type 2 Diabetes Adults: A Cross-Sectional Study. Nutrients 2024, 16, 2047. https://doi.org/10.3390/nu16132047
Gal AM, Arhire LI, Gherasim A, Graur M, Nita O, Dumitrascu O, Soimaru RM, Popa AD, Mihalache L. Association between Diet Quality and Eating Behavior in Type 2 Diabetes Adults: A Cross-Sectional Study. Nutrients. 2024; 16(13):2047. https://doi.org/10.3390/nu16132047
Chicago/Turabian StyleGal, Ana Maria, Lidia Iuliana Arhire, Andreea Gherasim, Mariana Graur, Otilia Nita, Oana Dumitrascu, Raluca Meda Soimaru, Alina Delia Popa, and Laura Mihalache. 2024. "Association between Diet Quality and Eating Behavior in Type 2 Diabetes Adults: A Cross-Sectional Study" Nutrients 16, no. 13: 2047. https://doi.org/10.3390/nu16132047
APA StyleGal, A. M., Arhire, L. I., Gherasim, A., Graur, M., Nita, O., Dumitrascu, O., Soimaru, R. M., Popa, A. D., & Mihalache, L. (2024). Association between Diet Quality and Eating Behavior in Type 2 Diabetes Adults: A Cross-Sectional Study. Nutrients, 16(13), 2047. https://doi.org/10.3390/nu16132047







