Dietary Exposure to Acrylamide Has Negative Effects on the Gastrointestinal Tract: A Review
Abstract
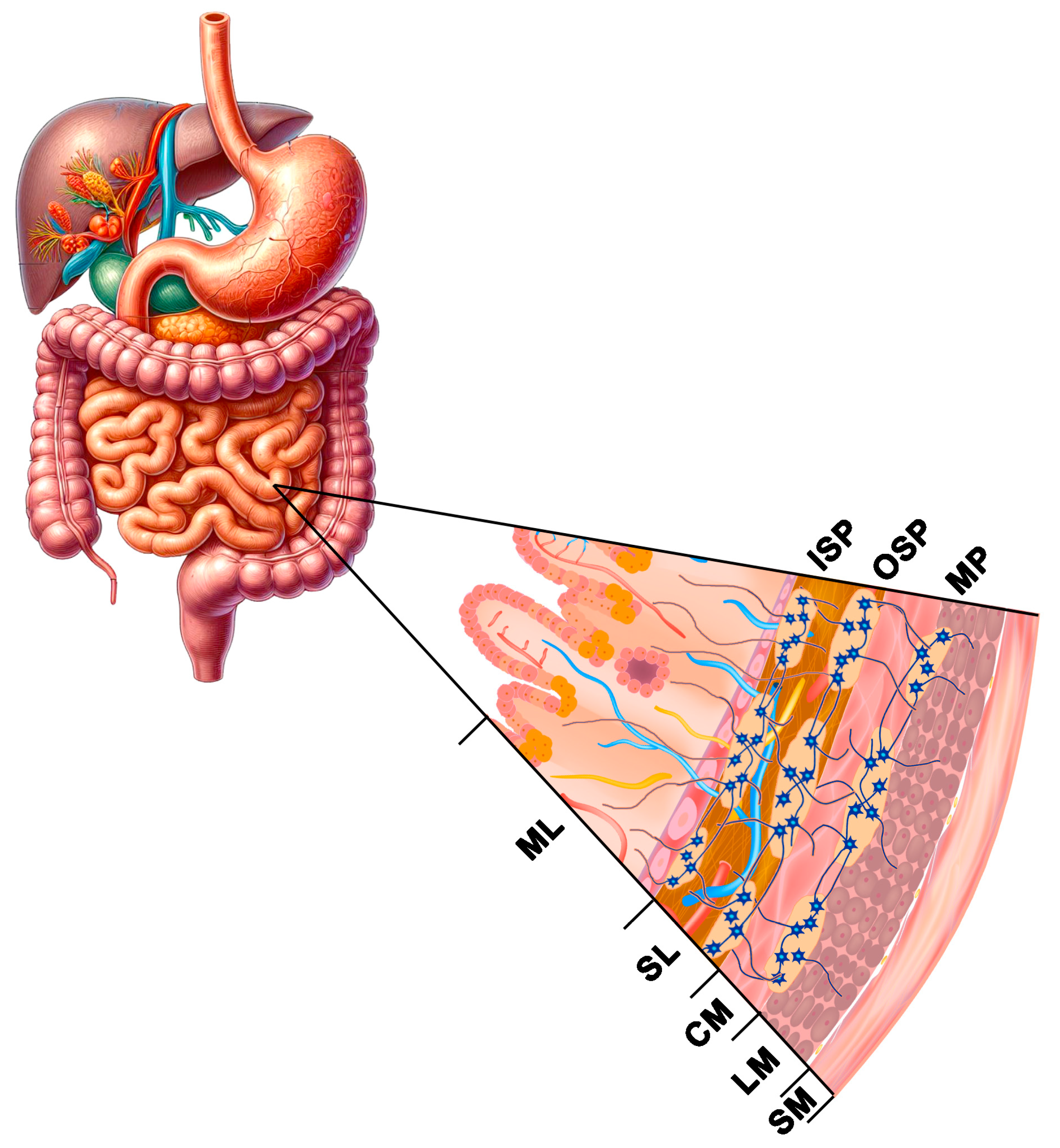
1. Introduction
2. Impact of Acrylamide on the Gastrointestinal Tract Morphology and the Functional Features of the Intestine
| GI Tract Segment | Species | Dose of ACR and Time of Exposure | The Result of Study | References |
|---|---|---|---|---|
| stomach | rats | 30 mg/kg of b.w./day for 4 weeks | gastric mucosa erosions, reduced mucosa thickness, inflammatory infiltration, apoptosis | [41] |
| stomach, liver | rats | 0.05% in drinking water for 40 days | increased lipid peroxidation (MDA level), decreased level of GSH | [42] |
| jejunum | mice | 0.5 mg/kg of b.w./day for 90 days | a decrease in the length of intestinal villi, the depth of crypts, the number of active crypts and the size of the intestine absorption surface; a reduced number of proliferating cells, an increased number of apoptotic cells | [37] |
| ileum, colon | rats | 25 mg/kg of b.w./day for 21 days | degeneration and shortening of intestinal villi, damage of ileum mucosa, inflammatory cell infiltration; degeneration of surface epithelium and Liberkühn crypts in the colon | [38] |
| ileum | rats | 0, 30, 45 and 60 mg/kg of b.w./day for 5 days | decreased ANAE-positive peripheral blood lymphocyte levels in a dose dependent manner | [56] |
| ileum | rats | 0, 125, 150, and 175 mg/kg of b.w in a single dose | reduced lymphoid follicles of the ileal Peyer’s patches (IPPs), regressed germinal centres (GCs), ANAE-positive lymphocyte depletion in IPPs | [56] |
| ileum | pig | 0.5 and 5 μg/kg of b.w./day for 28 days | an increase in the level of pro-inflammatory cytokines (interleukin 1 β (IL-1β), IL-6 and tumour necrosis factor-α (TNF-α)) synthesised in the IPP | [39] |
| small intestine, liver | rats | 25 mg/kg of b.w./day for 21 days | damage to the intestinal villi structure, the reduced cell density in the lamina propria, damaged intestinal epithelium, strong vacuolisation and infiltration of eosinophils in the liver | [45] |
| small intestine | mice | 0, 10 and 100 mg/kg of b.w./day for 28 days | reduced the expression of intestinal tight junction proteins, disrupted the permeability of the intestine, which impaired the intestinal epithelial barrier | [53] |
| small intestine | rats | 3 mg/kg of b.w./day for 0, 5, 10 and 15 days in utero | a decrease in the length, thickness and volume of the villi and the depth of crypts, an increase in the villi height to crypt depth ratio, a decrease in the crypt and mucosal membrane thickness, decreased expression of tight junction proteins (E-adherin, occludin), decreased activity of intestinal enzymes, increased apoptosis | [36] |
| small intestine | guinea pigs | 3 mg/kg of b.w./day for last 35 days of gestation | increased thickness of myenteron and submucosa, mucosa fractal dimension and the depth of crypts in the duodenum, increased the number of total, divided and inactive crypt, and the number of damaged villi in the duodenum and jejunum, increased the total villi number in the jejunum, the decrease of villi epithelium thickness and active crypt number in the jejunum, decreased goblet cells number and intact villi number in the duodenum and mucosa thickness and crypts width in the jejunum, higher size of nerve plexuses in duodenum, decreased expression of cadherin, increased apoptotic cell number | [54] |
| small intestine | rats | 20 mg/kg of b.w./day for last 10 days of gestation and during lactation | disturbances in intestinal enzymes activities (acid phosphatase, alkaline phosphatase, beta-glucuronidase, citrate synthase and lactic dehydrogenase) | [40] |
| Caco-2 cells | human | 2.5 mM ACR for 24 h | decreased cell viability, mitochondrial membrane potential (MMP) collapse | [46] |
| Caco-2 cells | human | 7.5 mmol/L ACR for 4–20 h | decreased transepithelial electrical resistance (TEER) value; mitochondrial membrane potential (MMP) collapse, decreased FITC-dextran 4 kDa permeability, increased apoptosis, increased lactic dehydrogenase (LDH) release, decreased expression of claudin-1, occludin and zonula occludens-1 | [52] |
| HepG2 cells | human | 2.5 mM ACR for 24 h | decreased cell viability, mitochondrial membrane potential (MMP) collapse | [47] |
| IEC-6 cells | rats | 1.25–10 mmol/L for 24 h | decreased cell viability, increased intercellular permeability and release of lactic dehydrogenase, destroyed tight junctions | [48] |
| IEC-6 cells | rats | 2.5 mM ACR for 24 h | decreased cell viability, decreased transepithelial resistance (TEER) value of the cell monolayer, suppressed protein expression of the tight junction proteins (occludin, claudin-1, and zonula occludens-1) | [51] |
3. Acrylamide Interaction with Gut Microbiome
4. Impact of Acrylamide on the Enteric Nervous System
5. Potential Carcinogenic Effect
6. Strategies for Decreasing ACR Levels in Food Products and Limiting Its Toxic Effect on the Gastrointestinal Tract
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of Acrylamide, a Carcinogen Formed in Heated Foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef] [PubMed]
- Blank, I.; Robert, F.; Goldmann, T.; Pollien, P.; Varga, N.; Devaud, S.; Saucy, F.; Huynh-Ba, T.; Stadler, R.H. Mechanisms of Acrylamide Formation: Maillard-Induced Transformation of Asparagine. In Chemistry and Safety of Acrylamide in Food; Friedman, M., Mottram, D., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2005; Volume 561, pp. 171–189. ISBN 978-0-387-23920-0. [Google Scholar]
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide Is Formed in the Maillard Reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, A.; Tanaka, Y.; Hengel, M.; Shibamoto, T. Gas Chromatographic Investigation of Acrylamide Formation in Browning Model Systems. J. Agric. Food Chem. 2003, 51, 3999–4003. [Google Scholar] [CrossRef] [PubMed]
- Claus, A.; Weisz, G.M.; Schieber, A.; Carle, R. Pyrolytic Acrylamide Formation from Purified Wheat Gluten and Gluten-supplemented Wheat Bread Rolls. Mol. Nutr. Food Res. 2006, 50, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Capuano, E.; Fogliano, V. Acrylamide and 5-Hydroxymethylfurfural (HMF): A Review on Metabolism, Toxicity, Occurrence in Food and Mitigation Strategies. LWT—Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- LoPachin, R.M. The Changing View of Acrylamide Neurotoxicity. NeuroToxicology 2004, 25, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-S.; McDaniel, L.P.; Manjanatha, M.G.; Shelton, S.D.; Doerge, D.R.; Mei, N. Mutagenicity of Acrylamide and Glycidamide in the Testes of Big Blue Mice. Toxicol. Sci. 2010, 117, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.; Mehta, R.; Curran, I.; Raju, J. Dietary Acrylamide Exposure in F344 Rats and Colon Tumor-Bearing Nude Nu/Nu Mice: Dataset of Gene Expression of Cancer Pathway Targets and Methylation Status of Tumor Suppressor Genes in Colon Mucosae and Tumors. Data Brief 2019, 27, 104763. [Google Scholar] [CrossRef]
- Abd Al Haleem, E.N.; Hasan, W.Y.S.; Arafa, H.M.M. Therapeutic Effects of Thymoquinone or Capsaicin on Acrylamide-Induced Reproductive Toxicity in Rats Mediated by Their Effect on Oxidative Stress, Inflammation, and Tight Junction Integrity. Drug Chem. Toxicol. 2022, 45, 2328–2340. [Google Scholar] [CrossRef]
- Lindeman, B.; Johansson, Y.; Andreassen, M.; Husøy, T.; Dirven, H.; Hofer, T.; Knutsen, H.K.; Caspersen, I.H.; Vejrup, K.; Paulsen, R.E.; et al. Does the Food Processing Contaminant Acrylamide Cause Developmental Neurotoxicity? A Review and Identification of Knowledge Gaps. Reprod. Toxicol. 2021, 101, 93–114. [Google Scholar] [CrossRef]
- World Health Organization International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1994. [Google Scholar]
- Sumner, S.C.J. Acrylamide: A Comparison of Metabolism and Hemoglobin Adducts in Rodents Following Dermal, Intraperitoneal, Oral, or Inhalation Exposure. Toxicol. Sci. 2003, 75, 260–270. [Google Scholar] [CrossRef]
- Sumner, S.C.J.; Fennell, T.R.; Moore, T.A.; Chanas, B.; Gonzalez, F.; Ghanayem, B.I. Role of Cytochrome P450 2E1 in the Metabolism of Acrylamide and Acrylonitrile in Mice. Chem. Res. Toxicol. 1999, 12, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Doroshyenko, O.; Fuhr, U.; Kunz, D.; Frank, D.; Kinzig, M.; Jetter, A.; Reith, Y.; Lazar, A.; Taubert, D.; Kirchheiner, J.; et al. In Vivo Role of Cytochrome P 450 2E1 and Glutathione-S-Transferase Activity for Acrylamide Toxicokinetics in Humans. Cancer Epidemiol. Biomark. Prev. 2009, 18, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Settels, E.; Bernauer, U.; Palavinskas, R.; Klaffke, H.S.; Gundert-Remy, U.; Appel, K.E. Human CYP2E1 Mediates the Formation of Glycidamide from Acrylamide. Arch. Toxicol. 2008, 82, 717–727. [Google Scholar] [CrossRef]
- Zödl, B.; Schmid, D.; Wassler, G.; Gundacker, C.; Leibetseder, V.; Thalhammer, T.; Ekmekcioglu, C. Intestinal Transport and Metabolism of Acrylamide. Toxicology 2007, 232, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, G.J.; Miller, E.; Sapienza, P.P.; Michel, T.C.; Inskeep, P.B. Comparative Tissue Distribution and Excretion of [1-14C]Acrylamide in Beagle Dogs and Miniature Pigs. Food Chem. Toxicol. 1987, 25, 871–875. [Google Scholar] [CrossRef]
- Schabacker, J.; Schwend, T.; Wink, M. Reduction of Acrylamide Uptake by Dietary Proteins in a Caco-2 Gut Model. J. Agric. Food Chem. 2004, 52, 4021–4025. [Google Scholar] [CrossRef] [PubMed]
- Marlowe, C.; Clark, M.J.; Mast, R.W.; Friedman, M.A.; Waddell, W.J. The Distribution of [14C]Acrylamide in Male and Pregnant Swiss-Webster Mice Studied by Whole-Body Autoradiography. Toxicol. Appl. Pharmacol. 1986, 86, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Fuhr, U.; Boettcher, M.I.; Kinzig-Schippers, M.; Weyer, A.; Jetter, A.; Lazar, A.; Taubert, D.; Tomalik-Scharte, D.; Pournara, P.; Jakob, V.; et al. Toxicokinetics of Acrylamide in Humans after Ingestion of a Defined Dose in a Test Meal to Improve Risk Assessment for Acrylamide Carcinogenicity. Cancer Epidemiol. Biomark. Prev. 2006, 15, 266–271. [Google Scholar] [CrossRef]
- Abramsson-Zetterberg, L.; Wong, J.; Ilbäck, N.-G. Acrylamide Tissue Distribution and Genotoxic Effects in a Common Viral Infection in Mice. Toxicology 2005, 211, 70–76. [Google Scholar] [CrossRef]
- Schettgen, T.; Kotting, B.; Hornig, M.; Beckmann, M.W.; Weiss, T.; Drexler, H.; Angerer, J. Trans-Placental Exposure of Neonates to Acrylamide? A Pilot Study. Int. Arch. Occup. Environ. Health 2004, 77, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Wajda, Ł.; Tarko, T.; Sroka, P.; Satora, P. A Review of the Interactions between Acrylamide, Microorganisms and Food Components. Food Funct. 2016, 7, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry, Biochemistry, and Safety of Acrylamide. A Review. J. Agric. Food Chem. 2003, 51, 4504–4526. [Google Scholar] [CrossRef] [PubMed]
- Kopp, E.K.; Dekant, W. Toxicokinetics of Acrylamide in Rats and Humans following Single Oral Administration of Low Doses. Toxicol. Appl. Pharmacol. 2009, 235, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, I.; Sana, M.; Chakraborty, I.; Rahman, M.H.; Biswas, R.; Mazumder, N. Dietary Acrylamide: A Detailed Review on Formation, Detection, Mitigation, and Its Health Impacts. Foods 2024, 13, 556. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on Acrylamide in Food. EFSA J. 2015, 13, 4104. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Benford, D.; Bignami, M.; Chipman, J.K.; Ramos Bordajandi, L. Assessment of the Genotoxicity of Acrylamide. EFSA J. 2022, 20, 7293. [Google Scholar] [CrossRef]
- Tran, N.L.; Barraj, L.M.; Murphy, M.M.; Bi, X. Dietary Acrylamide Exposure and Hemoglobin Adducts—National Health and Nutrition Examination Survey (2003–04). Food Chem. Toxicol. 2010, 48, 3098–3108. [Google Scholar] [CrossRef]
- Vikström, A.C.; Warholm, M.; Paulsson, B.; Axmon, A.; Wirfält, E.; Törnqvist, M. Hemoglobin Adducts as a Measure of Variations in Exposure to Acrylamide in Food and Comparison to Questionnaire Data. Food Chem. Toxicol. 2012, 50, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Vesper, H.W.; Slimani, N.; Hallmans, G.; Tjønneland, A.; Agudo, A.; Benetou, V.; Bingham, S.; Boeing, H.; Boutron-Ruault, M.-C.; Bueno-de-Mesquita, H.B.; et al. Cross-Sectional Study on Acrylamide Hemoglobin Adducts in Subpopulations from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. J. Agric. Food Chem. 2008, 56, 6046–6053. [Google Scholar] [CrossRef]
- Timmermann, C.; Mølck, S.; Kadawathagedara, M.; Bjerregaard, A.; Törnqvist, M.; Brantsæter, A.; Pedersen, M. A Review of Dietary Intake of Acrylamide in Humans. Toxics 2021, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.M. An Overview of the Gastrointestinal System. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; ISBN 978-0-409-90077-4. [Google Scholar]
- Wu, M.M.; Liao, B.; Xia, I.F.; Luk, P.K.; Wong, K.; Kwok, K.W. Food Emulsifiers Increase Toxicity of Food Contaminants in Three Human GI Tract Cell Lines. Food Chem. Toxicol. 2024, 185, 114499. [Google Scholar] [CrossRef]
- Muszyński, S.; Hułas-Stasiak, M.; Dobrowolski, P.; Arciszewski, M.B.; Hiżewska, L.; Donaldson, J.; Mozel, S.; Rycerz, K.; Kapica, M.; Puzio, I.; et al. Maternal Acrylamide Exposure Changes Intestinal Epithelium, Immunolocalization of Leptin and Ghrelin and Their Receptors, and Gut Barrier in Weaned Offspring. Sci. Rep. 2023, 13, 10286. [Google Scholar] [CrossRef]
- Dobrowolski, P.; Huet, P.; Karlsson, P.; Eriksson, S.; Tomaszewska, E.; Gawron, A.; Pierzynowski, S.G. Potato Fiber Protects the Small Intestinal Wall against the Toxic Influence of Acrylamide. Nutrition 2012, 28, 428–435. [Google Scholar] [CrossRef]
- Gedik, S.; Erdemli, M.; Gul, M.; Yigitcan, B.; Gozukara Bag, H.; Aksungur, Z.; Altinoz, E. Investigation of the Protective Effects of Crocin on Acrylamide Induced Small and Large Intestine Damage in Rats. Biotech. Histochem. 2018, 93, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Palus, K.; Obremski, K.; Bulc, M.; Całka, J. The Impact of Low and High Doses of Acrylamide on the Intramural Neurons of the Porcine Ileum. Food Chem. Toxicol. 2019, 132, 110673. [Google Scholar] [CrossRef] [PubMed]
- Walden, R.; Squibb, R.E.; Schiller, C.M. Effects of Prenatal and Lactational Exposure to Acrylamide on the Development of Intestinal Enzymes in the Rat. Toxicol. Appl. Pharmacol. 1981, 58, 363–369. [Google Scholar] [CrossRef] [PubMed]
- El-Mehi, A.E.; El-Sherif, N.M. Influence of Acrylamide on the Gastric Mucosa of Adult Albino Rats and the Possible Protective Role of Rosemary. Tissue Cell 2015, 47, 273–283. [Google Scholar] [CrossRef]
- Sadek, K. Antioxidant and Immunostimulant Effect of Carica Papaya Linn. Aqueous Extract in Acrylamide Intoxicated Rats. Acta Inform. Medica 2012, 20, 180. [Google Scholar] [CrossRef]
- Claus, A.; Carle, R.; Schieber, A. Acrylamide in Cereal Products: A Review. J. Cereal Sci. 2008, 47, 118–133. [Google Scholar] [CrossRef]
- Yousef, M.I.; El-Demerdash, F.M. Acrylamide-Induced Oxidative Stress and Biochemical Perturbations in Rats. Toxicology 2006, 219, 133–141. [Google Scholar] [CrossRef]
- Altinoz, E.; Turkoz, Y.; Vardi, N. The Protective Effect of N-Acetylcysteine against Acrylamide Toxicity in Liver and Small and Large Intestine Tissues. Bratisl. Med. J. 2015, 116, 252–258. [Google Scholar] [CrossRef]
- Chen, W.; Su, H.; Xu, Y.; Bao, T.; Zheng, X. Protective Effect of Wild Raspberry (Rubus Hirsutus Thunb.) Extract against Acrylamide-Induced Oxidative Damage Is Potentiated after Simulated Gastrointestinal Digestion. Food Chem. 2016, 196, 943–952. [Google Scholar] [CrossRef]
- Chen, W.; Su, H.; Xu, Y.; Jin, C. In Vitro Gastrointestinal Digestion Promotes the Protective Effect of Blackberry Extract against Acrylamide-Induced Oxidative Stress. Sci. Rep. 2017, 7, 40514. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, X.-H. Chemical Features of the Oligochitosan-Glycated Caseinate Digest and Its Enhanced Protection on Barrier Function of the Acrylamide-Injured IEC-6 Cells. Food Chem. 2019, 290, 246–254. [Google Scholar] [CrossRef]
- Su, Y.; Cheng, S.; Ding, Y.; Wang, L.; Sun, M.; Man, C.; Zhang, Y.; Jiang, Y. A Comparison of Study on Intestinal Barrier Protection of Polysaccharides from Hericium Erinaceus before and after Fermentation. Int. J. Biol. Macromol. 2023, 233, 123558. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Gao, H.; Ge, S.; Yao, X.; Liu, C.; Tan, X. Chronotoxicity of Acrylamide in Mice Fed a High-Fat Diet: The Involvement of Liver CYP2E1 Upregulation and Gut Leakage. Molecules 2023, 28, 5132. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, Q.; Zhao, X.-H.; Zhang, N. The Impact of Heat Treatment of Quercetin and Myricetin on Their Activities to Alleviate the Acrylamide-Induced Cytotoxicity and Barrier Loss in IEC-6 Cells. Plant Foods Hum. Nutr. 2022, 77, 436–442. [Google Scholar] [CrossRef]
- Yan, F.; Chen, W.; Zhao, L.; Lu, Q.; Wang, C.; Liu, R. Procyanidin A 1 and Its Digestive Products Prevent Acrylamide-Induced Intestinal Barrier Dysfunction via the MAPK-Mediated MLCK Pathway. Food Funct. 2021, 12, 11956–11965. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.; Ye, L.; Lin, X.; Huang, S.; Yue, W.; Wu, X. Aggravation of Food Allergy Symptoms by Treatment with Acrylamide in a Mouse Model. Food Chem. Toxicol. 2023, 176, 113808. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, E.; Dobrowolski, P.; Puzio, I.; Prost, L.; Kurlak, P.; Sawczuk, P.; Badzian, B.; Hulas-Stasiak, M.; Kostro, K. Acrylamide-Induced Prenatal Programming of Intestine Structure in Guinea Pig. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2014, 65, 107–115. [Google Scholar]
- Jung, C.; Hugot, J.-P.; Barreau, F. Peyer’s Patches: The Immune Sensors of the Intestine. Int. J. Inflamm. 2010, 2010, 823710. [Google Scholar] [CrossRef]
- Yener, Y.; Sur, E.; Telatar, T.; Oznurlu, Y. The Effect of Acrylamide on Alpha-Naphthyl Acetate Esterase Enzyme in Blood Circulating Lymphocytes and Gut Associated Lymphoid Tissues in Rats. Exp. Toxicol. Pathol. 2013, 65, 143–146. [Google Scholar] [CrossRef]
- Zaidi, S.I.A.; Raisuddin, S.; Singh, K.P.; Jafri, A.; Husain, R.; Husain, M.M.; Mall, S.A.; Seth, P.K.; Ray, P.K. Acrylamtoe Induced Immunosuppression in Rats and Its Modulan by 6-MFA, An Interferon Inducer. Immunopharmacol. Immunotoxicol. 1994, 16, 247–260. [Google Scholar] [CrossRef]
- O’Hara, A.M.; Shanahan, F. Gut Microbiota: Mining for Therapeutic Potential. Clin. Gastroenterol. Hepatol. 2007, 5, 274–284. [Google Scholar] [CrossRef]
- Bedade, D.K.; Singhal, R.S. Isolation and Characterization of Acrylamidase from Arthrobacter Sp. DBV1 and Its Ability to Biodegrade Acrylamide. Appl. Biochem. Biotechnol. 2017, 182, 570–585. [Google Scholar] [CrossRef]
- Shukor, M.Y.; Gusmanizar, N.; Azmi, N.A.; Hamid, M.; Ramli, J.; Shamaan, N.A.; Syed, M.A. Isolation and Characterization of an Acrylamide-Degrading Bacillus Cereus. J. Environ. Biol. 2009, 30, 57–64. [Google Scholar]
- Jain, N.; John, P.J.; Sharma, K.P.; Soni, I. Isolation and identification of acrylamide degrading bacteria from soil. Int. J. Appl. Biol. Pharm. Technol. 2012, 4, 179–187. [Google Scholar]
- Gusmanizar, N.; Shukor, Y.; Ramli, J.; Syed, M.A. Isolation and characterization of an acrylamide-degrading Burkholderia sp. Strain DRY27. J. Ris. Kim. 2015, 2, 34. [Google Scholar] [CrossRef]
- Bedade, D.K.; Singhal, R.S. Biodegradation of Acrylamide by a Novel Isolate, Cupriavidus Oxalaticus ICTDB921: Identification and Characterization of the Acrylamidase Produced. Bioresour. Technol. 2018, 261, 122–132. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Xu, J.-M.; Zheng, R.-C.; Zheng, Y.-G.; Shen, Y.-C. Improvement of Amidase Production by a Newly Isolated Delftia Tsuruhatensis ZJB-05174 through Optimization of Culture Medium. J. Microbiol. Biotechnol. 2008, 18, 1932–1937. [Google Scholar] [PubMed]
- Buranasilp, K.; Charoenpanich, J. Biodegradation of Acrylamide by Enterobacter Aerogenes Isolated from Wastewater in Thailand. J. Environ. Sci. China 2011, 23, 396–403. [Google Scholar] [CrossRef]
- Van Vliet, A.H.M.; Stoof, J.; Poppelaars, S.W.; Bereswill, S.; Homuth, G.; Kist, M.; Kuipers, E.J.; Kusters, J.G. Differential Regulation of Amidase- and Formamidase-Mediated Ammonia Production by the Helicobacter Pylori Fur Repressor. J. Biol. Chem. 2003, 278, 9052–9057. [Google Scholar] [CrossRef]
- Emmanuel Joshua Jebasingh, S.; Lakshmikandan, M.; Rajesh, R.P.; Raja, P. Biodegradation of Acrylamide and Purification of Acrylamidase from Newly Isolated Bacterium Moraxella Osloensis MSU11. Int. Biodeterior. Biodegrad. 2013, 85, 120–125. [Google Scholar] [CrossRef]
- Kumar, H.; Prasad, S.; Raj, J.; Bhalla, T.C. Constitutive Acetonitrile Hydrolysing Activity of Nocardia Globerula NHB-2: Optimization of Production and Reaction Conditions. Indian J. Exp. Biol. 2006, 44, 240–245. [Google Scholar]
- Sathesh Prabu, C.; Thatheyus, A.J. Biodegradation of Acrylamide Employing Free and Immobilized Cells of Pseudomonas aeruginosa. Int. Biodeterior. Biodegrad. 2007, 60, 69–73. [Google Scholar] [CrossRef]
- Chandrashekar, V.; Chandrashekar, C.; Shivakumar, R.; Bhattacharya, S.; Das, A.; Gouda, B.; Rajan, S.S. Assessment of Acrylamide Degradation Potential of Pseudomonas aeruginosa BAC-6 Isolated from Industrial Effluent. Appl. Biochem. Biotechnol. 2014, 173, 1135–1144. [Google Scholar] [CrossRef]
- Chacko, S.; Ramteke, P.W.; John, S.A. Amidase from plant growth promoting rhizobacterium. J. Bacteriol. 2009, 1, 46–50. [Google Scholar]
- Nawaz, M.S.; Khan, A.A.; Seng, J.E.; Leakey, J.E.; Siitonen, P.H.; Cerniglia, C.E. Purification and Characterization of an Amidase from an Acrylamide-Degrading Rhodococcus Sp. Appl. Environ. Microbiol. 1994, 60, 3343–3348. [Google Scholar] [CrossRef]
- Lakshmikandan, M.; Sivaraman, K.; Elaiya Raja, S.; Vasanthakumar, P.; Rajesh, R.P.; Sowparthani, K.; Jebasingh, S.E.J. Biodegradation of Acrylamide by Acrylamidase from Stenotrophomonas Acidaminiphila MSU12 and Analysis of Degradation Products by MALDI-TOF and HPLC. Int. Biodeterior. Biodegrad. 2014, 94, 214–221. [Google Scholar] [CrossRef]
- Hokama, S.; Honma, Y.; Toma, C.; Ogawa, Y. Oxalate-Degrading Enterococcus faecalis. Microbiol. Immunol. 2000, 44, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Lippolis, R.; Siciliano, R.; Mazzeo, M.; Abbrescia, A.; Gnoni, A.; Sardanelli, A.; Papa, S. Comparative Secretome Analysis of Four Isogenic Bacillus Clausii Probiotic Strains. Proteome Sci. 2013, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Bury-Moné, S.; Skouloubris, S.; Dauga, C.; Thiberge, J.-M.; Dailidiene, D.; Berg, D.E.; Labigne, A.; De Reuse, H. Presence of Active Aliphatic Amidases in Helicobacter Species Able to Colonize the Stomach. Infect. Immun. 2003, 71, 5613–5622. [Google Scholar] [CrossRef] [PubMed]
- Baker, W. A Sensitive Procedure for Screening Microorganisms for the Presence of Penicillin Amidase. Aust. J. Biol. Sci. 1984, 37, 257. [Google Scholar] [CrossRef] [PubMed]
- Baardseth, P.; Blom, H.; Skrede, G.; Mydland, L.T.; Skrede, A.; Slinde, E. Lactic Acid Fermentation Reduces Acrylamide Formation and Other Maillard Reactions in French Fries. J. Food Sci. 2006, 71, C28–C33. [Google Scholar] [CrossRef]
- Dominici, L.; Moretti, M.; Villarini, M.; Vannini, S.; Cenci, G.; Zampino, C.; Traina, G. In Vivo Antigenotoxic Properties of a Commercial Probiotic Supplement Containing Bifidobacteria. Int. J. Probiotics Prebiotics 2011, 6, 179–186. [Google Scholar]
- Seiquer, I.; Rubio, L.A.; Peinado, M.J.; Delgado-Andrade, C.; Navarro, M.P. Maillard Reaction Products Modulate Gut Microbiota Composition in Adolescents. Mol. Nutr. Food Res. 2014, 58, 1552–1560. [Google Scholar] [CrossRef]
- Santhanasabapathy, R.; Vasudevan, S.; Anupriya, K.; Pabitha, R.; Sudhandiran, G. Farnesol Quells Oxidative Stress, Reactive Gliosis and Inflammation during Acrylamide-Induced Neurotoxicity: Behavioral and Biochemical Evidence. Neuroscience 2015, 308, 212–227. [Google Scholar] [CrossRef]
- Sickles, D.W.; Stone, J.D.; Friedman, M.A. Fast Axonal Transport: A Site of Acrylamide Neurotoxicity? NeuroToxicology 2002, 23, 223–251. [Google Scholar] [CrossRef]
- An, L.; Li, G.; Si, J.; Zhang, C.; Han, X.; Wang, S.; Jiang, L.; Xie, K. Acrylamide Retards the Slow Axonal Transport of Neurofilaments in Rat Cultured Dorsal Root Ganglia Neurons and the Corresponding Mechanisms. Neurochem. Res. 2016, 41, 1000–1009. [Google Scholar] [CrossRef]
- Faria, M.; Ziv, T.; Gómez-Canela, C.; Ben-Lulu, S.; Prats, E.; Novoa-Luna, K.A.; Admon, A.; Piña, B.; Tauler, R.; Gómez-Oliván, L.M.; et al. Acrylamide Acute Neurotoxicity in Adult Zebrafish. Sci. Rep. 2018, 8, 7918. [Google Scholar] [CrossRef]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.-J. The Enteric Nervous System and Gastrointestinal Innervation: Integrated Local and Central Control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar] [CrossRef]
- Palus, K.; Całka, J. Influence of Acrylamide Administration on the Neurochemical Characteristics of Enteric Nervous System (ENS) Neurons in the Porcine Duodenum. Int. J. Mol. Sci. 2019, 21, 15. [Google Scholar] [CrossRef]
- Szymanska, K.; Gonkowski, S. Bisphenol A—Induced Changes in the Enteric Nervous System of the Porcine Duodenum. NeuroToxicology 2018, 66, 78–86. [Google Scholar] [CrossRef]
- Belai, A.; Burnstock, G. Acrylamide-Induced Neuropathic Changes in Rat Enteric Nerves: Similarities with Effects of Streptozotocin-Diabetes. J. Auton. Nerv. Syst. 1996, 58, 56–62. [Google Scholar] [CrossRef]
- Palus, K.; Makowska, K.; Całka, J. Alterations in Galanin-Like Immunoreactivity in the Enteric Nervous System of the Porcine Stomach Following Acrylamide Supplementation. Int. J. Mol. Sci. 2019, 20, 3345. [Google Scholar] [CrossRef]
- Palus, K.; Bulc, M.; Całka, J. Changes in VIP-, SP- and CGRP- like Immunoreactivity in Intramural Neurons within the Pig Stomach Following Supplementation with Low and High Doses of Acrylamide. NeuroToxicology 2018, 69, 47–59. [Google Scholar] [CrossRef]
- Palus, K.; Bulc, M.; Całka, J. Effect of Acrylamide Supplementation on the CART-, VAChT-, and nNOS-Immunoreactive Nervous Structures in the Porcine Stomach. Animals 2020, 10, 555. [Google Scholar] [CrossRef]
- Karpiesiuk, A.; Całka, J.; Palus, K. Acrylamide-Induced Changes in the Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Immunoreactivity in Small Intestinal Intramural Neurons in Pigs. Int. J. Environ. Res. Public Health 2023, 20, 3272. [Google Scholar] [CrossRef] [PubMed]
- Palus, K.; Makowska, K.; Całka, J. Acrylamide-Induced Alterations in the Cocaine- and Amphetamine-Regulated Peptide Transcript (CART)-like Immunoreactivity within the Enteric Nervous System of the Porcine Small Intestines. Ann. Anat.-Anat. Anz. 2018, 219, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Palus, K.; Bulc, M.; Całka, J. Effect of Acrylamide Supplementation on the Population of Vasoactive Intestinal Peptide (VIP)-Like Immunoreactive Neurons in the Porcine Small Intestine. Int. J. Mol. Sci. 2020, 21, 9691. [Google Scholar] [CrossRef]
- Bulc, M.; Całka, J.; Palus, K. Administration of Different Doses of Acrylamide Changed the Chemical Coding of Enteric Neurons in the Jejunum in Gilts. Int. J. Environ. Res. Public Health 2022, 19, 14514. [Google Scholar] [CrossRef] [PubMed]
- Eisenbrand, G. Revisiting the Evidence for Genotoxicity of Acrylamide (AA), Key to Risk Assessment of Dietary AA Exposure. Arch. Toxicol. 2020, 94, 2939–2950. [Google Scholar] [CrossRef]
- Kontaş Yedier, S.; Atlı Şekeroğlu, Z.; Şekeroğlu, V.; Aydın, B. Cytotoxic, Genotoxic, and Carcinogenic Effects of Acrylamide on Human Lung Cells. Food Chem. Toxicol. 2022, 161, 112852. [Google Scholar] [CrossRef]
- Başaran, B.; Çuvalcı, B.; Kaban, G. Dietary Acrylamide Exposure and Cancer Risk: A Systematic Approach to Human Epidemiological Studies. Foods 2023, 12, 346. [Google Scholar] [CrossRef] [PubMed]
- Besaratinia, A.; Pfeifer, G.P. A Review of Mechanisms of Acrylamide Carcinogenicity. Carcinogenesis 2007, 28, 519–528. [Google Scholar] [CrossRef]
- Martins, C.; Oliveira, N.G.; Pingarilho, M.; Gamboa Da Costa, G.; Martins, V.; Marques, M.M.; Beland, F.A.; Churchwell, M.I.; Doerge, D.R.; Rueff, J.; et al. Cytogenetic Damage Induced by Acrylamide and Glycidamide in Mammalian Cells: Correlation with Specific Glycidamide-DNA Adducts. Toxicol. Sci. 2006, 95, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Shipp, A.; Lawrence, G.; Gentry, R.; McDonald, T.; Bartow, H.; Bounds, J.; Macdonald, N.; Clewell, H.; Allen, B.; Van Landingham, C. Acrylamide: Review of Toxicity Data and Dose-Response Analyses for Cancer and Noncancer Effects. Crit. Rev. Toxicol. 2006, 36, 481–608. [Google Scholar] [CrossRef]
- Sickles, D.W.; Sperry, A.O.; Testino, A.; Friedman, M. Acrylamide Effects on Kinesin-Related Proteins of the Mitotic/Meiotic Spindle. Toxicol. Appl. Pharmacol. 2007, 222, 111–121. [Google Scholar] [CrossRef]
- Zhang, X. Simultaneous Exposure to Dietary Acrylamide and Corn Oil Developed Carcinogenesis through Cell Proliferation and Inhibition of Apoptosis by Regulating P53 -Mediated Mitochondria-Dependent Signaling Pathway. Toxicol. Ind. Health 2009, 25, 101–109. [Google Scholar] [CrossRef]
- Zhang, X. Long-Term Exposure to Various Types of Dietary Fat Modulates Acrylamide-Induced Preneoplastic Lesions of Colon Mucosa through Wnt/β -Catenin Signaling in Rats. Toxicol. Mech. Methods 2009, 19, 285–291. [Google Scholar] [CrossRef]
- Raju, J.; Sondagar, C.; Roberts, J.; Aziz, S.A.; Caldwell, D.; Vavasour, E.; Mehta, R. Dietary Acrylamide Does Not Increase Colon Aberrant Crypt Foci Formation in Male F344 Rats. Food Chem. Toxicol. 2011, 49, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Raju, J.; Roberts, J.; Sondagar, C.; Kapal, K.; Aziz, S.A.; Caldwell, D.; Mehta, R. Negligible Colon Cancer Risk from Food-Borne Acrylamide Exposure in Male F344 Rats and Nude (Nu/Nu) Mice-Bearing Human Colon Tumor Xenografts. PLoS ONE 2013, 8, e73916. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Katsonouri, A.; Christodoulou, M.-I.; Papageorgis, P. In Vivo Investigation of the Effect of Dietary Acrylamide and Evaluation of Its Clinical Relevance in Colon Cancer. Toxics 2023, 11, 856. [Google Scholar] [CrossRef]
- Lin, Y.; Lagergren, J.; Lu, Y. Dietary Acrylamide Intake and Risk of Esophageal Cancer in a Population-based Case-control Study in Sweden. Int. J. Cancer 2011, 128, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Lujan-Barroso, L.; González, C.A.; Slimani, N.; Obón-Santacana, M.; Ferrari, P.; Freisling, H.; Overvad, K.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; Racine, A.; et al. Dietary Intake of Acrylamide and Esophageal Cancer Risk in the European Prospective Investigation into Cancer and Nutrition Cohort. Cancer Causes Control 2014, 25, 639–646. [Google Scholar] [CrossRef]
- Liu, Z.; Tse, L.A.; Ho, S.C.; Wu, S.; Chen, B.; Chan, D.; Wong, S.Y. Dietary Acrylamide Exposure Was Associated with Increased Cancer Mortality in Chinese Elderly Men and Women: A 11-Year Prospective Study of Mr. and Ms. OS Hong Kong. J. Cancer Res. Clin. Oncol. 2017, 143, 2317–2326. [Google Scholar] [CrossRef]
- Rice, J.M. The Carcinogenicity of Acrylamide. Mutat. Res. Toxicol. Environ. Mutagen. 2005, 580, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Song, S.S.; Liu, M.; Park, S. Association of Fried Food Intake with Gastric Cancer Risk: A Systemic Review and Meta-Analysis of Case–Control Studies. Nutrients 2023, 15, 2982. [Google Scholar] [CrossRef]
- Bušová, M.; Bencko, V.; Veszelits Laktičová, K.; Holcátová, I.; Vargová, M. Risk of Exposure to Acrylamide. Cent. Eur. J. Public Health 2020, 28, S43–S46. [Google Scholar] [CrossRef]
- Rifai, L.; Saleh, F.A. A Review on Acrylamide in Food: Occurrence, Toxicity, and Mitigation Strategies. Int. J. Toxicol. 2020, 39, 93–102. [Google Scholar] [CrossRef] [PubMed]
- The FoodDrinkEurope Acrylamide “Toolbox” 2019. 2019. Available online: https://www.fooddrinkeurope.eu/wp-content/uploads/2021/05/FoodDrinkEurope_Acrylamide_Toolbox_2019.pdf (accessed on 23 June 2024).
- Capuano, E.; Ferrigno, A.; Acampa, I.; Serpen, A.; Açar, Ö.Ç.; Gökmen, V.; Fogliano, V. Effect of Flour Type on Maillard Reaction and Acrylamide Formation during Toasting of Bread Crisp Model Systems and Mitigation Strategies. Food Res. Int. 2009, 42, 1295–1302. [Google Scholar] [CrossRef]
- ALjahdali, N.; Carbonero, F. Impact of Maillard Reaction Products on Nutrition and Health: Current Knowledge and Need to Understand Their Fate in the Human Digestive System. Crit. Rev. Food Sci. Nutr. 2019, 59, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Kwolek-Mirek, M.; Zadrąg-Tęcza, R.; Bednarska, S.; Bartosz, G. Yeast Saccharomyces Cerevisiae Devoid of Cu,Zn-Superoxide Dismutase as a Cellular Model to Study Acrylamide Toxicity. Toxicol. Vitr. 2011, 25, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Piwowar, A.; Żurawska-Płaksej, E.; Bizoń, A.; Sawicka, E.; Płaczkowska, S.; Prescha, A. The Impact of Dietary Nitrates and Acrylamide Intake on Systemic Redox Status in Healthy Young Adults. Int. J. Occup. Med. Environ. Health 2023, 36, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Catalgol, B.; Ozhan, G.; Alpertunga, B. Acrylamide-Induced Oxidative Stress in Human Erythrocytes. Hum. Exp. Toxicol. 2009, 28, 611–617. [Google Scholar] [CrossRef]
- Chen, W.; Feng, L.; Shen, Y.; Su, H.; Li, Y.; Zhuang, J.; Zhang, L.; Zheng, X. Myricitrin Inhibits Acrylamide-Mediated Cytotoxicity in Human Caco-2 Cells by Preventing Oxidative Stress. BioMed Res. Int. 2013, 2013, 724183. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Jiang, L.; Geng, C.; Yao, X. Preventive effects of curcumin on acrylamide-induced DNA damage in HepG2 cells. Wei Sheng Yan Jiu 2009, 38, 392–395. [Google Scholar]
- Qu, D.; Liu, C.; Jiang, M.; Feng, L.; Chen, Y.; Han, J. After In Vitro Digestion, Jackfruit Flake Affords Protection against Acrylamide-Induced Oxidative Damage. Molecules 2019, 24, 3322. [Google Scholar] [CrossRef]
- Rivas-Jimenez, L.; Ramírez-Ortiz, K.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Garcia, H.S.; Hernandez-Mendoza, A. Evaluation of Acrylamide-Removing Properties of Two Lactobacillus Strains under Simulated Gastrointestinal Conditions Using a Dynamic System. Microbiol. Res. 2016, 190, 19–26. [Google Scholar] [CrossRef]
- Albedwawi, A.; Al Sakkaf, R.; Yusuf, A.; Osaili, T.; Al-Nabulsi, A.; Liu, S.-Q.; Palmisano, G.; Ayyash, M. Acrylamide Elimination by Lactic Acid Bacteria: Screening, Optimization, In Vitro Digestion, and Mechanism. Microorganisms 2022, 10, 557. [Google Scholar] [CrossRef] [PubMed]
- Torres-Gregorio, M.; Santiago-López, L.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Garcia, H.S.; Hernandez-Mendoza, A. Evaluation of Acrylamide-removing Properties of Bacterial Consortia under Simulated Gastrointestinal Conditions. J. Sci. Food Agric. 2021, 101, 5049–5055. [Google Scholar] [CrossRef] [PubMed]

| Microogranism | Enzyme Name (s) | Reference |
|---|---|---|
| Arthrobacter sp. DBV1 | Acrylamidase | [59] |
| Bacillus cereus strain DRY135 | Aliphatic amidase | [60] |
| Bacillus clausii strain 1779 | Aliphatic amidase | [61] |
| Burkholderia sp. strain DR.Y27 | Amidases of short-chain aliphatic | [62] |
| Cupriavidus oxalaticus ICTDB921 | Acrylamidase | [63] |
| Delftia tsuruhatensis ZJB-05174 | R-Enantio-selective amidase | [64] |
| Enterobacter aerogenes | Aliphatic amidase | [65] |
| Helicobacter pylori 26695 | Amidase AmiE | [66] |
| Moraxiella osloensis MSU11 | Aliphatic amidase | [67] |
| Nocardia globerula NHB-2 | Amidase | [68] |
| Pseudomonas aeruginosa | Aliphatic amidase | [69] |
| Pseudomonas aeruginosa BAC-6 | Aliphatic amidase | [70] |
| Pseudomonas putida MTCC 6809 | Extracellular amidase | [71] |
| Rodococcus sp. | Aliphatic amidase | [72] |
| Stenotrophomonas acidaminiphila MSU12 | Acrylamidase | [73] |
| Part of GI Tract | Species | Dose of ACR and Time of Exposure | Effect | References |
|---|---|---|---|---|
| duodenum | guinea pigs | 3 mg/kg of b.w./day for last 35 days of gestation | increased surface area and diameter of Auerbach’s and Meissner’s plexuses | [54] |
| jejunum | mice | 0.5 mg/kg of b.w./day for 90 days | increased intensity of neurofilament staining, circularity of the nerve ganglia, and the neurofilament integrated density and the cross-sectional area of the nerve ganglion | [37] |
| ileum | rats | 50 mg/kg of b.w./day for 10 days | decrease in the density of catecholamine-containing nerve fibres and tissue content of noradrenaline in the myenteric plexus, decrease in tissue content and immunoreactivity of CGRP, an increase in VIP immunoreactivity in the myenteric plexus, decrease in CGRP immunoreactivity and an increase in VIP and NPY immunoreactivity in the submucous plexus | [88] |
| stomach | pig | 0.5 and 5 μg/kg of b.w./day for 28 days | Low dose: an increase in the population of neurons immunoreactive to GAL, CART, VAChT, VIP, and SP in submucosal plexuses in the corpus and an increase in the number of GAL-, nNOS-, and VIP-immunoreactive in myenteric plexuses neurons in the cardia, corpus and pylorus, VAChT-, and SP-immunoreactive in the corpus and pylorus, and in the case of CART the changes were significant only in the corpus, and CGRP only in the pylorus. High dose: an increase in the number of GAL-, CART-, VAChT-, VIP-, SP-, and nNOS- immunopositive neurons in submucosal plexuses in the corpus of the stomach and an increase in the number of myenteric plexuses neurons immunopositive for GAL, CART, VAChT, VIP, SP, CGRP, and nNOS in the cardia, corpus, and pylorus. | [89,90,91] |
| duodenum | pig | 0.5 and 5 μg/kg of b.w./day for 28 days | Low dose: an increased population of PACAP-, CART-, CGRP-, VAChT-, GAL- and SP- positive neurons in the myenteric plexuses; an increase in the number of VAChT-, GAL- and SP-positive neurons in the OSP; increased the population of CGRP-, VAChT-, VIP-, GAL-, and SP-immunoreactive neurons in the ISP. High dose: an increased population of PACAP-, CART-, CGRP-, VAChT-, VIP-, nNOS-, GAL-, and SP-immunoreactive neurons in the MP, OSP and ISP. | [86,92,93,94] |
| jejunum | pig | 0.5 and 5 μg/kg of b.w./day for 28 days | Low dose: increased immunoreactivity with respect to PACAP, CART, CGRP, VAChT, GAL, and SP in the MP; an increased number of PACAP-immunoreactive neurons in the OSP; an increase in the number of PACAP-, VIP-, and GAL-immunoreactive and a decrease in the nNOS- positive neurons in the ISP. High dose: an increase in the population of PACAP-, CART-, CGRP-, VAChT-, VIP-, GAL-, and SP-immunoreactive and a decrease in that of nNOS- positive neurons in the MP, OSP, and ISP. | [86,92,93,94,95] |
| ileum | pig | 0.5 and 5 μg/kg of b.w./day for 28 days | Low dose: an increase in the number of CART-, CGRP-, VIP-, GAL-, and SP-immunopositive and a decrease in the number of nNOS- positive in the MP; an increase in immunoreactivity with respect to VAChT, VIP, and SP and a decrease in the number of nNOS- immunoreactive neurons in the OSP; increased number of CGRP-, VAChT-, VIP-, and GAL-immunoreactive neurons the ISP. High dose: an increase in the number of PACAP-, CART-, CGRP-, VAChT-, VIP-, GAL-, and SP- immunoreactive and decreased the number of nNOS- positive neurons in the MP, OSP, and ISP. | [39,86,92,93,94] |
| Part of GI Tract | Species | Dose of ACR and Time of Exposure | Effect | References |
|---|---|---|---|---|
| colon | rats | ACR in dose 10 mg/kg of b.w./day + diets supplemented with 10% corn oil for 8 weeks and then only 10% corn oil for 48 weeks | colonic aberrant crypt foci and colon cancer invasion; apoptosis was decreased and cell proliferation was increased in colonic mucosa; mitochondrial wt p53 was significantly inhibited through decreased mitochondrial localization of wt p53 and increased cytosolic p53, resulting in the up-regulation of Bcl-2 and the down-regulation of Bax in the mitochondria, inhibition of the release of cytochrome-c from the mitochondria into the cytosol and protein level of caspase-3 | [103] |
| colon | rats | ACR in dose 5 mg/kg of b.w./day + diet supplemented with 10% corn, olive, beef, or fish oil for 8 weeks and then diets supplemented with 10% oil for other 40 weeks | ACR and diet with corn oil and beef tallow enhanced colonic aberrant crypt foci formation, increased BrdU incorporation, expression of cytosolic beta-catenin and cyclin D1 and decreased apoptosis in the colon mucosa; ACR and diet with beef tallow increased expressions of Wnt2 and Wnt3; ACR and diet with corn oil increased expressions of Wnt5a; ACR and diet with olive and fish oil weakened the colonic aberrant crypt foci formation | [104] |
| colon | rats | subcutaneous injection of azoxymethane and ACR at 0, 5, 10 or 50 mg/kg diet and low fat (7% corn oil) or high fat (23.9% corn oil) for 8 weeks | ACR does not increase the risk of developing azoxymethane-induced precancerous lesions of the colon in rats the highest tested dose of ACR (50 mg/kg diet) had significantly lower total colonic aberrant crypt foci and lower large colonic aberrant crypt foci compared with their respective controls | [105] |
| rats | 0.5, 1.0 or 2.0 mg/kg diet for 2 weeks and then subcutaneous injection with azoxymethane once a week for 2 weeks and diet with ACR for 20 weeks | ACR alone at doses corresponding to actual levels in food does not induce cancer in rats, but it can intensify azoxymethane-induced cancers when administered at larger doses | [106] | |
| colon | mice | subcutaneous injection in the right shank with HT-29 human colon adenocarcinoma cells and after 3 weeks diet with ACR (0.5, 1.0 or 2.0 mg/kg diet) for 4 weeks | no differences in the growth of human colon tumour xenografts between acrylamide-treated and control mice | [106] |
| colon | mice | ACR in a dose 0.1 mg/kg of b.w./day for 4 weeks | genes implicated in RNA metabolism, processing and formation of the ribosomal subunits and protein translation and metabolism are upregulated in ACR-exposed colon tissue | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palus, K. Dietary Exposure to Acrylamide Has Negative Effects on the Gastrointestinal Tract: A Review. Nutrients 2024, 16, 2032. https://doi.org/10.3390/nu16132032
Palus K. Dietary Exposure to Acrylamide Has Negative Effects on the Gastrointestinal Tract: A Review. Nutrients. 2024; 16(13):2032. https://doi.org/10.3390/nu16132032
Chicago/Turabian StylePalus, Katarzyna. 2024. "Dietary Exposure to Acrylamide Has Negative Effects on the Gastrointestinal Tract: A Review" Nutrients 16, no. 13: 2032. https://doi.org/10.3390/nu16132032
APA StylePalus, K. (2024). Dietary Exposure to Acrylamide Has Negative Effects on the Gastrointestinal Tract: A Review. Nutrients, 16(13), 2032. https://doi.org/10.3390/nu16132032







