Early Methylglyoxal Exposure Leads to Worsened Cardiovascular Function in Young Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Models
2.1.1. Protocol 1—Early Glycotoxin Exposure through Breast Milk
2.1.2. Protocol 2—Early Glycotoxin Exposure through Maternal GLO-1 Inhibition during Lactation
2.2. Plasma Biochemical Measurements
2.3. Ex Vivo Experiments
2.3.1. Langendorff Heart
2.3.2. Isolated Aorta Rings
2.4. Morphological Analysis
2.4.1. Measurement of Cardiomyocyte Diameter and Intima–Media Thickness in Aortas
2.4.2. Perivascular Fibrosis Measurement
2.4.3. Interstitial Fibrosis Measurement
2.5. Statistical Analysis
3. Results
3.1. Effects of Early Methylglyoxal Exposure during Lactation on the Phenotype of Young Offspring
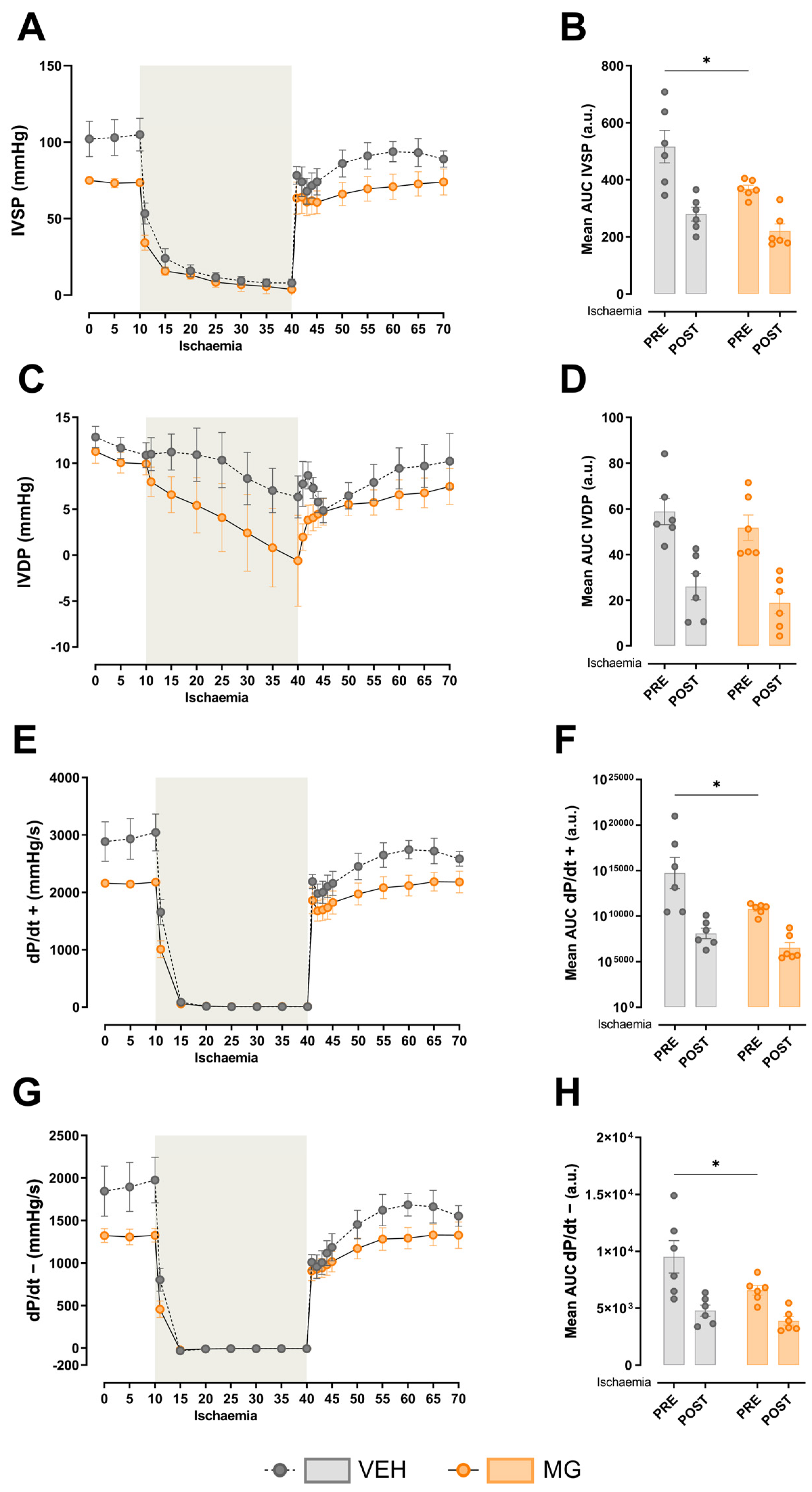
3.2. Effects of Early Methylglyoxal Exposure during Lactation on Left Ventricular Cardiac Function
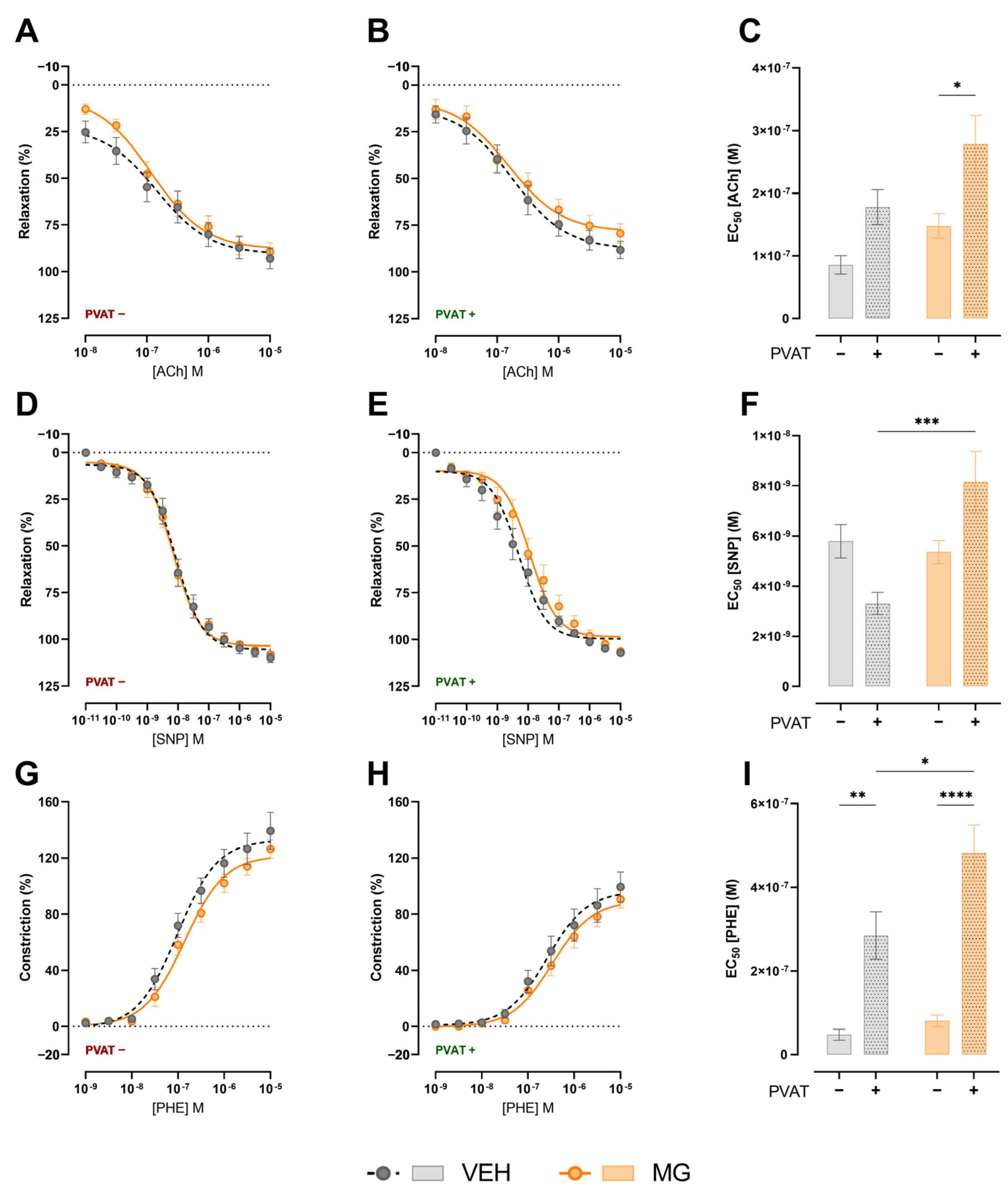
3.3. Effects of Early Exposure to Methylglyoxal during Lactation on Vasomotricity
3.4. Effects of Early Exposure to Methylglyoxal during Lactation on Cardiac and Aorta Morphology
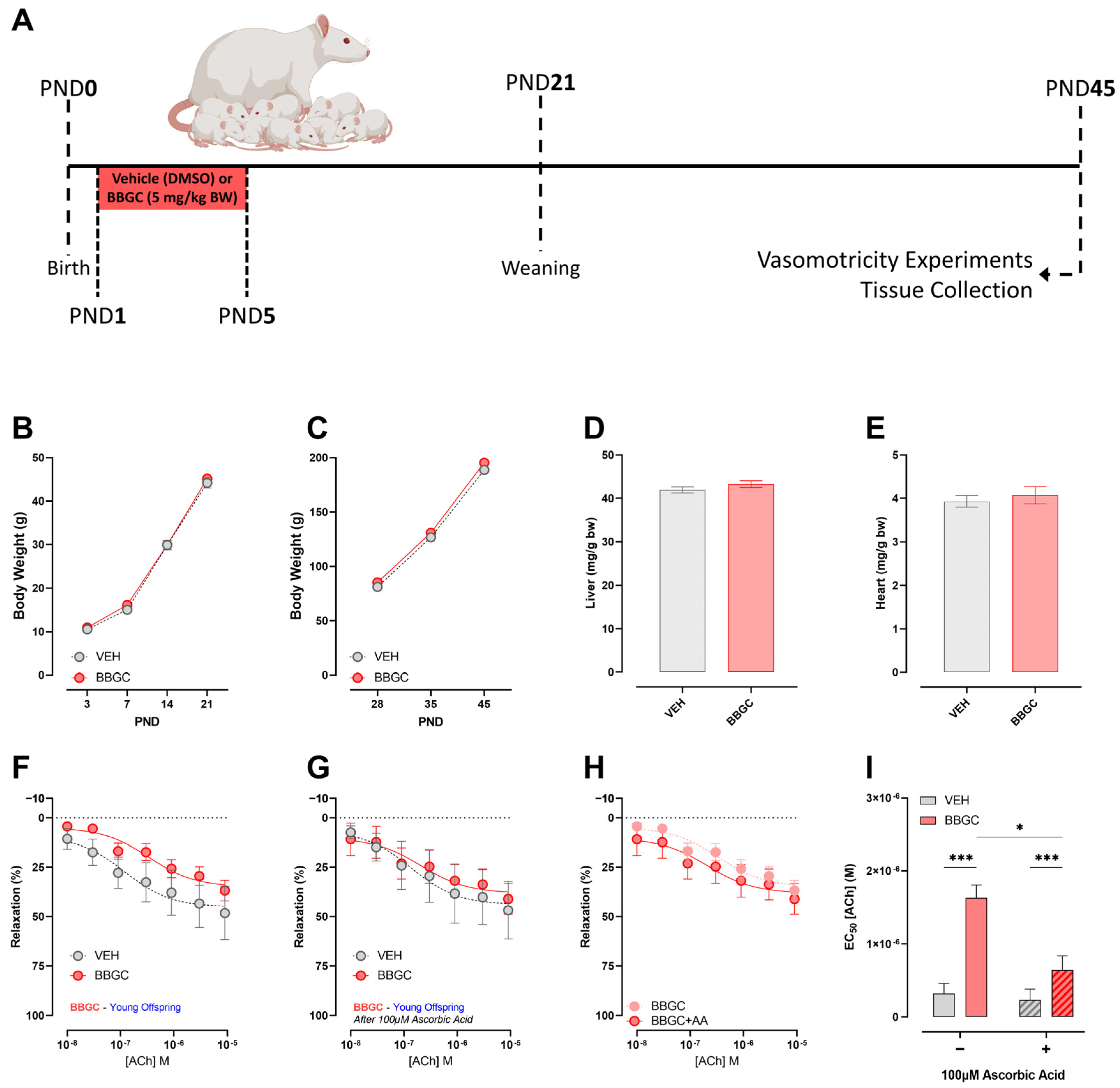
3.5. Effects of Early Exposure to Endogenous Glycotoxins during Lactation on Offspring Development and Vasomotricity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global Burden of Obesity in 2005 and Projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Bashir, M.; Dabbous, Z.; Baagar, K.; Elkhatib, F.; Ibrahim, A.; Brich, S.A.; Abdel-Rahman, M.E.; Konje, J.C.; Abou-Samra, A.B. Type 2 Diabetes Mellitus in Pregnancy: The Impact of Maternal Weight and Early Glycaemic Control on Outcomes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 233, 53–57. [Google Scholar] [CrossRef]
- Plagemann, A.; Harder, T.; Rodekamp, E.; Kohlhoff, R. Rapid Neonatal Weight Gain Increases Risk of Childhood Overweight in Offspring of Diabetic Mothers. J. Perinat. Med. 2012, 40, 557–563. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Matafome, P.; Rodrigues, T.; Sena, C.; Seiça, R. Methylglyoxal in Metabolic Disorders: Facts, Myths, and Promises. Med. Res. Rev. 2017, 37, 368–403. [Google Scholar] [CrossRef] [PubMed]
- Francisco, F.A.; Saavedra, L.P.J.J.; Ferreira-Junior, M.D.; Cavalcante, K.V.N.N.; Moreira, V.M.; Prates, K.V.; Borges, S.C.; Buttow, N.C.; Ribeiro, T.A.; Pedrino, G.R.; et al. Early Postnatal Exposure of Rat Pups to Methylglyoxal Induces Oxidative Stress, Inflammation and Dysmetabolism at Adulthood. J. Dev. Orig. Health Dis. 2022, 13, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Deng, Q.; Babcock, S.A.; He, E.Y.; Ren, J.; Zhang, Y. Inhibition of Advanced Glycation Endproduct (AGE) Rescues against Streptozotocin-Induced Diabetic Cardiomyopathy: Role of Autophagy and ER Stress. Toxicol. Lett. 2018, 284, 10–20. [Google Scholar] [CrossRef]
- Brouwers, O.; Niessen, P.M.; Ferreira, I.; Miyata, T.; Scheffer, P.G.; Teerlink, T.; Schrauwen, P.; Brownlee, M.; Stehouwer, C.D.; Schalkwijk, C.G. Overexpression of Glyoxalase-I Reduces Hyperglycemiainduced Levels of Advanced Glycation End Products and Oxidative Stress in Diabetic Rats. J. Biol. Chem. 2011, 286, 1374–1380. [Google Scholar] [CrossRef]
- Crisóstomo, J.; Matafome, P.; Santos-Silva, D.; Rodrigues, L.; Sena, C.M.; Pereira, P.; Seiça, R. Methylglyoxal Chronic Administration Promotes Diabetes-like Cardiac Ischaemia Disease in Wistar Normal Rats. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1223–1230. [Google Scholar] [CrossRef]
- Sena, C.M.; Matafome, P.; Crisóstomo, J.; Rodrigues, L.; Fernandes, R.; Pereira, P.; Seiça, R.M. Methylglyoxal Promotes Oxidative Stress and Endothelial Dysfunction. Pharmacol. Res. 2012, 65, 497–506. [Google Scholar] [CrossRef]
- Wang, X.; Gong, Y.; Zhou, B.; Yang, J.; Cheng, Y.; Zhao, J.; Qi, M. Ursolic Acid Ameliorates Oxidative Stress, Inflammation and Fibrosis in Diabetic Cardiomyopathy Rats. Biomed. Pharmacother. 2018, 97, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Tikellis, C.; Pickering, R.J.; Tsorotes, D.; Huet, O.; Cooper, M.E.; Jandeleit-Dahm, K.; Thomas, M.C. Dicarbonyl Stress in the Absence of Hyperglycemia Increases Endothelial Inflammation and Atherogenesis Similar to That Observed in Diabetes. Diabetes 2014, 63, 3915–3925. [Google Scholar] [CrossRef]
- Nam, D.H.; Han, J.H.; Lee, T.J.; Shishido, T.; Lim, J.H.; Kim, G.Y.; Woo, C.H. CHOP Deficiency Prevents Methylglyoxal-Induced Myocyte Apoptosis and Cardiac Dysfunction. J. Mol. Cell. Cardiol. 2015, 85, 168–177. [Google Scholar] [CrossRef]
- Blackburn, N.J.R.; Vulesevic, B.; McNeill, B.; Cimenci, C.E.; Ahmadi, A.; Gonzalez-Gomez, M.; Ostojic, A.; Zhong, Z.; Brownlee, M.; Beisswenger, P.J.; et al. Methylglyoxal-Derived Advanced Glycation End Products Contribute to Negative Cardiac Remodeling and Dysfunction Post-Myocardial Infarction. Basic Res. Cardiol. 2017, 112, 57. [Google Scholar] [CrossRef]
- Lee, J.H.; Parveen, A.; Do, M.H.; Kang, M.C.; Yumnam, S.; Kim, S.Y. Molecular Mechanisms of Methylglyoxal-Induced Aortic Endothelial Dysfunction in Human Vascular Endothelial Cells. Cell Death Dis. 2020, 11, 403. [Google Scholar] [CrossRef]
- Azul, L.; Leandro, A.; Boroumand, P.; Klip, A.; Seiça, R.; Sena, C.M. Increased Inflammation, Oxidative Stress and a Reduction in Antioxidant Defense Enzymes in Perivascular Adipose Tissue Contribute to Vascular Dysfunction in Type 2 Diabetes. Free Radic. Biol. Med. 2020, 146, 264–274. [Google Scholar] [CrossRef]
- Wang, J.; Polaki, V.; Chen, S.; Bihl, J.C. Exercise Improves Endothelial Function Associated with Alleviated Inflammation and Oxidative Stress of Perivascular Adipose Tissue in Type 2 Diabetic Mice. Oxid. Med. Cell. Longev. 2020, 2020, 8830537. [Google Scholar] [CrossRef]
- Lowe, W.L.; Lowe, L.P.; Kuang, A.; Catalano, P.M.; Nodzenski, M.; Talbot, O.; Tam, W.H.; Sacks, D.A.; McCance, D.; Linder, B.; et al. Maternal Glucose Levels during Pregnancy and Childhood Adiposity in the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study. Diabetologia 2019, 62, 598–610. [Google Scholar] [CrossRef]
- Moon, J.H.; Jang, H.C. Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications. Diabetes Metab. J. 2022, 46, 3–14. [Google Scholar] [CrossRef]
- Cerychova, R.; Bohuslavova, R.; Papousek, F.; Sedmera, D.; Abaffy, P.; Benes, V.; Kolar, F.; Pavlinkova, G. Adverse Effects of Hif1a Mutation and Maternal Diabetes on the Offspring Heart. Cardiovasc. Diabetol. 2018, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.D.; Sabey, K.H.; Knutson, A.J.; Gandy, T.C.T.; Louwagie, E.J.; Lauterboeck, L.; Mdaki, K.S.; Baack, M.L. Diabetic Pregnancy and Maternal High-Fat Diet Impair Mitochondrial Dynamism in the Developing Fetal Rat Heart by Sex-Specific Mechanisms. Int. J. Mol. Sci. 2019, 20, 3090. [Google Scholar] [CrossRef] [PubMed]
- Toop, C.R.; Muhlhausler, B.S.; O’Dea, K.; Gentili, S. Impact of Perinatal Exposure to Sucrose or High Fructose Corn Syrup (HFCS-55) on Adiposity and Hepatic Lipid Composition in Rat Offspring. J. Physiol. 2017, 595, 4379–4398. [Google Scholar] [CrossRef]
- Csongová, M.; Gurecká, R.; Koborová, I.; Celec, P.; Domonkos, E.; Uličná, O.; Somoza, V.; Šebeková, K. The Effects of a Maternal Advanced Glycation End Product-Rich Diet on Somatic Features, Reflex Ontogeny and Metabolic Parameters of Offspring Mice. Food Funct. 2018, 9, 3432–3446. [Google Scholar] [CrossRef] [PubMed]
- Francisco, F.A.; Barella, L.F.; da Silveira, S.S.; Saavedra, L.P.J.; Prates, K.V.; Alves, V.S.; da Franco, C.C.S.; Miranda, R.A.; Ribeiro, T.A.; Tófolo, L.P.; et al. Methylglyoxal Treatment in Lactating Mothers Leads to Type 2 Diabetes Phenotype in Male Rat Offspring at Adulthood. Eur. J. Nutr. 2018, 57, 477–486. [Google Scholar] [CrossRef]
- Roest, P.A.M.; Molin, D.G.M.; Schalkwijk, C.G.; Ven Iperen, L.; Wentzel, P.; Eriksson, U.J.; Gittenberger-de Groot, A.C. Specific Local Cardiovascular Changes of n ε-(Carboxymethyl) Lysine, Vascular Endothelial Growth Factor, and Smad2 in the Developing Embryos Coincide with Maternal Diabetes-Induced Congenital Heart Defects. Diabetes 2009, 58, 1222–1228. [Google Scholar] [CrossRef]
- Mericq, V.; Piccardo, C.; Cai, W.; Chen, X.; Zhu, L.; Striker, G.E.; Vlassara, H.; Uribarri, J. Maternally Transmitted and Food-Derived Glycotoxins: A Factor Preconditioning the Young to Diabetes? Diabetes Care 2010, 33, 2232–2237. [Google Scholar] [CrossRef]
- Sousa, D.; Rocha, M.; Amaro, A.; Ferreira-Junior, M.D.; Cavalcante, K.V.N.; Monteiro-Alfredo, T.; Barra, C.; Rosendo-Silva, D.; Saavedra, L.P.J.; Magalhães, J.; et al. Exposure to Obesogenic Environments during Perinatal Development Modulates Offspring Energy Balance Pathways in Adipose Tissue and Liver of Rodent Models. Nutrients 2023, 15, 1281. [Google Scholar] [CrossRef] [PubMed]
- Amaro, A.; Sousa, D.; Sá-Rocha, M.; Ferreira-Junior, M.D.; Barra, C.; Monteiro, T.; Mathias, P.; Gomes, R.M.; Baptista, F.I.; Matafome, P. Sex-Specificities in Offspring Neurodevelopment and Behaviour upon Maternal Glycation: Putative Underlying Neurometabolic and Synaptic Changes. Life Sci. 2023, 321, 121597. [Google Scholar] [CrossRef]
- Hadi, A.M.; Mouchaers, K.T.B.B.; Schalij, I.; Grunberg, K.; Meijer, G.A.; Vonk-Noordegraaf, A.; Van Der Laarse, W.J.; Beliën, J.A.M.M. Rapid Quantification of Myocardial Fibrosis: A New Macro-Based Automated Analysis. Anal. Cell. Pathol. 2010, 33, 257–269. [Google Scholar] [CrossRef][Green Version]
- Ornoy, A.; Reece, E.A.; Pavlinkova, G.; Kappen, C.; Miller, R.K. Effect of Maternal Diabetes on the Embryo, Fetus, and Children: Congenital Anomalies, Genetic and Epigenetic Changes and Developmental Outcomes. Birth Defects Res. Part C Embryo Today Rev. 2015, 105, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Peila, C.; Gazzolo, D.; Bertino, E.; Cresi, F.; Coscia, A. Influence of Diabetes during Pregnancy on Human Milk Composition. Nutrients 2020, 12, 185. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating Glycotoxins and Dietary Advanced Glycation Endproducts: Two Links to Inflammatory Response, Oxidative Stress, and Aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.; Dhar, I.; Jiang, B.; Desai, K.M.; Wu, L. Chronic Methylglyoxal Infusion by Minipump Causes Pancreatic β-Cell Dysfunction and Induces Type 2 Diabetes in Sprague-Dawley Rats. Diabetes 2011, 60, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Onishi, A.; Akimoto, T.; Morishita, Y.; Hirahara, I.; Inoue, M.; Kusano, E.; Nagata, D. Peritoneal Fibrosis Induced by Intraperitoneal Methylglyoxal Injection: The Role of Concurrent Renal Dysfunction. Am. J. Nephrol. 2014, 40, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.; Matafome, P.; Crisóstomo, J.; Santos-Silva, D.; Sena, C.; Pereira, P.; Seiça, R. Advanced Glycation End Products and Diabetic Nephropathy: A Comparative Study Using Diabetic and Normal Rats with Methylglyoxal-Induced Glycation. J. Physiol. Biochem. 2014, 70, 173–184. [Google Scholar] [CrossRef]
- Castelhano, J.; Ribeiro, B.; Sanches, M.; Graça, B.; Saraiva, J.; Oliveiros, B.; Neves, C.; Rodrigues, T.; Sereno, J.; Gonçalves, S.; et al. A Rat Model of Enhanced Glycation Mimics Cardiac Phenotypic Components of Human Type 2 Diabetes: A Translational Study Using MRI. J. Diabetes Complicat. 2020, 34, 107554. [Google Scholar] [CrossRef]
- Almeida, F.; Santos-Silva, D.; Rodrigues, T.; Matafome, P.; Crisóstomo, J.; Sena, C.; Gonçalves, L.; Seiça, R. Pyridoxamine Reverts Methylglyoxal-Induced Impairment of Survival Pathways during Heart Ischemia. Cardiovasc. Ther. 2013, 31, e79–e85. [Google Scholar] [CrossRef]
- Molica, F.; Morel, S.; Kwak, B.R.; Rohner-Jeanrenaud, F.; Steffens, S. Adipokines at the Crossroad between Obesity and Cardiovascular Disease. Thromb. Haemost. 2015, 113, 553–566. [Google Scholar] [CrossRef]
- Moazzen, H.; Lu, X.; Ma, N.L.; Velenosi, T.J.; Urquhart, B.L.; Wisse, L.J.; Gittenberger-de Groot, A.C.; Feng, Q. N-Acetylcysteine Prevents Congenital Heart Defects Induced by Pregestational Diabetes. Cardiovasc. Diabetol. 2014, 13, 46. [Google Scholar] [CrossRef]
- Bartekova, M.; Barancik, M.; Ferenczyova, K.; Dhalla, N.S. Beneficial Effects of N-Acetylcysteine and N-Mercaptopropionylglycine on Ischemia Reperfusion Injury in the Heart. Curr. Med. Chem. 2018, 25, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Giam, B.; Chu, P.-Y.; Kuruppu, S.; Smith, A.I.; Horlock, D.; Kiriazis, H.; Du, X.-J.; Kaye, D.M.; Rajapakse, N.W. N-Acetylcysteine Attenuates the Development of Cardiac Fibrosis and Remodeling in a Mouse Model of Heart Failure. Physiol. Rep. 2016, 4, e12757. [Google Scholar] [CrossRef] [PubMed]
- Balci, Y.I.; Acer, S.; Yagci, R.; Kucukatay, V.; Sarbay, H.; Bozkurt, K.; Polat, A. N-Acetylcysteine Supplementation Reduces Oxidative Stress for Cytosine Arabinoside in Rat Model. Int. Ophthalmol. 2017, 37, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Juurlink, B.H.J. Increased Methylglyoxal and Oxidative Stress in Hypertensive Rat Vascular Smooth Muscle Cells. Hypertension 2002, 39, 809–814. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira-Junior, M.D.; Cavalcante, K.V.N.; Costa, J.M.; Bessa, A.S.M.; Amaro, A.; de Castro, C.H.; Xavier, C.H.; Silva, S.; Fonseca, D.A.; Matafome, P.; et al. Early Methylglyoxal Exposure Leads to Worsened Cardiovascular Function in Young Rats. Nutrients 2024, 16, 2029. https://doi.org/10.3390/nu16132029
Ferreira-Junior MD, Cavalcante KVN, Costa JM, Bessa ASM, Amaro A, de Castro CH, Xavier CH, Silva S, Fonseca DA, Matafome P, et al. Early Methylglyoxal Exposure Leads to Worsened Cardiovascular Function in Young Rats. Nutrients. 2024; 16(13):2029. https://doi.org/10.3390/nu16132029
Chicago/Turabian StyleFerreira-Junior, Marcos Divino, Keilah Valéria N. Cavalcante, Jaqueline M. Costa, Amanda S. M. Bessa, Andreia Amaro, Carlos Henrique de Castro, Carlos Henrique Xavier, Sónia Silva, Diogo A. Fonseca, Paulo Matafome, and et al. 2024. "Early Methylglyoxal Exposure Leads to Worsened Cardiovascular Function in Young Rats" Nutrients 16, no. 13: 2029. https://doi.org/10.3390/nu16132029
APA StyleFerreira-Junior, M. D., Cavalcante, K. V. N., Costa, J. M., Bessa, A. S. M., Amaro, A., de Castro, C. H., Xavier, C. H., Silva, S., Fonseca, D. A., Matafome, P., & Gomes, R. M. (2024). Early Methylglyoxal Exposure Leads to Worsened Cardiovascular Function in Young Rats. Nutrients, 16(13), 2029. https://doi.org/10.3390/nu16132029








