Exploring the Impact of French Raw-Milk Cheeses on Oxidative Process Using Caenorhabditis elegans and Human Leukocyte Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Cheese Selection
2.2. Reagents and Solvents
2.3. Obtention of Cheese Fractions
2.4. In Vivo Study on Caenorhabditis elegans
2.4.1. Growth of Escherichia coli and Heat-Killed Preparation
2.4.2. Caenorhabditis elegans Maintenance
2.4.3. Synchronization of Caenorhabditis elegans
2.4.4. Caenorhabditis elegans Culture Conditions for Assays
2.4.5. Survival Rate of Caenorhabditis elegans on Oxidative Medium
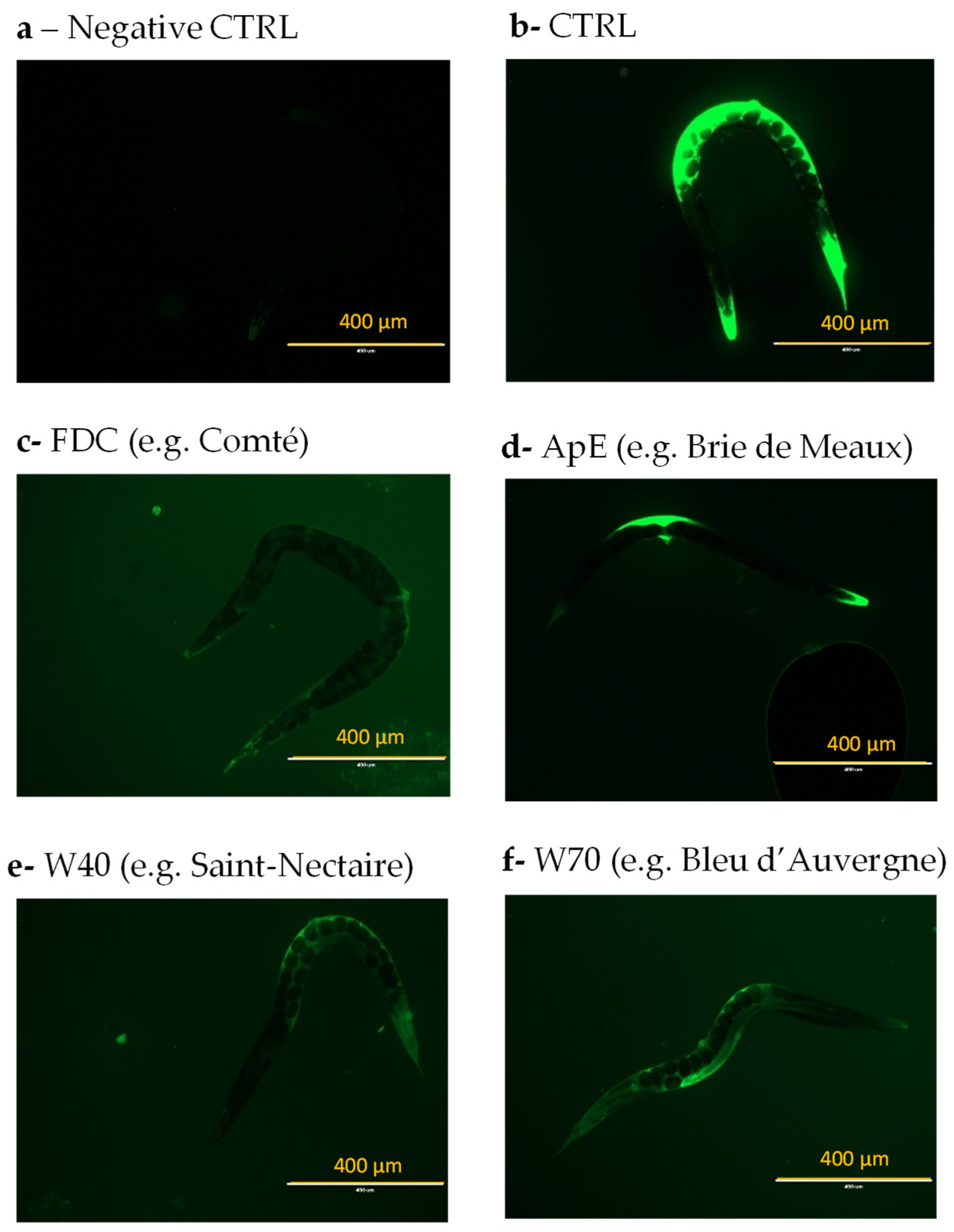
2.4.6. Quantification of Intracellular ROS in Caenorhabditis elegans under Oxidative Conditions
2.4.7. Quantitative Analyses of the daf-16, skn-1, ctl-2, and sod-3 Gene Expression in Caenorhabditis elegans
2.5. In Vitro Analyses of Human Leukocytes
2.5.1. Leukocyte Obtention
2.5.2. Kinetics of ROS Production by Leukocytes
2.5.3. Leukocyte Viability MTT Assay
2.6. Statistical Analysis
3. Results
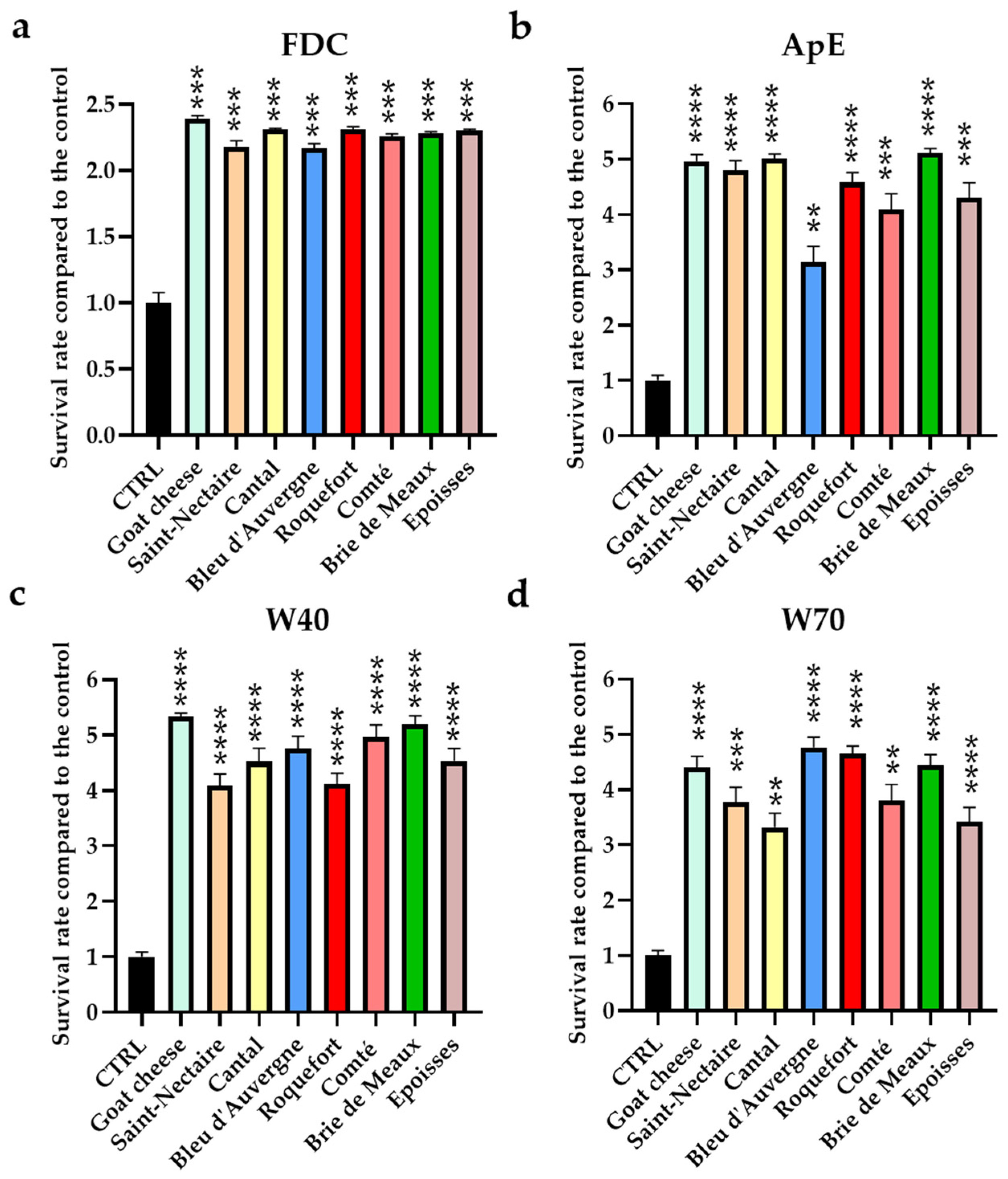
3.1. Assessment of the Protective Effect of Cheese Fractions in C. elegans under Oxidative Conditions
3.2. Effect of Cheeses on ROS Accumulation in C. elegans on Oxidative Condition
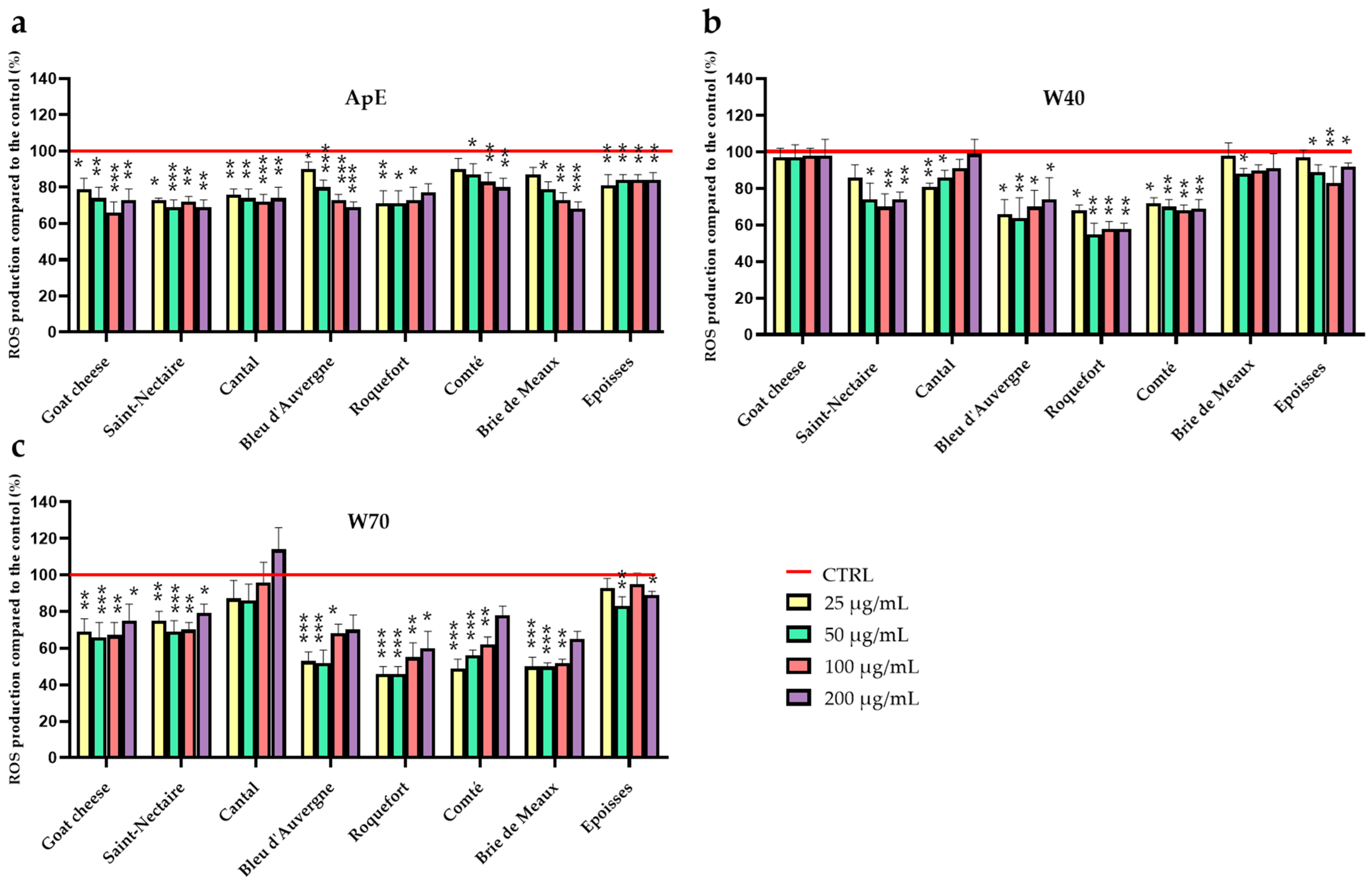
3.3. Impact of Cheese Extracts on ROS Production in Human Leukocytes
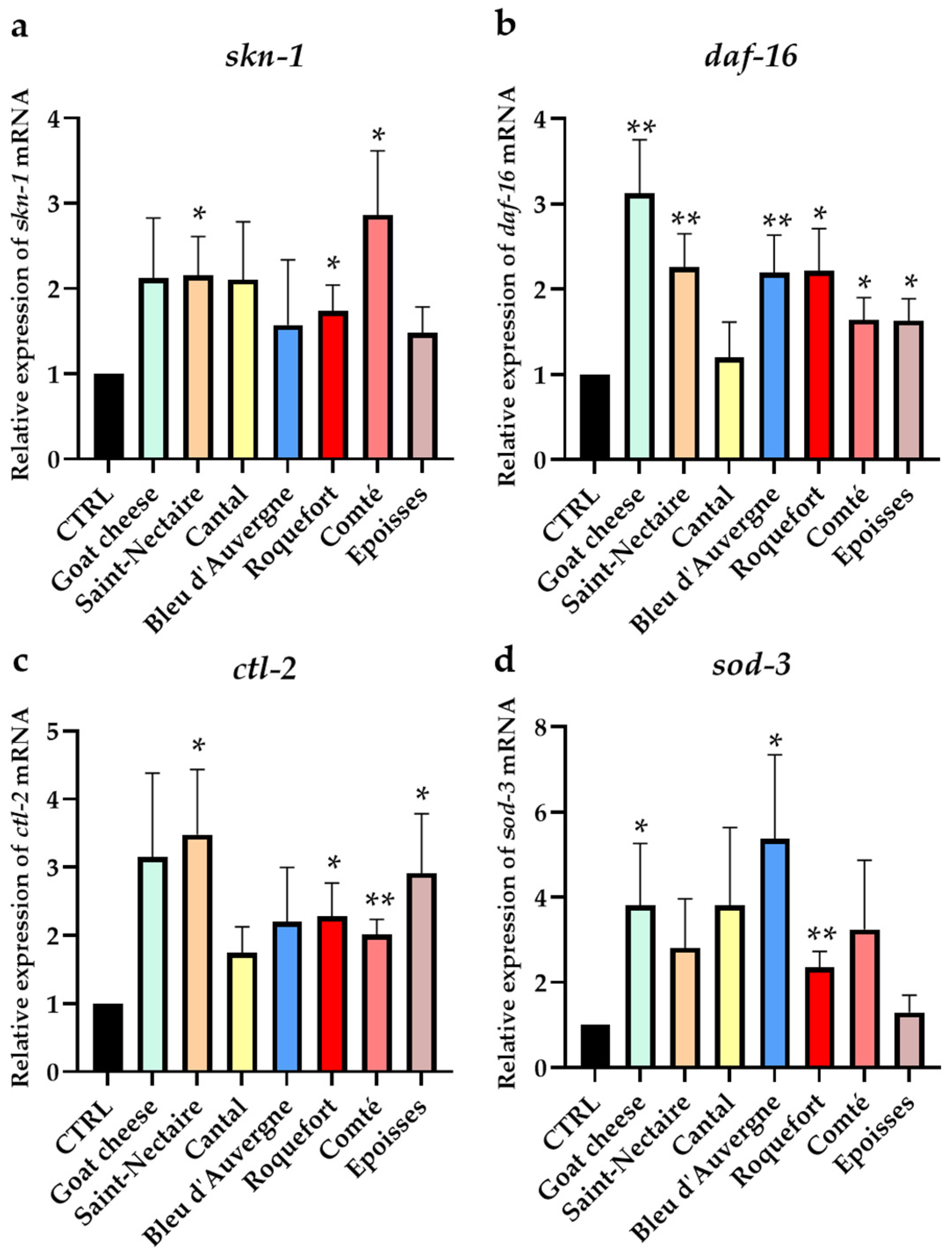
3.4. Effect of Cheese Supplementation on ROS Elimination in C. elegans through Antioxidant Pathway Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Lodovici, M.; Bigagli, E. Oxidative Stress and Air Pollution Exposure. J. Toxicol. 2011, 2011, 487074. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial Dysfunction and Oxidative Stress in Aging and Cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, Oxidative Stress and Autophagy in Skin Aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/Oxidative Stress Signaling in Osteoarthritis. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging; Springer: Berlin/Heidelberg, Germany, 2023; Volume 97, ISBN 0123456789. [Google Scholar]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health Benefits of Fermented Foods. Crit. Rev. Food Sci. Nutr. 2017, 59, 506–527. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health Benefits of Fermented Foods: Microbiota and Beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of Plant-Based Milk Alternatives for Improved Flavour and Nutritional Value. Appl. Microbiol. Biotechnol. 2019, 103, 9263–9275. [Google Scholar] [CrossRef] [PubMed]
- Pua, A.; Tang, V.C.Y.; Goh, R.M.V.; Sun, J.; Lassabliere, B.; Liu, S.Q. Ingredients, Processing, and Fermentation: Addressing the Organoleptic Boundaries of Plant-Based Dairy Analogues. Foods 2022, 11, 875. [Google Scholar] [CrossRef] [PubMed]
- Voidarou, C.; Antoniadou, M.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Lagiou, A.; Bezirtzoglou, E. Fermentative Foods: Microbiology, Biochemistry, Potential Human Health Benefits and Public Health Issues. Foods 2021, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Storm, E. Fermented Foods in Medicine: An Evaluation of Their Uses and Effects on the Human Gut Microbiota. Life Excit. Biol. 2020, 8, 34–53. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.; El-Maksoud, A.A.A.; Ambrosio, G.M.; de Gouveia, F.G. Recent Development in Antioxidant of Milk and Its Products. In Recent Developments in Antioxidants from Natural Sources; IntechOpen: London, UK, 2023. [Google Scholar]
- Park, Y.W.; Nam, M.S. Bioactive Peptides in Milk and Dairy Products: A Review. Korean J. Food Sci. Anim. Resour. 2015, 35, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant Properties of Milk and Dairy Products: A Comprehensive Review of the Current Knowledge. Lipids Health Dis. 2019, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, S.; Body, J.J.; Bruyère, O.; Bergmann, P.; Brandi, M.L.; Cooper, C.; Devogelaer, J.P.; Gielen, E.; Goemaere, S.; Kaufman, J.M.; et al. Effects of Dairy Products Consumption on Health: Benefits and Beliefs—A Commentary from the Belgian Bone Club and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases. Calcif. Tissue Int. 2016, 98, 1–17. [Google Scholar] [CrossRef]
- Tunick, M.H.; Van Hekken, D.L. Dairy Products and Health: Recent Insights. J. Agric. Food Chem. 2015, 63, 9381–9388. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mann, B.; Kumar, R.; Sangwan, R.B. Antioxidant Activity of Cheddar Cheeses at Different Stages of Ripening. Int. J. Dairy Technol. 2009, 62, 339–347. [Google Scholar] [CrossRef]
- Almena-Aliste, M.; Mietton, B. Cheese Classification, Characterization, and Categorization: A Global Perspective. Microbiol. Spectr. 2014, 2, CM-0003-2012. [Google Scholar] [CrossRef]
- Nam, J.H.; Cho, Y.S.; Rackerby, B.; Goddik, L.; Park, S.H. Shifts of Microbiota during Cheese Production: Impact on Production and Quality. Appl. Microbiol. Biotechnol. 2021, 105, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.V.; Sharma, V.; Bector, B.S. Effect of Ripening on Total Conjugated Linoleic Acid and Its Isomers in Buffalo Cheddar Cheese. Int. J. Dairy Technol. 2006, 59, 257–260. [Google Scholar] [CrossRef]
- Díaz-Castro, J.; Pérez-Sánchez, L.J.; Ramírez López-Frías, M.; López-Aliaga, I.; Nestares, T.; Alférez, M.J.M.; Ojeda, M.L.; Campos, M.S. Influence of Cow or Goat Milk Consumption on Antioxidant Defence and Lipid Peroxidation during Chronic Iron Repletion. Br. J. Nutr. 2012, 108, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Santiago-López, L.; Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Liceaga, A.M.; González-Córdova, A.F. Invited Review: Bioactive Compounds Produced during Cheese Ripening and Health Effects Associated with Aged Cheese Consumption. J. Dairy Sci. 2018, 101, 3742–3757. [Google Scholar] [CrossRef] [PubMed]
- Barac, M.; Pesic, M.; Zilic, S.; Smiljanic, M.; Stanojevic, S.; Vasic, M.; Despotovic, S.; Vucic, T.; Kostic, A. Protein Profiles and Total Antioxidant Capacity of Water-Soluble and Water-Insoluble Fractions of White Brined Goat Cheese at Different Stages of Ripening. Int. J. Food Sci. Technol. 2016, 51, 1140–1149. [Google Scholar] [CrossRef]
- Bottesini, C.; Paolella, S.; Lambertini, F.; Galaverna, G.; Tedeschi, T.; Dossena, A.; Marchelli, R.; Sforza, S. Antioxidant Capacity of Water Soluble Extracts from Parmigiano-Reggiano Cheese. Int. J. Food Sci. Nutr. 2013, 64, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, S.R.; Phillips, M.; Kailasapathy, K. Identification of Bioactive Peptides in Commercial Cheddar Cheese. Food Res. Int. 2010, 43, 1545–1548. [Google Scholar] [CrossRef]
- Hernández-Galán, L.; Cardador-Martínez, A.; Picque, D.; Spinnler, H.E.; López-del-Castillo Lozano, M.; Martín del Campo, S.T. ACEI and Antioxidant Peptides Release during Ripening of Mexican Cotija Hard Cheese. J. Food Res. 2016, 5, 85. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Torres-Llanez, M.J.; Hernández-Mendoza, A.; Reyes-Díaz, R.; Vallejo-Cordoba, B.; González-Córdova, A.F. Antioxidant Capacity and Identification of Radical Scavenging Peptides from Crema de Chiapas, Fresco and Cocido Cheeses. J. Food Sci. Technol. 2022, 59, 2705–2713. [Google Scholar] [CrossRef]
- Cardin, G.; Poupet, C.; Bonnet, M.; Veisseire, P.; Ripoche, I.; Chalard, P.; Chauder, A.; Saunier, E.; Priam, J.; Rios, L. A Mechanistic Study of the Antiaging Effect of Raw-Milk Cheese Extracts. Nutrients 2021, 13, 897. [Google Scholar] [CrossRef]
- Zhu, A.; Zheng, F.; Zhang, W.; Li, L.; Li, Y.; Hu, H.; Wu, Y.; Bao, W.; Li, G.; Wang, Q.; et al. Oxidation and Antioxidation of Natural Products in the Model Organism Caenorhabditis elegans. Antioxidants 2022, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Cardin, G.; Ripoche, I.; Poupet, C.; Bonnet, M.; Veisseire, P.; Chalard, P.; Chauder, A.; Saunier, E.; Priam, J.; Bornes, S.; et al. Development of an Innovative Methodology Combining Chemical Fractionation and in vivo Analysis to Investigate the Biological Properties of Cheese. PLoS ONE 2020, 15, e0242370. [Google Scholar] [CrossRef] [PubMed]
- Poupet, C.; Saraoui, T.; Veisseire, P.; Bonnet, M.; Dausset, C.; Gachinat, M.; Camarès, O.; Chassard, C.; Nivoliez, A.; Bornes, S. Lactobacillus Rhamnosus Lcr35 as an Effective Treatment for Preventing Candida Albicans Infection in the Invertebrate Model Caenorhabditis elegans: First Mechanistic Insights. PLoS ONE 2019, 14, e0216184. [Google Scholar] [CrossRef] [PubMed]
- Veisseire, P.; Bonnet, M.; Saraoui, T.; Poupet, C.; Camarès, O.; Gachinat, M.; Callon, C.; Febvre, G.; Chassard, C.; Bornes, S. Investigation into in vitro and in vivo Caenorhabditis elegans Models to Select Cheese Yeasts as Probiotic Candidates for Their Preventive Effects against Salmonella Typhimurium. Microorganisms 2020, 8, 922. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The Genetics of Caenorhabditis elegans. Methods 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Porta-de-la-Riva, M.; Fontrodona, L.; Villanueva, A.; Cerón, J. Basic Caenorhabditis elegans Methods: Synchronization and Observation. J. Vis. Exp. 2012, 64, e4019. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, I.; Setoyama, O.; Urakawa, A.; Sugawara, M.; Jia, Y.; Komiya, Y.; Nagasao, J.; Arihara, K. Lysine-Glucose Maillard Reaction Products Promote Longevity and Stress Tolerance in Caenorhabditis elegans via the Insulin/IGF-1 Signaling Pathway. J. Funct. Foods 2021, 87, 104750. [Google Scholar] [CrossRef]
- Hoogewijs, D.; Houthoofd, K.; Matthijssens, F.; Vandesompele, J.; Vanfleteren, J.R. Selection and Validation of a Set of Reliable Reference Genes for Quantitative Sod Gene Expression Analysis in C. elegans. BMC Mol. Biol. 2008, 9, 9. [Google Scholar] [CrossRef]
- Cholet, J.; Decombat, C.; Vareille-Delarbre, M.; Gainche, M.; Berry, A.; Senejoux, F.; Ripoche, I.; Delort, L.; Vermerie, M.; Fraisse, D.; et al. In vitro Anti-Inflammatory and Immunomodulatory Activities of an Extract from the Roots of Bupleurum Rotundifolium. Medicines 2019, 6, 101. [Google Scholar] [CrossRef]
- Chervet, A.; Nehme, R.; Decombat, C.; Longechamp, L.; Habanjar, O.; Rousset, A.; Fraisse, D.; Blavignac, C.; Filaire, E.; Berthon, J.Y.; et al. Exploring the Therapeutic Potential of Ampelopsis Grossedentata Leaf Extract as an Anti-Inflammatory and Antioxidant Agent in Human Immune Cells. Int. J. Mol. Sci. 2024, 25, 416. [Google Scholar] [CrossRef]
- Shi, P.; Huang, Z.; Chen, G. Rhein Induces Apoptosis and Cell Cycle Arrest in Human Hepatocellular Carcinoma BEL-7402 Cells. Am. J. Chin. Med. 2008, 36, 805–813. [Google Scholar] [CrossRef]
- GraphPad Prism 10 Statistics Guide—How the Dunn Method for Nonparametric Comparisons Works. Available online: https://www.graphpad.com/guides/prism/latest/statistics/stat_how_the_dunn_method_for_nonpar.htm (accessed on 8 June 2024).
- Dinno, A. Nonparametric Pairwise Multiple Comparisons in Independent Groups Using Dunn’s Test. Stata J. 2015, 15, 292–300. [Google Scholar] [CrossRef]
- The C. elegans Sequencing Consortium. Genome Sequence of the Nematode C. elegans: A Platform for Investigating Biology. Science 1998, 282, 2012–2018. [Google Scholar]
- Lant, B.; Storey, K.B. An Overview of Stress Response and Hypometabolic Strategies in Caenorhabditis elegans: Conserved and Contrasting Signals with the Mammalian System. Int. J. Biol. Sci. 2010, 6, 9–50. [Google Scholar] [CrossRef]
- Grompone, G.; Martorell, P.; Llopis, S.; González, N.; Genovés, S.; Mulet, A.P.; Fernández-Calero, T.; Tiscornia, I.; Bollati-Fogolín, M.; Chambaud, I.; et al. Anti-Inflammatory Lactobacillus Rhamnosus CNCM I-3690 Strain Protects against Oxidative Stress and Increases Lifespan in Caenorhabditis elegans. PLoS ONE 2012, 7, e0052493. [Google Scholar] [CrossRef]
- Jin, X.; He, Y.; Liu, Z.; Zhou, Y.; Chen, X.; Wang, G.; Sun, Z.; Zhao, J.; Zhang, H.; Chen, W. Lactic Acid Bacteria Exhibit Similar Antioxidant Capacities in: Caenorhabditis elegans- and Campylobacter Jejuni-Infected Mice. RSC Adv. 2020, 10, 3329–3342. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zheng, B.; Li, T.; Liu, R.H. Raspberry Extract Ameliorates Oxidative Stress in Caenorhabditis elegans via the SKN-1/Nrf2 Pathway. J. Funct. Foods 2020, 70, 103977. [Google Scholar] [CrossRef]
- Miranda-Vizuete, A.; Veal, E.A. Caenorhabditis elegans as a Model for Understanding ROS Function in Physiology and Disease. Redox Biol. 2017, 11, 708–714. [Google Scholar] [CrossRef]
- El-Benna, J.; Hurtado-Nedelec, M.; Marzaioli, V.; Marie, J.C.; Gougerot-Pocidalo, M.A.; Dang, P.M.C. Priming of the Neutrophil Respiratory Burst: Role in Host Defense and Inflammation. Immunol. Rev. 2016, 273, 180–193. [Google Scholar] [CrossRef]
- Damascena, H.L.; Silveira, W.A.A.; Castro, M.S.; Fontes, W. Neutrophil Activated by the Famous and Potent PMA (Phorbol Myristate Acetate). Cells 2022, 11, 2889. [Google Scholar] [CrossRef]
- Karlsson, A.; Nixon, J.B.; McPhail, L.C. Phorbol Myristate Acetate Induces Neutrophil NADPH-Oxidase Activity by Two Separate Signal Transduction Pathways: Dependent or Independent of Phosphatidylinositol 3-Kinase. J. Leukoc. Biol. 2000, 67, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Tullet, J.M.A.; Green, J.W.; Au, C.; Benedetto, A.; Thompson, M.A.; Clark, E.; Gilliat, A.F.; Young, A.; Schmeisser, K.; Gems, D. The SKN-1/Nrf2 Transcription Factor Can Protect against Oxidative Stress and Increase Lifespan in C. elegans by Distinct Mechanisms. Aging Cell 2017, 16, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, E.; Zhang, Y.; Saremi, B.; Yadavali, S.; Hakimi, A.; Dehghani, M.; Goodarzi, M.; Tu, X.; Robertson, S.; Lin, R.; et al. Hydralazine Induces Stress Resistance and Extends C. elegans Lifespan by Activating the NRF2/SKN-1 Signalling Pathway. Nat. Commun. 2017, 8, 2223. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Aballay, A. Regulation of DAF-16-Mediated Innate Immunity in Caenorhabditis elegans. J. Biol. Chem. 2009, 284, 35580–35587. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Kennedy, S.; Tolonen, A.C.; Ruvkun, G. DAF-16 Target Genes That Control C. elegans Life-Span and Metabolism. Science 2003, 300, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.I.; Fraser, A.; Kamath, R.S.; Ahringer, J.; Li, H.; Kenyon, C. Genes That Act Downstream of DAF-16 to Influence the Lifespan of Caenorhabditis elegans. Nature 2003, 424, 277–284. [Google Scholar] [CrossRef]
- Henderson, S.T.; Johnson, T.E. Daf-16 Integrates Developmental and Environmental Inputs to Mediate Aging in the Nematode Caenorhabditis elegans. Curr. Biol. 2001, 11, 1975–1980. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zhang, C.; Chen, T.; Zhou, L.; Huang, Y.; Yuan, M.; Li, T.; Ding, C. Oleuropein Enhances Stress Resistance and Extends Lifespan via Insulin/Igf-1 and Skn-1/Nrf2 Signaling Pathway in Caenorhabditis elegans. Antioxidants 2021, 10, 1697. [Google Scholar] [CrossRef] [PubMed]
- Lallès, J.P. Dairy Products and the French Paradox: Could Alkaline Phosphatases Play a Role? Med. Hypotheses 2016, 92, 7–11. [Google Scholar] [CrossRef]
- Petyaev, I.M.; Bashmakov, Y.K. Could Cheese Be the Missing Piece in the French Paradox Puzzle? Med. Hypotheses 2012, 79, 746–749. [Google Scholar] [CrossRef]
- Kawakami, H. The French Paradox: Was It Attributed to Cheese Consumption? 2023. Available online: https://www.oatext.com/pdf/DU-9-161.pdf (accessed on 7 June 2024).
- Biong, A.S.; Müller, H.; Seljeflot, I.; Veierød, M.B.; Pedersen, J.I. A Comparison of the Effects of Cheese and Butter on Serum Lipids, Haemostatic Variables and Homocysteine. Br. J. Nutr. 2004, 92, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Mennen, L.; Balkau, B. Tissue-Type Plasminogen Activator Antigen and Consumption of Dairy Products. The D.E.S.I.R. Study. Data from an Epidemiological Study on Insulin Resistance Syndrome. Thromb. Res. 1999, 94, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Abadía-García, L.; Cardador, A.; Martín del Campo, S.T.; Arvízu, S.M.; Castaño-Tostado, E.; Regalado-González, C.; García-Almendarez, B.; Amaya-Llano, S.L. Influence of Probiotic Strains Added to Cottage Cheese on Generation of Potentially Antioxidant Peptides, Anti-Listerial Activity, and Survival of Probiotic Microorganisms in Simulated Gastrointestinal Conditions. Int. Dairy J. 2013, 33, 191–197. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Jimenez, M.T.B.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef]
- Li, J.; Shen, X. Oxidative Stress and Adipokine Levels Were Significantly Correlated in Diabetic Patients with Hyperglycemic Crises. Diabetol. Metab. Syndr. 2019, 11, 13. [Google Scholar] [CrossRef]


| Name of the Cheese | Cheese-Making Process | Milk Origin | Paste | Rind | Ripening |
|---|---|---|---|---|---|
| Goat cheese | fresh cheese | goat | - | - | 3 weeks |
| Saint-Nectaire | uncooked pressed cheeses | cow | soft cheese | surface molds | 6 weeks |
| Cantal | uncooked cheeses | cow | hard cheese | washed rind | 12 to 17 weeks (“entre-deux”) |
| Bleu d’Auvergne | uncooked unpressed cheeses | cow | internal molds soft cheese | - | 8 weeks |
| Roquefort | uncooked unpressed cheeses | ewe | internal molds soft cheese | - | 8 weeks |
| Comté | cooked cheeses | cow | hard cheese | smear rind | 17 weeks |
| Brie de Meaux | uncooked unpressed cheeses | cow | soft cheese | bloomy rind | 5 to 6 weeks (“three-quarters ripened”) |
| Epoisses | uncooked unpressed cheeses | cow | soft cheese | washed rind | 4 weeks |
| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Y45F10D.4 | CGAGAACCCGCGAAATGTCGGA | CGGTTGCCAGGGAAGATGAGGC |
| skn-1 | GTTCAATCAACAACAGGTGGATCA | TGGATGTTGGGAACACTCTGTC |
| daf-16 | TTCAATGCAAGGAGCATTTG | AGCTGGAGAAACACGAGACG |
| ctl-2 | TCCCAGATGGGTACCGTCAT | TCACTCCTTGAGTTGGCTTGA |
| sod-3 | CAATTGCTCTCCAACCAGCG | ACCGAAGTCGCGCTTAATAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diet, A.; Poix, C.; Bonnet, M.; Coelho, C.; Ripoche, I.; Decombat, C.; Priam, J.; Saunier, E.; Chalard, P.; Bornes, S.; et al. Exploring the Impact of French Raw-Milk Cheeses on Oxidative Process Using Caenorhabditis elegans and Human Leukocyte Models. Nutrients 2024, 16, 1862. https://doi.org/10.3390/nu16121862
Diet A, Poix C, Bonnet M, Coelho C, Ripoche I, Decombat C, Priam J, Saunier E, Chalard P, Bornes S, et al. Exploring the Impact of French Raw-Milk Cheeses on Oxidative Process Using Caenorhabditis elegans and Human Leukocyte Models. Nutrients. 2024; 16(12):1862. https://doi.org/10.3390/nu16121862
Chicago/Turabian StyleDiet, Anna, Christophe Poix, Muriel Bonnet, Christian Coelho, Isabelle Ripoche, Caroline Decombat, Julien Priam, Etienne Saunier, Pierre Chalard, Stéphanie Bornes, and et al. 2024. "Exploring the Impact of French Raw-Milk Cheeses on Oxidative Process Using Caenorhabditis elegans and Human Leukocyte Models" Nutrients 16, no. 12: 1862. https://doi.org/10.3390/nu16121862
APA StyleDiet, A., Poix, C., Bonnet, M., Coelho, C., Ripoche, I., Decombat, C., Priam, J., Saunier, E., Chalard, P., Bornes, S., Caldefie-Chezet, F., & Rios, L. (2024). Exploring the Impact of French Raw-Milk Cheeses on Oxidative Process Using Caenorhabditis elegans and Human Leukocyte Models. Nutrients, 16(12), 1862. https://doi.org/10.3390/nu16121862







