Effects of Bifidobacterium longum 35624 in Children and Adolescents with Irritable Bowel Syndrome
Highlights
- Probiotic supplementation with Bifidobacterium longum 35624 reduced IBS symptoms and impact on daily life, indicating positive effects on quality of life.
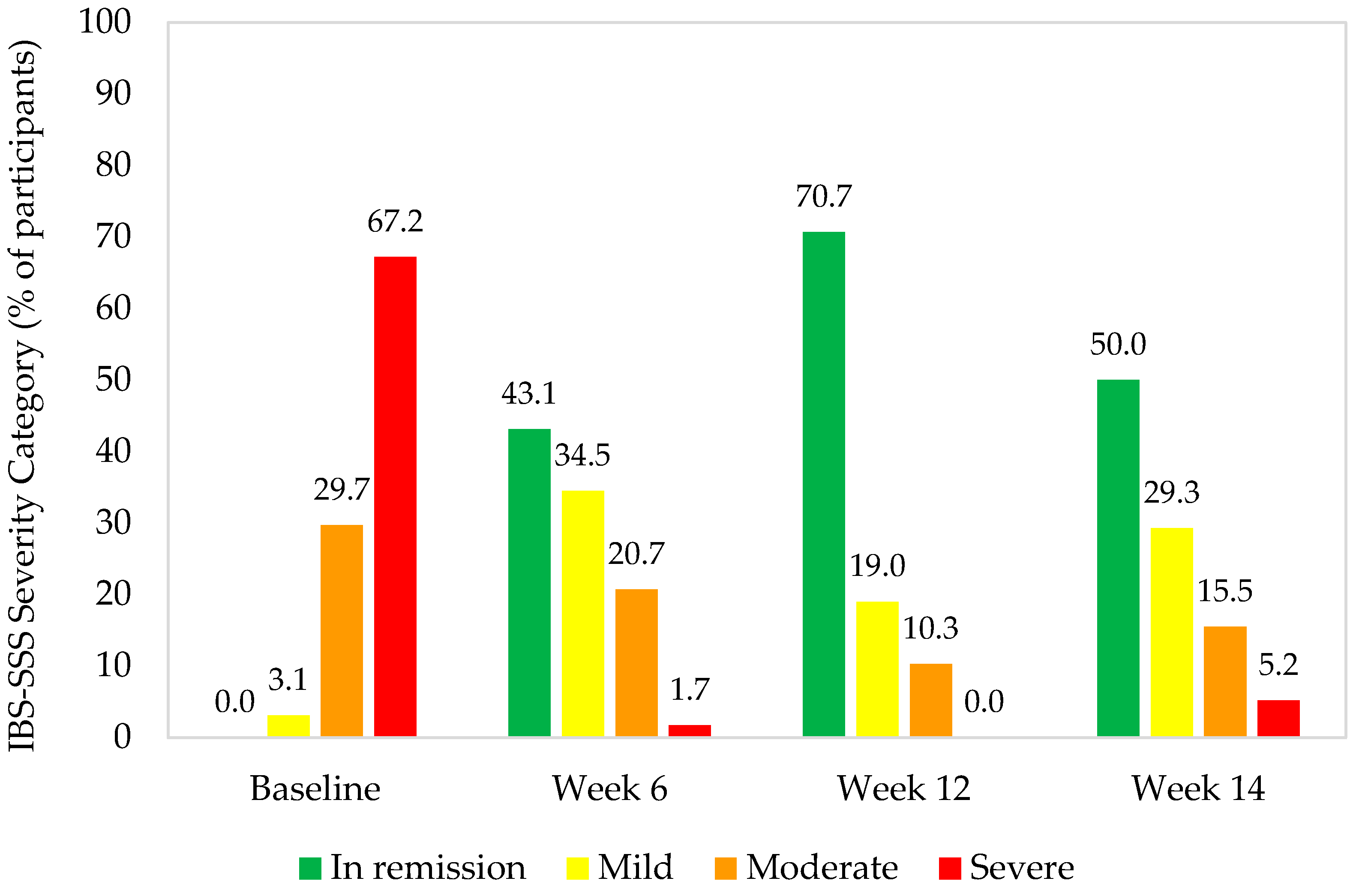
- There was a high response rate of participants to probiotic supplementation, with >93% experiencing clinically significant improvements in IBS-SSS.
- The distribution of IBS severity categories changed during probiotic supplementation, with decreases in the proportion of participants with severe or moderate symptoms and increases in those with mild symptoms or in remission.
- Following cessation of probiotic supplementation, composite IBS-SSS scores significantly increased, suggesting that a longer intake period may be necessary to maintain beneficial results.
Abstract
1. Introduction
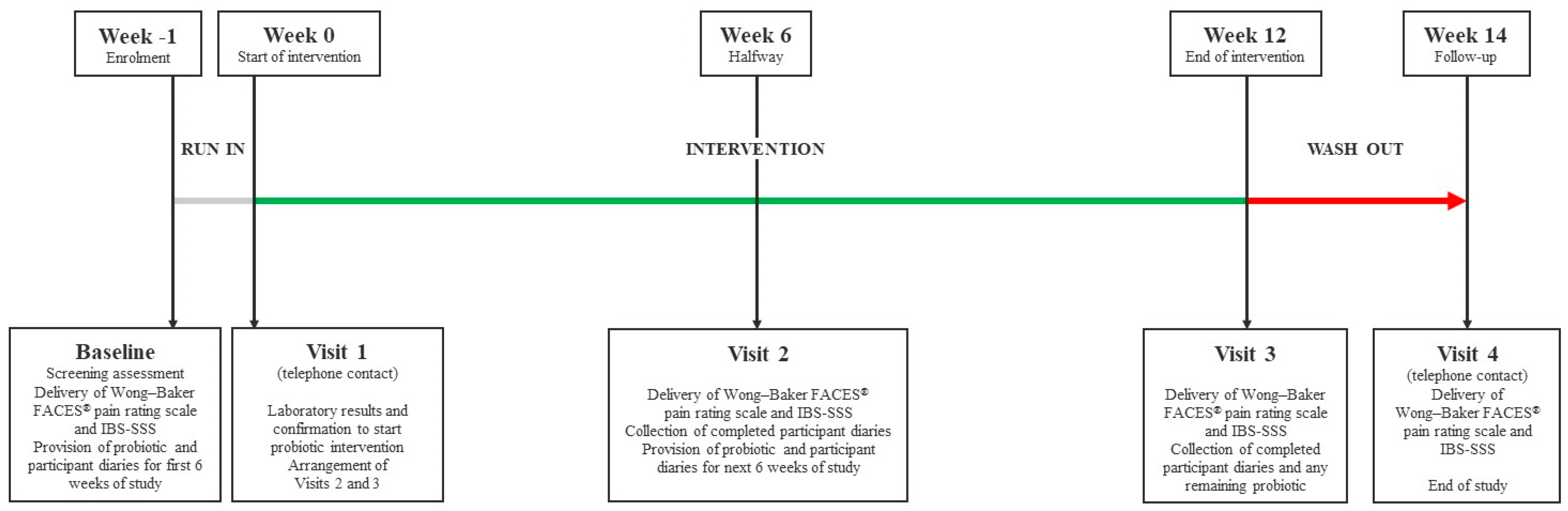
2. Materials and Methods
3. Results
3.1. Intention to Treat Analysis
3.1.1. Participant Characteristics
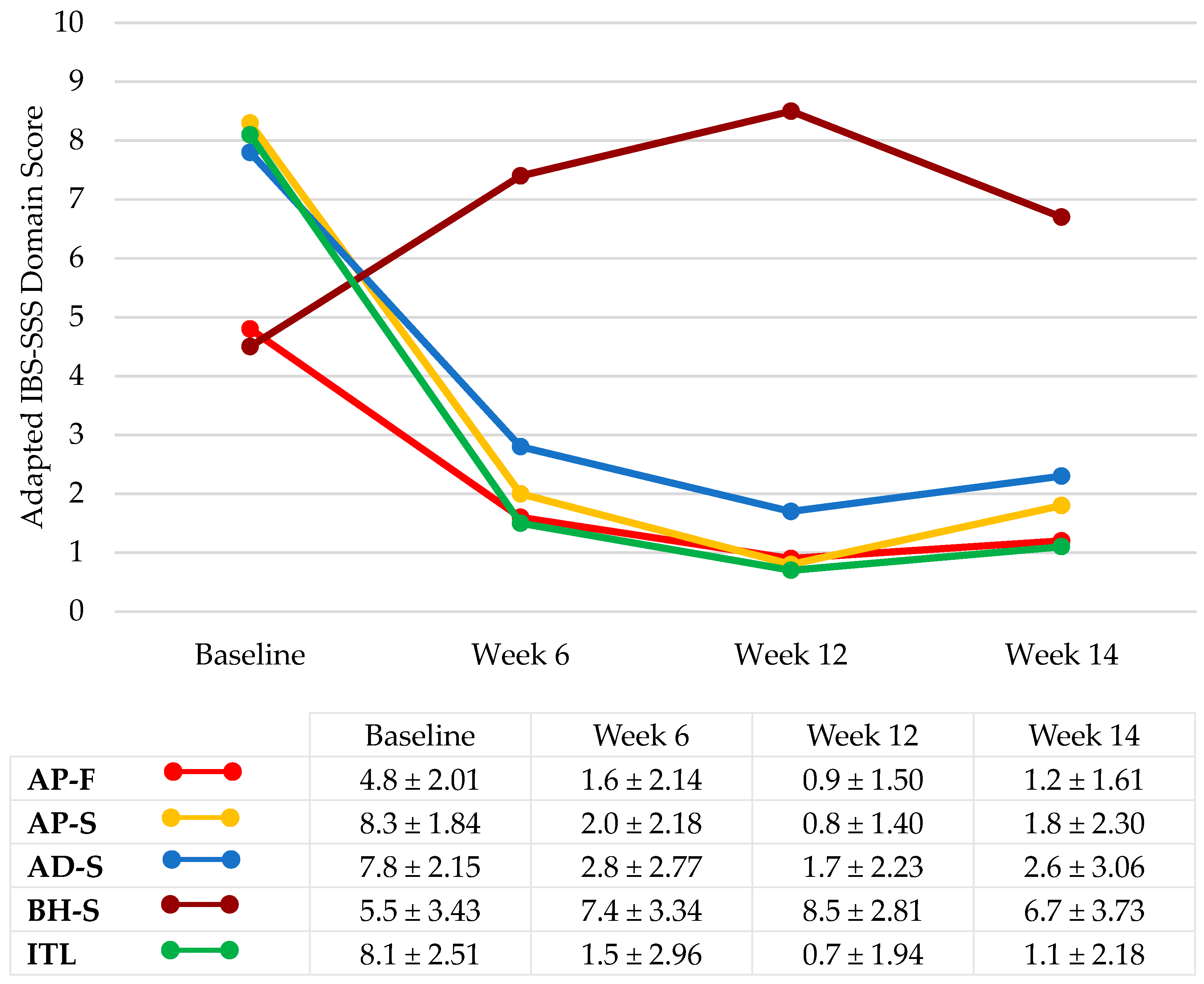
3.1.2. IBS Symptom Severity
3.1.3. Relationship between Baseline Vitamin D Status and IBS-SSS Treatment
3.1.4. Parent and Child Perspectives of IBS Symptoms
3.1.5. Stool Consistency
3.2. Per Protocol Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features, and Rome IV. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Hyams, J.S.; Di Lorenzo, C.; Saps, M.; Shulman, R.J.; Staiano, A.; van Tilburg, M. Functional Disorders: Children and Adolescents. Gastroenterology 2016, 150, 1456–1468. [Google Scholar] [CrossRef] [PubMed]
- Vernon-Roberts, A.; Alexander, I.; Day, A.S. Systematic Review of Pediatric Functional Gastrointestinal Disorders (Rome IV Criteria). J. Clin Med. 2021, 10, 5087. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Benítez, C.A.; Chanis, R.; Játiva, E.; Mejia, M.; Moreno, J.; Ramírez-Hernández, C.R.; Villamarín-Betancourt, E.A.; Gómez-Urrego, J.F. Coexistencia de trastornos gastrointestinales funcionales en lactantes y preescolares latinoamericanos. Rev. Colomb. Gastroenterol. 2019, 34, 370–375. [Google Scholar] [CrossRef]
- Schmulson, M.J.; Drossman, D.A. What Is New in Rome IV. J. Neurogastroenterol. Motil. 2017, 23, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Biglari, M.; Nasseri Moghaddam, S. Post-infectious Irritable Bowel Syndrome: A Narrative Review. Middle East J. Dig. Dis. 2019, 11, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Bin, Y.; Kang, L.; Lili, Y. Vitamin D status in irritable bowel syndrome and the impact of supplementation on symptoms: A systematic review and meta-analysis. Nutr. Hosp. 2022, 39, 1144–1152. [Google Scholar] [PubMed]
- Quigley, E.M.; Fried, M.; Gwee, K.A.; Khalif, I.; Hungin, A.P.; Lindberg, G.; Abbas, Z.; Fernandez, L.B.; Bhatia, S.J.; Schmulson, M.; et al. World Gastroenterology Organisation Global Guidelines Irritable Bowel Syndrome: A Global Perspective Update September 2015. J. Clin. Gastroenterol. 2016, 50, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Cruchet, S.; Furnes, R.; Maruy, A.; Hebel, E.; Palacios, J.; Medina, F.; Ramirez, N.; Orsi, M.; Rondon, L.; Sdepanian, V.; et al. The use of probiotics in pediatric gastroenterology: A review of the literature and recommendations by Latin-American experts. Paediatr. Drugs 2015, 17, 199–216. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, L.; McCarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O’sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F.; et al. Lactobacillus and Bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005, 128, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Lyseng-Williamson, K. Bifidobacterium infantis 35624 as a probiotic dietary supplement: A profile of its use. Drugs Ther. Perspect. 2017, 33, 368–374. [Google Scholar] [CrossRef]
- Whorwell, P.J.; Altringer, L.; Morel, J.; Bond, Y.; Charbonneau, D.; O’Mahony, L.; Kiely, B.; Shanahan, F.; Quigley, E.M.M. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am. J. Gastroenterol. 2006, 101, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Hoy-Schulz, Y.E.; Jannat, K.; Roberts, T.; Zaidi, S.H.; Unicomb, L.; Luby, S.; Parsonnet, J. Safety and acceptability of Lactobacillus reuteri DSM 17938 and Bifidobacterium longum subspecies infantis 35624 in Bangladeshi infants: A phase I randomized clinical trial. BMC Complement. Altern. Med. 2016, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Almansa, C.; García-Sánchez, R.; Barceló, M.; Díaz-Rubio, M.; Rey, E. Translation, cultural adaptation and validation of a Spanish version of the Irritable Bowel Syndrome Severity Score. Rev. Esp. Enferm. Dig. 2011, 103, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.M.; Wong, D.L. QUEST: A process of pain assessment in children (continuing education credit). Orthop. Nurs. 1987, 6, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Díaz, M.A.; Suárez Parga, J.M.; Pardo Merino, A.; García Vargas, M.; Pascual Renedo, V. Adaptación cultural al español y validación de la escala GSFQ (Gastrointestinal Short Form Questionnaire). Gastroenterol. Y Hepatol. 2009, 32, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Mínguez Pérez, M.; Benages Martínez, A. The Bristol scale-A useful system to assess stool form? Rev. Esp. Enferm. Dig. 2009, 101, 305–311. [Google Scholar] [PubMed]
- Vázquez-Frias, R.; Consuelo-Sánchez, A.; Acosta-Rodríguez-Bueno, C.P.; Blanco-Montero, A.; Robles, D.C.; Cohen, V.; Márquez, D.; Perez, M. Efficacy and Safety of the Adjuvant Use of Probiotic Bacillus clausii Strains in Pediatric Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Study. Paediatr. Drugs. 2023, 25, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Barraza-Ortiz, D.A.; Pérez-López, N.; Medina-López, V.M.; Minero-Alfaro, J.I.; Zamarripa-Dorsey, F.; Fernández-Martínez, N.d.C.; Llorente-Ramón, A.; Ramos-Aguilar, G.A. Combination of a Probiotic and an Antispasmodic Increases Quality of Life and Reduces Symptoms in Patients with Irritable Bowel Syndrome: A Pilot Study. Dig. Dis. 2021, 39, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Rajindrajith, S.; Devanarayana, N.M.; Crispus Perera, B.J.; Benninga, M.A. Childhood constipation as an emerging public health problem. World J. Gastroenterol. 2016, 22, 6864–6875. [Google Scholar] [CrossRef]
- Guiraldes, E. Pediatric Gastroenterology in Chile—A Personal Perspective. J. Pediatr. Gastroenterol. Nutr. 2001, 33, 231–232. [Google Scholar] [PubMed]
- Kaplan, A.I.; Mazor, Y.; Prott, G.M.; Sequeira, C.; Jones, M.P.; Malcolm, A. Experiencing multiple concurrent functional gastrointestinal disorders is associated with greater symptom severity and worse quality of life in chronic constipation and defecation disorders. Neurogastroenterol. Motil. 2023, 35, e14524. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, J.M.; Iglicki, F. Effect of Bifidobacterium longum 35624 on disease severity and quality of life in patients with irritable bowel syndrome. World J. Gastroenterol. 2022, 28, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Hulme, K.; Chilcot, J.; Smith, M.A. Doctor-patient relationship and quality of life in Irritable Bowel Syndrome: An exploratory study of the potential mediating role of illness perceptions and acceptance. Psychol. Health Med. 2018, 23, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Varni, J.W.; Lane, M.M.; Burwinkle, T.M.; Fontaine, E.N.; Youssef, N.N.; Schwimmer, J.B.; Pardee, P.E.; Pohl, J.F.; Easley, D.J. Health-related quality of life in pediatric patients with irritable bowel syndrome: A comparative analysis. J. Dev. Behav. Pediatr. 2006, 27, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Cassar, G.E.; Youssef, G.J.; Knowles, S.; Moulding, R.; Austin, D.W. Health-Related Quality of Life in Irritable Bowel Syndrome: A Systematic Review and Meta-analysis. Gastroenterol. Nurs. 2020, 43, E102–E122. [Google Scholar] [CrossRef] [PubMed]
- Solovyeva, O.; Nekrasova, A.; Topalova, I.; Ponomarenko, V.A.; Tsurtsumiia, D.B.; Ilyashevich, I.G. Long-term probiotic administration for irritable bowel syndrome: A legal need. Ter. Arkhiv. 2023, 95, 7. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bravo, F.; Duarte, L.; Arredondo-Olguín, M.; Iñiguez, G.; Castillo-Valenzuela, O. Vitamin D status and obesity in children from Chile. Eur. J. Clin. Nutr. 2022, 76, 899–901. [Google Scholar] [CrossRef]
- Giron, F.; Quigley, E.M.M. Pharmabiotic Manipulation of the Microbiota in Gastrointestinal Disorders: A Clinical Perspective. J. Neurogastroenterol. Motil. 2018, 24, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Groeger, D.; O’Mahony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.G.; Kiely, B.; Shanahan, F.; Quigley, E.M. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 2013, 4, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Weerts, Z.; Vork, L.; Mujagic, Z.; Keszthelyi, D.; Hesselink, M.A.M.; Kruimel, J.; Leue, C.; Muris, J.W.; Jonkers, D.M.A.E.; Masclee, A.A.M. Reduction in IBS symptom severity is not paralleled by improvement in quality of life in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2019, 31, e13629. [Google Scholar] [CrossRef] [PubMed]
- Martoni, C.J.; Srivastava, S.; Leyer, G.J. Lactobacillus acidophilus DDS-1 and Bifidobacterium lactis UABla-12 Improve Abdominal Pain Severity and Symptomology in Irritable Bowel Syndrome: Randomized Controlled Trial. Nutrients 2020, 12, 363. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | n = 64 |
|---|---|
| Demographics | |
| Female, n (%) | 42 (65.6) |
| Age (years), mean ± SD | 12.3 ± 2.9 |
| Resident of Santiago, n (%) | 47 (73.4) |
| Resident of Coyhaique, n (%) | 17 (26.6) |
| Anthropometrics, mean ± SD | |
| Weight (kg) | 45.7 ± 15.3 |
| Height (cm) | 148.1 ± 15.1 |
| BMI (kg/m2) | 20.3 ± 4.0 |
| Biochemistry, mean ± SD | |
| Serum vitamin D *, ng/mL (sufficiency ≥ 30 ng/mL) | 19.7 ± 6.50 |
| Concurrent pediatric DGBI (Rome IV criteria [5]), n (%) | |
| H2. Functional abdominal pain disorders | 64 (100.0) |
| H2a. Functional dyspepsia | 45 (70.3) |
| H2b. Irritable bowel syndrome (IBS) | 64 (100.0) |
| H2c. Abdominal migraine | 0 (0.0) |
| H2d. Functional abdominal pain not otherwise specified | 60 (93.8) |
| H3. Functional defecation disorders | 60 (93.8) |
| H3a. Functional constipation | 43 (67.2) |
| H3b. Non-retentive fecal incontinence | 9 (14.1) |
| Functional defecation disorder not otherwise specified | 8 (12.5) |
| IBS severity | |
| Participants in IBS remission (IBS-SSS < 75), n (%) | 0 (0.0) |
| Participants with mild IBS (IBS-SSS 75–175), n (%) | 2 (3.1) |
| Participants with moderate IBS (IBS-SSS 176–300), n (%) | 19 (29.7) |
| Participants with severe IBS (IBS-SSS > 300), n (%) | 43 (67.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruchet Muñoz, S.; Verbeke Palma, S.; Lera Marqués, L.; Espinosa Pizarro, M.N.; Malig Mechasqui, J.; Sorensen, K. Effects of Bifidobacterium longum 35624 in Children and Adolescents with Irritable Bowel Syndrome. Nutrients 2024, 16, 1967. https://doi.org/10.3390/nu16121967
Cruchet Muñoz S, Verbeke Palma S, Lera Marqués L, Espinosa Pizarro MN, Malig Mechasqui J, Sorensen K. Effects of Bifidobacterium longum 35624 in Children and Adolescents with Irritable Bowel Syndrome. Nutrients. 2024; 16(12):1967. https://doi.org/10.3390/nu16121967
Chicago/Turabian StyleCruchet Muñoz, Sylvia, Sandra Verbeke Palma, Lydia Lera Marqués, María Nelly Espinosa Pizarro, Jacqueline Malig Mechasqui, and Katy Sorensen. 2024. "Effects of Bifidobacterium longum 35624 in Children and Adolescents with Irritable Bowel Syndrome" Nutrients 16, no. 12: 1967. https://doi.org/10.3390/nu16121967
APA StyleCruchet Muñoz, S., Verbeke Palma, S., Lera Marqués, L., Espinosa Pizarro, M. N., Malig Mechasqui, J., & Sorensen, K. (2024). Effects of Bifidobacterium longum 35624 in Children and Adolescents with Irritable Bowel Syndrome. Nutrients, 16(12), 1967. https://doi.org/10.3390/nu16121967






