Effects of a Ketogenic Diet on Body Composition in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial
Abstract
1. Introduction
2. Materials and Methods
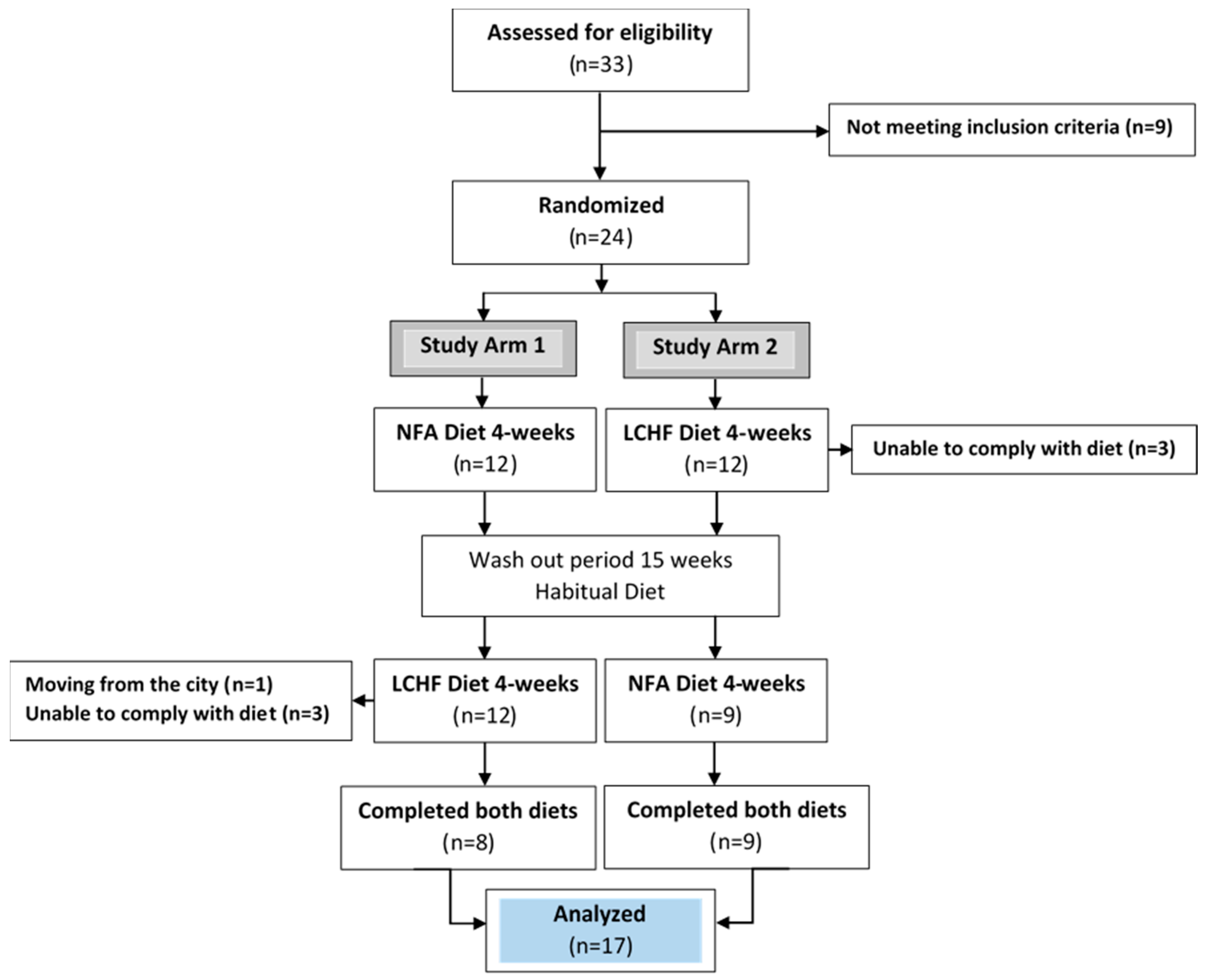
2.1. Study and Design Overview
2.2. Ethics
2.3. Eligibility Criteria, Participants, and Study Setting
2.4. Allocation and Blinding
2.5. Study Diets
2.6. Dietary Adherence and Physical Activity
2.7. Body Composition
2.8. Statistical Analyses and Power Calculations
3. Results
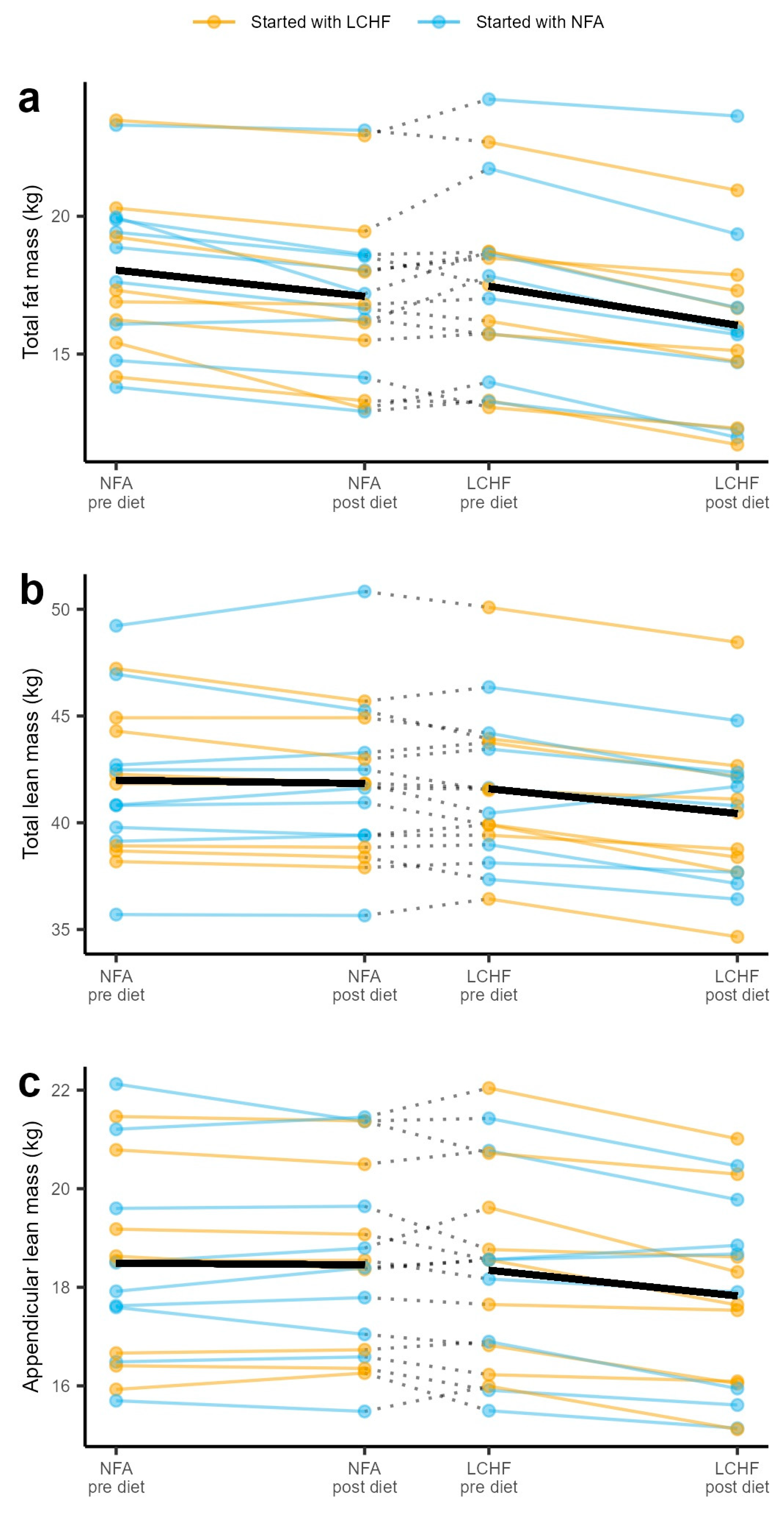
3.1. Dual-Energy X-ray Absorptiometry Parameters
3.2. Dual-Energy X-ray Absorptiometry Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Longo, R.; Peri, C.; Cricrì, D.; Coppi, L.; Caruso, D.; Mitro, N.; De Fabiani, E.; Crestani, M. Ketogenic Diet: A New Light Shining on Old but Gold Biochemistry. Nutrients 2019, 11, 2497. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Ratamess, N.A.; Faigenbaum, A.D.; Bush, J.A. Ergogenic Properties of Ketogenic Diets in Normal-Weight Individuals: A Systematic Review. J. Am. Coll. Nutr. 2020, 39, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Aragon, A.A.; Schoenfeld, B.J.; Wildman, R.; Kleiner, S.; VanDusseldorp, T.; Taylor, L.; Earnest, C.P.; Arciero, P.J.; Wilborn, C.; Kalman, D.S.; et al. International society of sports nutrition position stand: Diets and body composition. J. Int. Soc. Sports Nutr. 2017, 14, 16. [Google Scholar] [CrossRef]
- Varlamov, O.; Bethea, C.L.; Roberts, C.T., Jr. Sex-specific differences in lipid and glucose metabolism. Front. Endocrinol. 2014, 5, 241. [Google Scholar] [CrossRef] [PubMed]
- Durkalec-Michalski, K.; Nowaczyk, P.M.; Siedzik, K. Effect of a four-week ketogenic diet on exercise metabolism in CrossFit-trained athletes. J. Int. Soc. Sports Nutr. 2019, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Urbain, P.; Strom, L.; Morawski, L.; Wehrle, A.; Deibert, P.; Bertz, H. Impact of a 6-week non-energy-restricted ketogenic diet on physical fitness, body composition and biochemical parameters in healthy adults. Nutr. Metab. 2017, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.E.; Carrigan, C.T.; Margolis, L.M. High-Fat Ketogenic Diets and Physical Performance: A Systematic Review. Adv. Nutr. 2021, 12, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Burén, J.; Ericsson, M.; Damasceno, N.R.T.; Sjödin, A. A Ketogenic Low-Carbohydrate High-Fat Diet Increases LDL Cholesterol in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial. Nutrients 2021, 13, 814. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.A.; Varley, B.J.; Hartwig, T.B.; Chapman, P.; Rigney, M. A Low-Carbohydrate Ketogenic Diet Reduces Body Mass Without Compromising Performance in Powerlifting and Olympic Weightlifting Athletes. J. Strength. Cond. Res. 2018, 32, 3373–3382. [Google Scholar] [CrossRef]
- Mah, E.; Schulz, J.A.; Kaden, V.N.; Lawless, A.L.; Rotor, J.; Mantilla, L.B.; Liska, D.J. Cashew consumption reduces total and LDL cholesterol: A randomized, crossover, controlled-feeding trial. Am. J. Clin. Nutr. 2017, 105, 1070–1078. [Google Scholar] [CrossRef]
- Bähr, M.; Fechner, A.; Krämer, J.; Kiehntopf, M.; Jahreis, G. Lupin protein positively affects plasma LDL cholesterol and LDL:HDL cholesterol ratio in hypercholesterolemic adults after four weeks of supplementation: A randomized, controlled crossover study. Nutr. J. 2013, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Lafeber, M.; Grobbee, D.E.; Schrover, I.M.; Thom, S.; Webster, R.; Rodgers, A.; Visseren, F.L.; Bots, M.L.; Spiering, W. Comparison of a morning polypill, evening polypill and individual pills on LDL-cholesterol, ambulatory blood pressure and adherence in high-risk patients; a randomized crossover trial. Int. J. Cardiol. 2015, 181, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Sjödin, A.; Hellström, F.; Burén, J. Effects of a Ketogenic Low Carbohydrate High Fat Diet and a Diet Based on the Nordic Nutrition Recommendations: Study Protocol for an Explorative Randomized Controlled Trial with Crossover Design in Healthy Young Women. Available online: https://osf.io/t87yk/ (accessed on 12 December 2019).
- Sjödin, A.; Hellström, F.; Sehlstedt, E.; Svensson, M.; Burén, J. Effects of a Ketogenic Diet on Muscle Fatigue in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial. Nutrients 2020, 12, 955. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.W.; McPherson, G.C.; Ramsay, C.R.; Campbell, M.K. The method of minimization for allocation to clinical trials: A review. Control. Clin. Trials 2002, 23, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Treasure, T.; MacRae, K.D. Minimisation: The platinum standard for trials?: Randomisation doesn’t guarantee similarity of groups; minimisation does. bmj 1998, 317, 362–363. [Google Scholar] [CrossRef]
- Evans, S.; Royston, P.; Day, S. MINIM: Allocation by Minimisation in Clinical Trials [Computer Program]. Available online: http://www-users.york.ac.uk/~mb55/guide/minim.htm (accessed on 30 March 2011).
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity; Nordic Council of Ministers: Copenhagen, Denmark, 2014; p. 627. [Google Scholar]
- Kenward, M.G.; Roger, J.H. The use of baseline covariates in crossover studies. Biostatistics 2010, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kuchkuntla, A.R.; Shah, M.; Velapati, S.; Gershuni, V.M.; Rajjo, T.; Nanda, S.; Hurt, R.T.; Mundi, M.S. Ketogenic Diet: An Endocrinologist Perspective. Curr. Nutr. Rep. 2019, 8, 402–410. [Google Scholar] [CrossRef]
- Felig, P.; Marliss, E.; Owen, O.E.; Cahill, G.F., Jr. Blood glucose and cluconeogenesis in fasting man. Arch. Intern. Med. 1969, 123, 293–298. [Google Scholar] [CrossRef]
- Owen, O.E.; Morgan, A.P.; Kemp, H.G.; Sullivan, J.M.; Herrera, M.G.; Cahill, G.F., Jr. Brain metabolism during fasting. J. Clin. Investig. 1967, 46, 1589–1595. [Google Scholar] [CrossRef]
- Paoli, A.; Tinsley, G.M.; Mattson, M.P.; De Vivo, I.; Dhawan, R.; Moro, T. Common and divergent molecular mechanisms of fasting and ketogenic diets. Trends Endocrinol. Metab. 2024, 35, 125–141. [Google Scholar] [CrossRef]
- Szwed, A.; Kim, E.; Jacinto, E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol. Rev. 2021, 101, 1371–1426. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.L.; Carrigan, C.T.; Margolis, L.M. Body composition changes in physically active individuals consuming ketogenic diets: A systematic review. J. Int. Soc. Sports Nutr. 2021, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; White, I.R.; Lee, D.S.; van Buuren, S. Missing Data in Clinical Research: A Tutorial on Multiple Imputation. Can. J. Card. 2021, 37, 1322–1331. [Google Scholar] [CrossRef]
- Hall, K.D.; Chen, K.Y.; Guo, J.; Lam, Y.Y.; Leibel, R.L.; Mayer, L.E.; Reitman, M.L.; Rosenbaum, M.; Smith, S.R.; Walsh, B.T.; et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am. J. Clin. Nutr. 2016, 104, 324–333. [Google Scholar] [CrossRef]
- Phinney, S.D.; Bistrian, B.R.; Wolfe, R.R.; Blackburn, G.L. The human metabolic response to chronic ketosis without caloric restriction: Physical and biochemical adaptation. Metab. Clin. Exp. 1983, 32, 757–768. [Google Scholar] [CrossRef]
- Kreitzman, S.N.; Coxon, A.Y.; Szaz, K.F. Glycogen storage: Illusions of easy weight loss, excessive weight regain, and distortions in estimates of body composition. Am. J. Clin. Nutr. 1992, 56, 292s–293s. [Google Scholar] [CrossRef]
- Phinney, S.D. Ketogenic diets and physical performance. Nutr. Metab. 2004, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Bianco, A.; Grimaldi, K.A. The Ketogenic Diet and Sport: A Possible Marriage? Exerc. Sport. Sci. Rev. 2015, 43, 153–162. [Google Scholar] [CrossRef]
- Fernández-Elías, V.E.; Ortega, J.F.; Nelson, R.K.; Mora-Rodriguez, R. Relationship between muscle water and glycogen recovery after prolonged exercise in the heat in humans. Eur. J. Appl. Physiol. 2015, 115, 1919–1926. [Google Scholar] [CrossRef]
- Wilson, J.M.; Lowery, R.P.; Roberts, M.D.; Sharp, M.H.; Joy, J.M.; Shields, K.A.; Partl, J.M.; Volek, J.S.; D‘Agostino, D.P. Effects of Ketogenic Dieting on Body Composition, Strength, Power, and Hormonal Profiles in Resistance Training Men. J. Strength Cond. Res. 2020, 34, 3463–3474. [Google Scholar] [CrossRef]
- Volek, J.S.; Freidenreich, D.J.; Saenz, C.; Kunces, L.J.; Creighton, B.C.; Bartley, J.M.; Davitt, P.M.; Munoz, C.X.; Anderson, J.M.; Maresh, C.M.; et al. Metabolic characteristics of keto-adapted ultra-endurance runners. Metab. Clin. Exp. 2016, 65, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.C.; Noakes, T.D.; Chacko, S.K.; Swart, J.; Kohn, T.A.; Smith, J.A. Gluconeogenesis during endurance exercise in cyclists habituated to a long-term low carbohydrate high-fat diet. J. Physiol. 2016, 594, 4389–4405. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Cao, J.J.; Margolis, L.M.; Sauter, E.R.; Whigham, L.D.; McClung, J.P.; Rood, J.C.; Carbone, J.W.; Combs, G.F., Jr.; Young, A.J. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: A randomized controlled trial. FASEB J. 2013, 27, 3837–3847. [Google Scholar] [CrossRef] [PubMed]
- Mettler, S.; Mitchell, N.; Tipton, K.D. Increased protein intake reduces lean body mass loss during weight loss in athletes. Med. Sci. Sports Exerc. 2010, 42, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Longland, T.M.; Oikawa, S.Y.; Mitchell, C.J.; Devries, M.C.; Phillips, S.M. Higher compared with lower dietary protein during an energy deficit combined with intense exercise promotes greater lean mass gain and fat mass loss: A randomized trial. Am. J. Clin. Nutr. 2016, 103, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Brehm, B.J.; Spang, S.E.; Lattin, B.L.; Seeley, R.J.; Daniels, S.R.; D‘Alessio, D.A. The role of energy expenditure in the differential weight loss in obese women on low-fat and low-carbohydrate diets. J. Clin. Endocrinol. Metab. 2005, 90, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Noakes, M.; Foster, P.R.; Keogh, J.B.; James, A.P.; Mamo, J.C.; Clifton, P.M. Comparison of isocaloric very low carbohydrate/high saturated fat and high carbohydrate/low saturated fat diets on body composition and cardiovascular risk. Nutr. Metab. 2006, 3, 7. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Willoughby, D.S. Fat-Free Mass Changes During Ketogenic Diets and the Potential Role of Resistance Training. Int. J. Sport. Nutr. Exerc. Metab. 2016, 26, 78–92. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Bagheri, R.; Asbaghi, O.; Tinsley, G.M.; Kooti, W.; Abbasnezhad, A.; Afrisham, R.; Wong, A. Effects of resistance training combined with a ketogenic diet on body composition: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 5717–5732. [Google Scholar] [CrossRef]
- Thomson, D.M. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018, 19, 3125. [Google Scholar] [CrossRef]
- Angelidi, A.M.; Stefanakis, K.; Chou, S.H.; Valenzuela-Vallejo, L.; Dipla, K.; Boutari, C.; Ntoskas, K.; Tokmakidis, P.; Kokkinos, A.; Goulis, D.G.; et al. Relative Energy Deficiency in sport (REDs): Endocrine manifestations, pathophysiology and treatments. Endocr. Rev. 2024, bnae011. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Median | (Min–Max) |
|---|---|---|
| Age | 23.8 | (19.7–27.3) |
| Body weight (kg) | 60.8 | (50.8–70.9) |
| Body height (cm) | 167.6 | (158.0–178.2) |
| BMI (kg/m2) | 22.0 | (19.4–24.0) |
| Waist (cm) | 71.0 | (67.0–78.0) |
| Hip (cm) | 97.0 | (87.5–100.0) |
| WHR | 0.76 | (0.70–0.81) |
| VO2max (mL/kg/min) | 43.65 | (37.7–50.7) |
| Parameters | LCHF Diet | NFA Diet | ||
|---|---|---|---|---|
| (Mean ± SD) | (Mean ± SD) | |||
| Pre-Diet | Post-Diet | Pre-Diet | Post-Diet | |
| FMI | 6.2 ± 1.1 | 5.7 ± 1.0 | 6.4 ± 0.9 | 6.1 ± 1.0 |
| LMI | 14.8 ± 0.8 | 14.4 ± 0.7 | 15.0 ± 0.8 | 14.9 ± 0.8 |
| ALMI | 6.5 ± 0.5 | 6.3 ± 0.5 | 6.6 ± 0.5 | 6.6 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burén, J.; Svensson, M.; Liv, P.; Sjödin, A. Effects of a Ketogenic Diet on Body Composition in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial. Nutrients 2024, 16, 2030. https://doi.org/10.3390/nu16132030
Burén J, Svensson M, Liv P, Sjödin A. Effects of a Ketogenic Diet on Body Composition in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial. Nutrients. 2024; 16(13):2030. https://doi.org/10.3390/nu16132030
Chicago/Turabian StyleBurén, Jonas, Michael Svensson, Per Liv, and Anna Sjödin. 2024. "Effects of a Ketogenic Diet on Body Composition in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial" Nutrients 16, no. 13: 2030. https://doi.org/10.3390/nu16132030
APA StyleBurén, J., Svensson, M., Liv, P., & Sjödin, A. (2024). Effects of a Ketogenic Diet on Body Composition in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial. Nutrients, 16(13), 2030. https://doi.org/10.3390/nu16132030






