A Healthful Plant-Based Diet as an Alternative Dietary Approach in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease
Abstract
1. Introduction
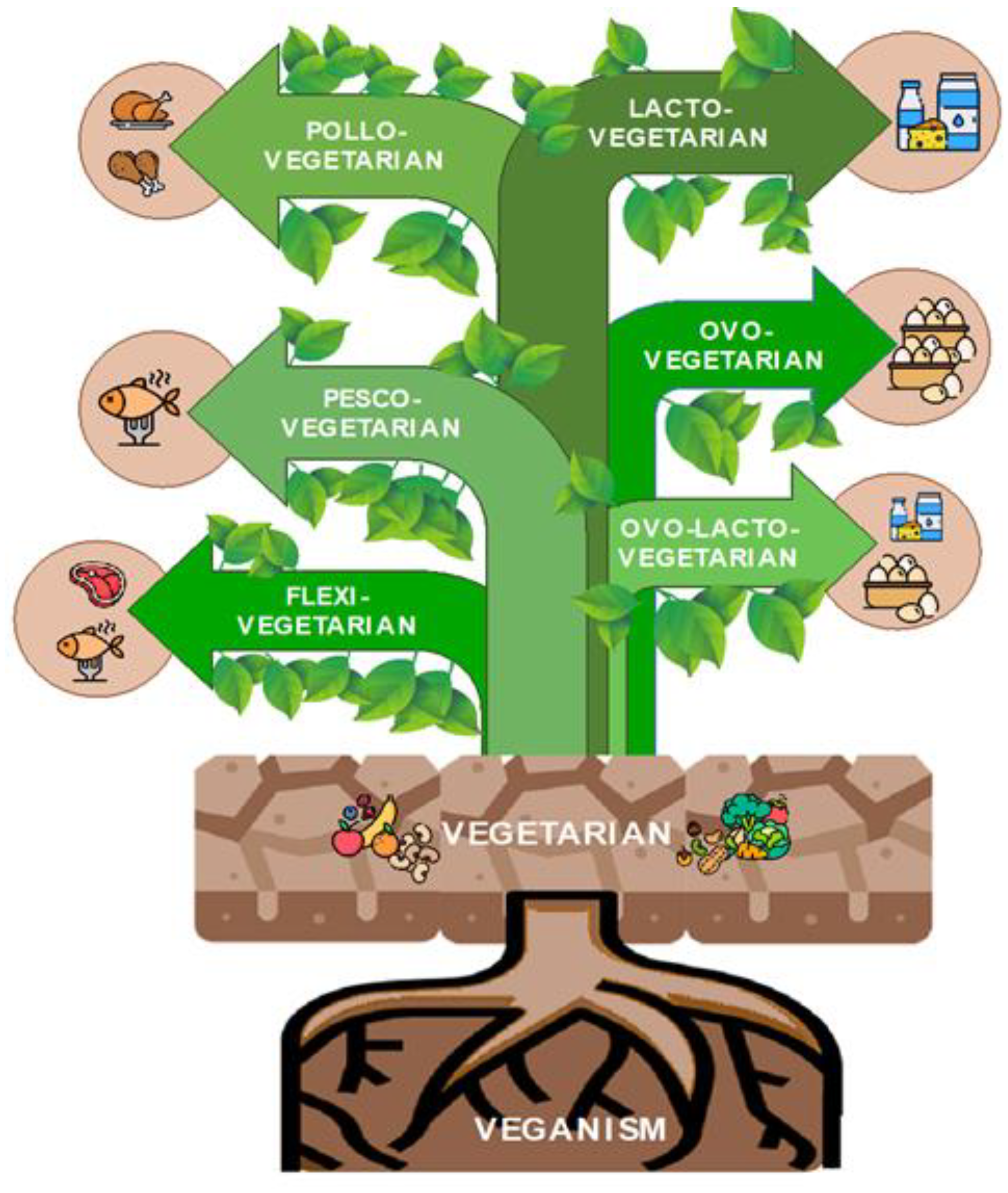
2. What Is a Plant-Based Diet?
3. Impact of Plant-Based Diets on MASLD Outcomes
| Authors—Year | Study Design | Hypothesis/Aim | Outcomes—Remarks |
|---|---|---|---|
| Choi S.H. et al., 2015 [52] | Cross-sectional and retrospective study comparing MASLD prevalence of 615 Buddhist priests and controls matched for age, sex, BMI and eventual MetS, who underwent routine health checks. | To assess the relationship between MASLD and vegetarian diets, taking into account MetS and obesity. | A vegetarian diet does not protect against MASLD. MASLD was significantly associated with male gender, WC, BMI, albumin, glucose, ALT, TG, low HDL and high LDL. |
| Mazidi M., Kengne A. 2018 [51] | Observational study in US adults from the 2005–2010 NHANES in 18,345 participants. | To investigate the influence of PDI, hPDI and uPDI on MASLD occurrence and liver function tests. | The results showed an inverse link between a healthy PBD and the likelihood of MASLD and more favorable liver function tests; whereas an unhealthy PBD would have the opposite effect. |
| Chiu T.H. et al., 2018 [73] | Cross-sectional study that included 2127 nonvegetarians and 1273 vegetarians who did not smoke or habitually drink alcohol and had no hepatitis B or hepatitis C. | To examine the association between vegetarian diets, principal food groups and MASLD, and then compare the extent of liver fibrosis between vegetarians and non-vegetarians in subjects with fatty liver. | A vegetarian diet resulted inversely related to fatty liver through reduced BMI. Substituting soy for meat/fish or whole grains for refined carbohydrates might be protective regardless of dietary pattern. |
| Kahleova H. et al., 2020 [47] | 16-week RCT with 244 participants randomized to the low-fat vegan diet or to the control group with no dietary changes. | To measure the impact of a low-fat vegan diet on body weight, IR, postprandial metabolism, and intramyocellular and hepatocellular lipid levels in overweight adults. | A low-fat vegan diet effectively reduces body weight and increases metabolism after meals, probably due to increased insulin sensitivity from reduced hepatocellular and intramyocellular fats. |
| Chiarioni G. et al., 2021 [74] | Prospective, pilot study on 32 patients with MASLD who accepted to adhere to a 6-month vegan diet. | To explore the effect of a vegan diet on liver chemistry in a group of patients with MASLD. | Improved hepatic enzymes in MASLD patients on a vegan diet with the decrease in body weight being a variable that did not seem to be critical for the outcome. |
| Li X., 2022 [50] | NHANES 2017–2018 cross-sectional study of 3900 US adults. | To investigate through transient elastography the association between the overall PDI, hPDI and uPDI, and MASLD. | A healthful PBD was inversely related to the odds of MASLD, also after adjusting for BMI. Unhealthful PBDs, on the other hand, showed a positive association with MASLD. |
| Garousi N. et al., 2023 [75] | RCT with 75 overweight/obese adults with MASLD, randomly allocated to 3-month LOV-D or SWL-D groups. | To compare the effects of a LOV-D vs. a SWL-D on obese/overweight adults with MASLD. | Adherence to a 3-month LOV-D resulted in improved MASLD, anthropometric measures, glycemic-related markers and lipid profiles. |
| Lv Y. et al., 2023 [37] | Longitudinal cohort study on 159,222 participants in the UK Biobank. | To study the link between PDIs and MASLD risk and whether these associations might be modified by the genetic risk of MASLD. | Higher intake of healthful PBDs was linked to a lower MASLD risk and hepatic fat content calculated by MRI-PDFF, while unhealthful PBDs were linked to higher MASLD risk and intrahepatic steatosis. |
4. Implementation of a Plant-Based Diet in MASLD Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Horn, P.; Wong, V.W.S.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL–EASD–EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). J. Hepatol. 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, T.H.; Sheron, N.; Zelber-Sagi, S.; Carrieri, P.; Dusheiko, G.; Bugianesi, E.; Pryke, R.; Hutchinson, S.J.; Sangro, B.; Martin, N.K.; et al. The EASL–Lancet Liver Commission: Protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet 2022, 399, 61–116. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Brizi, M.; Morselli-Labate, A.M.; Bianchi, G.; Bugianesi, E.; McCullough, A.J.; Forlani, G.; Melchionda, N. Association of nonalcoholic fatty liver disease with insulin resistance. Am. J. Med. 1999, 107, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.; Gao, L.; Yao, Z.; Shao, S.; Wang, X.; Proud, C.G.; Zhao, J. Hepatic selective insulin resistance at the intersection of insulin signaling and metabolic dysfunction-associated steatotic liver disease. Cell Metab. 2024, 36, 947–968. [Google Scholar] [CrossRef] [PubMed]
- Rosso, C.; Caviglia, G.P.; Birolo, G.; Armandi, A.; Pennisi, G.; Pelusi, S.; Younes, R.; Liguori, A.; Perez-Diaz-Del-Campo, N.; Nicolosi, A.; et al. Impact of PNPLA3 rs738409 Polymorphism on the Development of Liver-Related Events in Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 3314–3321.e3. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Petroni, M.L.; Brodosi, L.; Bugianesi, E.; Marchesini, G. Management of Non-Alcoholic Fatty Liver Disease. BMJ 2021, 372, m4747. [Google Scholar] [CrossRef]
- Satapathy, S.; Sanyal, A. Epidemiology and Natural History of Nonalcoholic Fatty Liver Disease. Semin. Liver Dis. 2015, 35, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zou, B.; Yeo, Y.H.; Li, J.; Huang, D.Q.; Wu, Y.; Yang, H.; Liu, C.; Kam, L.Y.; Tan, X.X.E.; et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 739–752. [Google Scholar] [CrossRef]
- Kosmalski, M.; Frankowski, R.; Ziółkowska, S.; Różycka-Kosmalska, M.; Pietras, T. What’s New in the Treatment of Non-Alcoholic Fatty Liver Disease (NAFLD). J. Clin. Med. 2023, 12, 1852. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with Diet, Physical Activity and Exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Moss, K.; Gitman, V.; Sanchez, M.I.P.; Oczkowski, S.; Armstrong, D.; Jayakumar, S.; Karvellas, C.J.; Selzner, N.; Dionne, J. Evidence related to a vegetarian diet and metabolic dysfunction-associated steatotic liver disease: Protocol for a scoping review. BMJ Open 2024, 14, e079750. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Urbistondo, D.; Perez-Diaz-Del-Campo, N.; Landecho, M.F.; Martínez, J.A. Alcohol Drinking Impacts on Adiposity and Steatotic Liver Disease: Concurrent Effects on Metabolic Pathways and Cardiovascular Risks. Curr. Obes. Rep. 2024; ahead of print. [Google Scholar]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.M.; Johnson, N.A.; Burdon, C.A.; Cohn, J.S.; O’Connor, H.T.; George, J. Omega-3 supplementation and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012, 56, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, S.H.; Mansoori, A.; Hosseinzadeh, M. Relationship between dietary patterns and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2021, 36, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Grinshpan, L.S.; Ivancovsky-Wajcman, D.; Goldenshluger, A.; Gepner, Y. One size does not fit all; practical, personal tailoring of the diet to NAFLD patients. Liver Int. 2022, 42, 1731–1750. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.S.; Tresserra-Rimbau, A.; Karavasiloglou, N.; Jennings, A.; Cantwell, M.; Hill, C.; Perez-Cornago, A.; Bondonno, N.P.; Murphy, N.; Rohrmann, S.; et al. Association of Healthful Plant-based Diet Adherence with Risk of Mortality and Major Chronic Diseases Among Adults in the UK. JAMA Netw. Open 2023, 6, e234714. [Google Scholar] [CrossRef]
- Bugianesi, E.; McCullough, A.J.; Marchesini, G. Insulin resistance: A metabolic pathway to chronic liver disease. Hepatology 2005, 42, 987–1000. [Google Scholar] [CrossRef]
- Salehin, S.; Rasmussen, P.; Mai, S.; Mushtaq, M.; Agarwal, M.; Hasan, S.M.; Salehin, S.; Raja, M.; Gilani, S.; Khalife, W.I. Plant Based Diet and Its Effect on Cardiovascular Disease. Int. J. Environ. Res. Public Health 2023, 20, 3337. [Google Scholar] [CrossRef] [PubMed]
- Corrin, T.; Papadopoulos, A. Understanding the attitudes and perceptions of vegetarian and plant-based diets to shape future health promotion programs. Appetite 2017, 109, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Fehér, A.; Gazdecki, M.; Véha, M.; Szakály, M.; Szakály, Z. A Comprehensive Review of the Benefits of and the Barriers to the Switch to a Plant-Based Diet. Sustainability 2020, 12, 4136. [Google Scholar] [CrossRef]
- Storz, M.A. What makes a plant-based diet? A review of current concepts and proposal for a standardized plant-based dietary intervention checklist. Eur. J. Clin. Nutr. 2022, 76, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, S.M.; Rosenfeld, D.L.; Moreira, A.V.B.; Zandonadi, R.P. Plant-based and vegetarian diets: An overview and definition of these dietary patterns. Eur. J. Nutr. 2023, 62, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and Vegetable Intake and the Risk of Cardiovascular Disease, Total Cancer and All-Cause Mortality-a Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Hemler, E.C.; Hu, F.B. Plant-Based Diets for Cardiovascular Disease Prevention: All Plant Foods Are Not Created Equal. Curr. Atheroscler. Rep. 2019, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Hu, F.B. Plant-based diets and cardiovascular health. Trends Cardiovasc. Med. 2018, 28, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cueto, F.J.; Rini, L.; Faber, I.; Rasmussen, M.A.; Bechtold, K.-B.; Schouteten, J.J.; De Steur, H. How barriers towards plant-based food consumption differ according to dietary lifestyle: Findings from a consumer survey in 10 EU countries. Int. J. Gastron. Food Sci. 2022, 29, 100587. [Google Scholar] [CrossRef]
- Bryant, C.J. We Can’t Keep Meating Like This: Attitudes towards Vegetarian and Vegan Diets in the United Kingdom. Sustainability 2019, 11, 6844. [Google Scholar] [CrossRef]
- Havermans, R.C.; Rutten, G.; Bartelet, D. Adolescent’s Willingness to Adopt a More Plant-Based Diet: A Theory-Based Interview Study. Front. Nutr. 2021, 8, 688131. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, W.; Li, S.; Tu, H.; Jia, J.; Zhao, W.; Xu, A.; Xu, W.; Tsai, M.K.; Chu, D.T.-W.; et al. Association between plant-based dietary pattern and biological aging trajectory in a large prospective cohort. BMC Med. 2023, 21, 310. [Google Scholar] [CrossRef]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Rosenfeld, R.M.; Juszczak, H.M.; Wong, M.A. Scoping review of the association of plant-based diet quality with health outcomes. Front. Nutr. 2023, 10, 1211535. [Google Scholar] [CrossRef]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Coresh, J.; Rebholz, C.M. Plant-Based Diets Are Associated with a Lower Risk of Incident Cardiovascular Disease, Cardiovascular Disease Mortality, and All-Cause Mortality in a General Population of Middle-Aged Adults. J. Am. Heart Assoc. 2019, 8, e012865. [Google Scholar] [CrossRef]
- Lv, Y.; Rong, S.; Deng, Y.; Bao, W.; Xia, Y.; Chen, L. Plant-based diets, genetic predisposition and risk of non-alcoholic fatty liver disease. BMC Med. 2023, 21, 351. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Dinu, M.; Pagliai, G.; Cesari, F.; Gori, A.M.; Sereni, A.; Becatti, M.; Fiorillo, C.; Marcucci, R.; Casini, A. Low-Calorie Vegetarian Versus Mediterranean Diets for Reducing Body Weight and Improving Cardiovascular Risk Profile. Circulation 2018, 137, 1103–1113. [Google Scholar] [CrossRef]
- Buss, J. Limitations of Body Mass Index to Assess Body Fat. Workplace Health Saf. 2014, 62, 264. [Google Scholar] [CrossRef] [PubMed]
- González-Muniesa, P.; Mártinez-González, M.-A.; Hu, F.B.; Després, J.-P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A. Obesity. Nat. Rev. Dis. Prim. 2017, 3, 17034. [Google Scholar] [CrossRef]
- Ronca, A.; Pellegrini, N.; Pagliai, G.; Dinu, M.; Manfredini, M.; Incerti, M.; Favari, E.; Sofi, F. Effects of a dietary intervention with Mediterranean vs lacto-ovo vegetarian diets on HDL function: Results from the CARDIVEG study. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 651–658. [Google Scholar] [CrossRef]
- Kahleova, H.; Fleeman, R.; Hlozkova, A.; Holubkov, R.; Barnard, N.D. A plant-based diet in overweight individuals in a 16-week randomized clinical trial: Metabolic benefits of plant protein. Nutr. Diabetes 2018, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Hlozkova, A.; Fleeman, R.; Fletcher, K.; Holubkov, R.; Barnard, N.D. Fat Quantity and Quality, as Part of a Low-Fat, Vegan Diet, Are Associated with Changes in Body Composition, Insulin Resistance, and Insulin Secretion. A 16-Week Randomized Controlled Trial. Nutrients 2019, 11, 615. [Google Scholar] [CrossRef]
- Wiśniewska, K.; Okręglicka, K.M.; Nitsch-Osuch, A.; Oczkowski, M. Plant-Based Diets and Metabolic Syndrome Components: The Questions That Still Need to Be Answered—A Narrative Review. Nutrients 2024, 16, 165. [Google Scholar] [CrossRef] [PubMed]
- Crosby, L.; Rembert, E.; Levin, S.; Green, A.; Ali, Z.; Jardine, M.; Nguyen, M.; Elliott, P.; Goldstein, D.; Freeman, A.; et al. Changes in Food and Nutrient Intake and Diet Quality on a Low-Fat Vegan Diet Are Associated with Changes in Body Weight, Body Composition, and Insulin Sensitivity in Overweight Adults: A Randomized Clinical Trial. J. Acad. Nutr. Diet. 2022, 122, 1922–1939.e0. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.S.; Puglisi, M.; Malysheva, O.; Caudill, M.A.; Sholola, M.; Cooperstone, J.L.; Fernandez, M.L. Eggs Improve Plasma Biomarkers in Patients with Metabolic Syndrome Following a Plant-Based Diet—A Randomized Crossover Study. Nutrients 2022, 14, 2138. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Petersen, K.F.; Shulman, G.I.; Alwarith, J.; Rembert, E.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. Effect of a Low-Fat Vegan Diet on Body Weight, Insulin Sensitivity, Postprandial Metabolism, and Intramyocellular and Hepatocellular Lipid Levels in Overweight Adults. JAMA Netw. Open 2020, 3, e2025454. [Google Scholar] [CrossRef]
- Li, H.-Y.; Gan, R.-Y.; Shang, A.; Mao, Q.-Q.; Sun, Q.-C.; Wu, D.-T.; Geng, F.; He, X.-Q.; Li, H.-B. Plant-Based Foods and Their Bioactive Compounds on Fatty Liver Disease: Effects, Mechanisms, and Clinical Application. Oxidative Med. Cell. Longev. 2021, 2021, 6621644. [Google Scholar] [CrossRef]
- Tuso, P.J.; Ismail, M.H.; Ha, B.P.; Bartolotto, C. Nutritional update for physicians: Plant-based diets. Perm. J. 2013, 17, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peng, Z.; Li, M.; Zeng, X.; Li, H.; Zhu, Y.; Chen, H.; Hu, A.; Zhao, Q.; Zhang, Z.; et al. A Healthful Plant-Based Diet Is Associated with Lower Odds of Nonalcoholic Fatty Liver Disease. Nutrients 2022, 14, 4099. [Google Scholar] [CrossRef]
- Mazidi, M.; Kengne, A.P. Higher adherence to plant-based diets are associated with lower likelihood of fatty liver. Clin. Nutr. 2019, 38, 1672–1677. [Google Scholar] [CrossRef]
- Choi, S.H.; Oh, D.J.; Kwon, K.H.; Lee, J.K.; Koh, M.-S.; Lee, J.H.; Kang, H.W. A vegetarian diet does not protect against nonalcoholic fatty liver disease (NAFLD): A cross-sectional study between Buddhist priests and the general population. Turk. J. Gastroenterol. 2015, 26, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Berná, G.; Romero-Gomez, M. The role of nutrition in non-alcoholic fatty liver disease: Pathophysiology and management. Liver Int. 2020, 40 (Suppl. S1), 102–108. [Google Scholar] [CrossRef] [PubMed]
- Naomi, N.D.; Ngo, J.; Brouwer-Brolsma, E.M.; Buso, M.E.C.; Soedamah-Muthu, S.S.; Pérez-Rodrigo, C.; Harrold, J.A.; Halford, J.C.G.; Raben, A.; Geleijnse, J.M.; et al. Sugar-sweetened beverages, low/no-calorie beverages, fruit juice and non-alcoholic fatty liver disease defined by fatty liver index: The SWEET project. Nutr. Diabetes 2023, 13, 6. [Google Scholar] [CrossRef]
- Tian, A.; Sun, Z.; Zhang, M.; Li, J.; Pan, X.; Chen, P. Associations between dietary fatty acid patterns and non-alcoholic fatty liver disease in typical dietary population: A UK biobank study. Front. Nutr. 2023, 10, 1117626. [Google Scholar] [CrossRef] [PubMed]
- Šmíd, V.; Dvořák, K.; Šedivý, P.; Kosek, V.; Leníček, M.; Dezortová, M.; Hajšlová, J.; Hájek, M.; Vítek, L.; Bechyňská, K.; et al. Effect of Omega-3 Polyunsaturated Fatty Acids on Lipid Metabolism in Patients with Metabolic Syndrome and NAFLD. Hepatol. Commun. 2022, 6, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Depner, C.M.; Philbrick, K.A.; Jump, D.B. Docosahexaenoic Acid Attenuates Hepatic Inflammation, Oxidative Stress, and Fibrosis without Decreasing Hepatosteatosis in a Ldlr Mouse Model of Western Diet-Induced Nonalcoholic Steatohepatitis. J. Nutr. 2013, 143, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Lamaziere, A.; Wolf, C.; Barbe, U.; Bausero, P.; Visioli, F. Lipidomics of hepatic lipogenesis inhibition by omega 3 fatty acids. Prostaglandins, Leukot. Essent. Fat. Acids 2013, 88, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, H.; Zhang, Y.; Rao, S.; Mo, Y.; Zhang, H.; Liang, S.; Zhang, Z.; Yang, W. Dietary fiber intake and non-alcoholic fatty liver disease: The mediating role of obesity. Front. Public Health 2023, 10, 1038435. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.J.; O’neil, C.E.; Greenway, F.L. Dietary fiber and satiety: The effects of oats on satiety. Nutr. Rev. 2016, 74, 131–147. [Google Scholar] [CrossRef]
- Waddell, I.S.; Orfila, C. Dietary fiber in the prevention of obesity and obesity-related chronic diseases: From epidemiological evidence to potential molecular mechanisms. Crit. Rev. Food Sci. Nutr. 2023, 63, 8752–8767. [Google Scholar] [CrossRef]
- Slavin, J.L. Dietary fiber and body weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Howarth, N.C.; Saltzman, E.; Roberts, S.B. Dietary Fiber and Weight Regulation. Nutr. Rev. 2001, 59, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Schoeneck, M.; Iggman, D. The effects of foods on LDL cholesterol levels: A systematic review of the accumulated evidence from systematic reviews and meta-analyses of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Goszcz, K.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Bioactive Polyphenols and Cardiovascular Disease: Chemical Antagonists, Pharmacological Agents or Xenobiotics That Drive an Adaptive Response? Br. J. Pharmacol. 2017, 174, 1209–1225. [Google Scholar] [CrossRef]
- Marino, M.; Del Bo’, C.; Martini, D.; Porrini, M.; Riso, P. A Review of Registered Clinical Trials on Dietary (Poly)Phenols: Past Efforts and Possible Future Directions. Foods 2020, 9, 1606. [Google Scholar] [CrossRef] [PubMed]
- Tressera-Rimbau, A.; Arranz, S.; Eder, M.; Vallverdú-Queralt, A. Dietary Polyphenols in the Prevention of Stroke. Oxidative Med. Cell. Longev. 2017, 2017, 7467962. [Google Scholar] [CrossRef] [PubMed]
- Medina-Remón, A.; Tresserra-Rimbau, A.; Pons, A.; Tur, J.; Martorell, M.; Ros, E.; Buil-Cosiales, P.; Sacanella, E.; Covas, M.; Corella, D.; et al. Effects of total dietary polyphenols on plasma nitric oxide and blood pressure in a high cardiovascular risk cohort. The PREDIMED randomized trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Forouhi, N.G.; Sharp, S.J.; González, C.A.; Buijsse, B.; Guevara, M.; van der Schouw, Y.T.; Amiano, P.; Boeing, H.; Bredsdorff, L.; et al. The Association Between Dietary Flavonoid and Lignan Intakes and Incident Type 2 Diabetes in European Populations. Diabetes Care 2013, 36, 3961–3970. [Google Scholar] [CrossRef] [PubMed]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.; Hasan, P.M.; Shamsi, A. Role of polyphenols in combating Type 2 Diabetes and insulin resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef]
- Rodriguez-Ramiro, I.; Vauzour, D.; Minihane, A.M. Polyphenols and non-alcoholic fatty liver disease: Impact and mechanisms. Proc. Nutr. Soc. 2016, 75, 47–60. [Google Scholar] [CrossRef]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.H.; Lin, M.-N.; Pan, W.-H.; Chen, Y.-C.; Lin, C.-L. Vegetarian Diet, Food Substitution, and Nonalcoholic Fatty Liver. Ci Ji Yi Xue Za Zhi = Tzu-Chi Med. J. 2018, 30, 102–109. [Google Scholar]
- Chiarioni, G.; Popa, S.L.; Dalbeni, A.; Senore, C.; Leucuta, D.C.; Baroni, L.; Fantin, A. Vegan Diet Advice Might Benefit Liver Enzymes in Nonalcoholic Fatty Liver Disease: An Open Observational Pilot Study. J. Gastrointest. Liver Dis. 2021, 30, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Garousi, N.; Tamizifar, B.; Pourmasoumi, M.; Feizi, A.; Askari, G.; Clark, C.C.T.; Entezari, M.H. Effects of lacto-ovo-vegetarian diet vs. standard-weight-loss diet on obese and overweight adults with non-alcoholic fatty liver disease: A randomised clinical trial. Arch. Physiol. Biochem. 2023, 129, 975–983. [Google Scholar] [CrossRef]
- Perez-Diaz-Del-Campo, N.; Dileo, E.; Castelnuovo, G.; Nicolosi, A.; Guariglia, M.; Caviglia, G.P.; Rosso, C.; Armandi, A.; Bugianesi, E. A nutrigenetic precision approach for the management of non-alcoholic fatty liver disease. Clin. Nutr. 2023, 42, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Rosina, F.; Gambino, R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of randomised trials. Diabetologia 2012, 55, 885–904. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Jun, D.W.; Lee, H.Y.; Moon, J.H. Critical appraisal for low-carbohydrate diet in nonalcoholic fatty liver disease: Review and meta-analyses. Clin. Nutr. 2019, 38, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Perez-Diaz-Del-Campo, N.; Castelnuovo, G.; Caviglia, G.P.; Armandi, A.; Rosso, C.; Bugianesi, E. Role of Circadian Clock on the Pathogenesis and Lifestyle Management in Non-Alcoholic Fatty Liver Disease. Nutrients 2022, 14, 5053. [Google Scholar] [CrossRef] [PubMed]
- Hemler, E.C.; Hu, F.B. Plant-Based Diets for Personal, Population, and Planetary Health. Adv. Nutr. 2019, 10, S275–S283. [Google Scholar] [CrossRef]
- Crous-Bou, M.; Molinuevo, J.-L.; Sala-Vila, A. Plant-Rich Dietary Patterns, Plant Foods and Nutrients, and Telomere Length. Adv. Nutr. 2019, 10 (Suppl. S4), S296–S303. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- Flint, M.; Bowles, S.; Lynn, A.; Paxman, J.R. Novel plant-based meat alternatives: Future opportunities and health considerations. Proc. Nutr. Soc. 2023, 82, 370–385. [Google Scholar] [CrossRef]
- Jayedi, A.; Zeraattalab-Motlagh, S.; Jabbarzadeh, B.; Hosseini, Y.; Jibril, A.T.; Shahinfar, H.; Mirrafiei, A.; Hosseini, F.; Shab-Bidar, S. Dose-dependent effect of carbohydrate restriction for type 2 diabetes management: A systematic review and dose-response meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2022, 116, 40–56. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Nitzan-Kaluski, D.; Goldsmith, R.; Webb, M.; Blendis, L.; Halpern, Z.; Oren, R. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): A population based study. J. Hepatol. 2007, 47, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J. Nutrition Concerns and Health Effects of Vegetarian Diets. Nutr. Clin. Pract. 2010, 25, 613–620. [Google Scholar] [CrossRef]
- Marsh, K.; Zeuschner, C.; Saunders, A. Health Implications of a Vegetarian Diet: A Review. Am. J. Lifestyle Med. 2012, 6, 250–267. [Google Scholar] [CrossRef]
- Gilsing, A.M.J.; Crowe, F.L.; Lloyd-Wright, Z.; Sanders, T.A.B.; Appleby, P.N.; Allen, N.E.; Key, T.J. Serum concentrations of vitamin B12 and folate in British male omnivores, vegetarians and vegans: Results from a cross-sectional analysis of the EPIC-Oxford cohort study. Eur. J. Clin. Nutr. 2010, 64, 933–939. [Google Scholar] [CrossRef]
- Zeuschner, C.L.; Hokin, B.D.; Marsh, K.A.; Saunders, A.V.; Reid, M.A.; Ramsay, M.R. Vitamin B12 and Vegetarian Diets. Med. J. Aust. 2013, 199, S27–S32. [Google Scholar] [CrossRef]
- Springmann, M.; Wiebe, K.; Mason-D’Croz, D.; Sulser, T.B.; Rayner, M.; Scarborough, P. Health and nutritional aspects of sustainable diet strategies and their association with environmental impacts: A global modelling analysis with country-level detail. Lancet Planet. Health 2018, 2, e451–e461. [Google Scholar] [CrossRef] [PubMed]
- WHO Plant-Based Diets and Their Impact on Health, Sustainability and the Environment. Available online: https://www.who.int/europe/publications/i/item/WHO-EURO-2021-4007-43766-61591 (accessed on 29 May 2024).
- Gibson, A.A.; Sainsbury, A. Strategies to Improve Adherence to Dietary Weight Loss Interventions in Research and Real-World Settings. Behav. Sci. 2017, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Tran, E.; Dale, H.F.; Jensen, C.; Lied, G.A. Effects of Plant-Based Diets on Weight Status: A Systematic Review. Diabetes, Metab. Syndr. Obesity Targets Ther. 2020, 13, 3433–3448. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castelnuovo, G.; Perez-Diaz-del-Campo, N.; Rosso, C.; Armandi, A.; Caviglia, G.P.; Bugianesi, E. A Healthful Plant-Based Diet as an Alternative Dietary Approach in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease. Nutrients 2024, 16, 2027. https://doi.org/10.3390/nu16132027
Castelnuovo G, Perez-Diaz-del-Campo N, Rosso C, Armandi A, Caviglia GP, Bugianesi E. A Healthful Plant-Based Diet as an Alternative Dietary Approach in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease. Nutrients. 2024; 16(13):2027. https://doi.org/10.3390/nu16132027
Chicago/Turabian StyleCastelnuovo, Gabriele, Nuria Perez-Diaz-del-Campo, Chiara Rosso, Angelo Armandi, Gian Paolo Caviglia, and Elisabetta Bugianesi. 2024. "A Healthful Plant-Based Diet as an Alternative Dietary Approach in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease" Nutrients 16, no. 13: 2027. https://doi.org/10.3390/nu16132027
APA StyleCastelnuovo, G., Perez-Diaz-del-Campo, N., Rosso, C., Armandi, A., Caviglia, G. P., & Bugianesi, E. (2024). A Healthful Plant-Based Diet as an Alternative Dietary Approach in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease. Nutrients, 16(13), 2027. https://doi.org/10.3390/nu16132027







