A Diet Lacking Selenium, but Not Zinc, Copper or Manganese, Induces Anticancer Activity in Mice with Metastatic Cancers
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Drugs
2.2. Animals
2.3. In Vivo Cancer Models
2.4. Diet Preparation and Composition
2.5. Statistical Analysis
3. Results
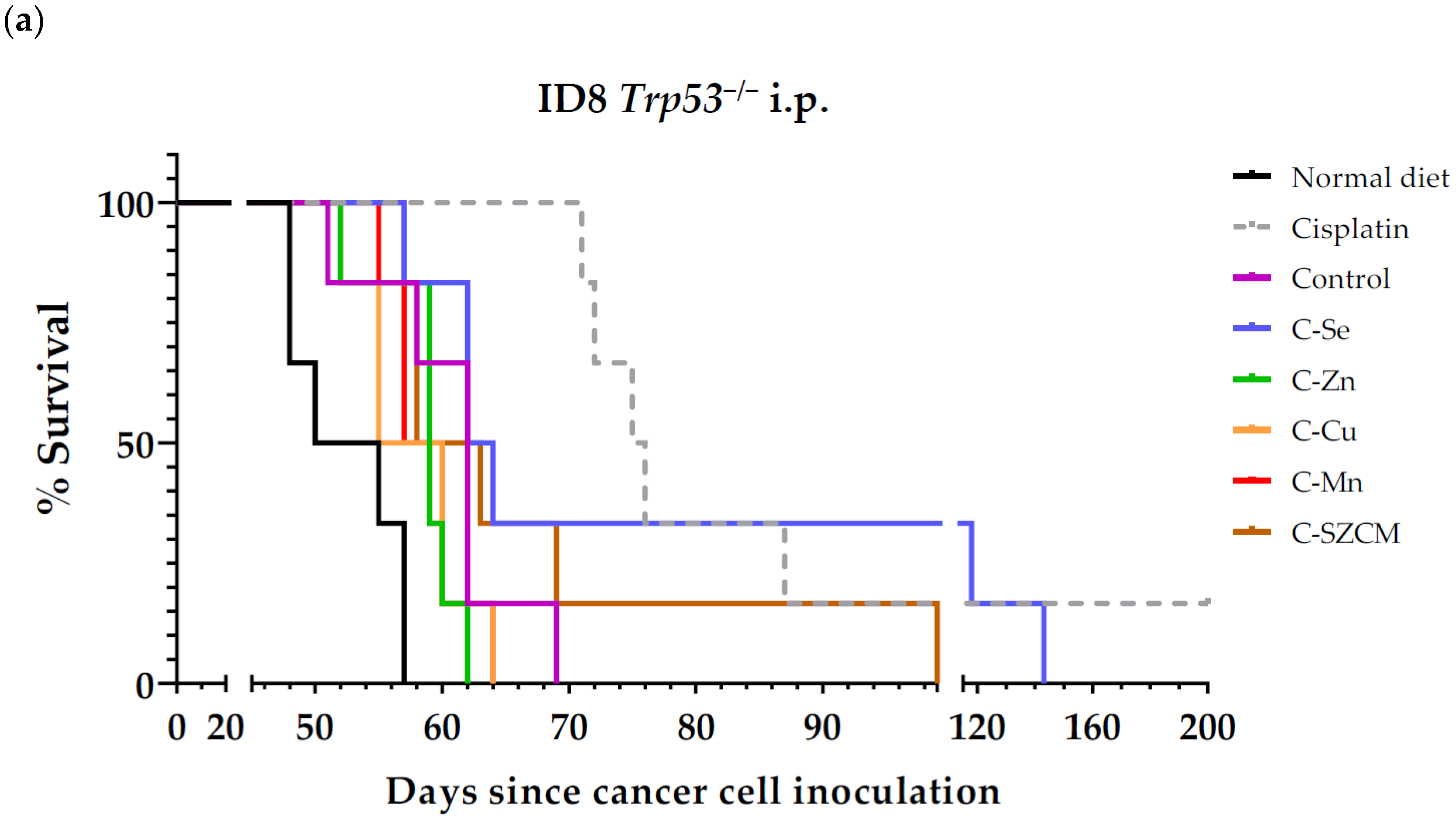
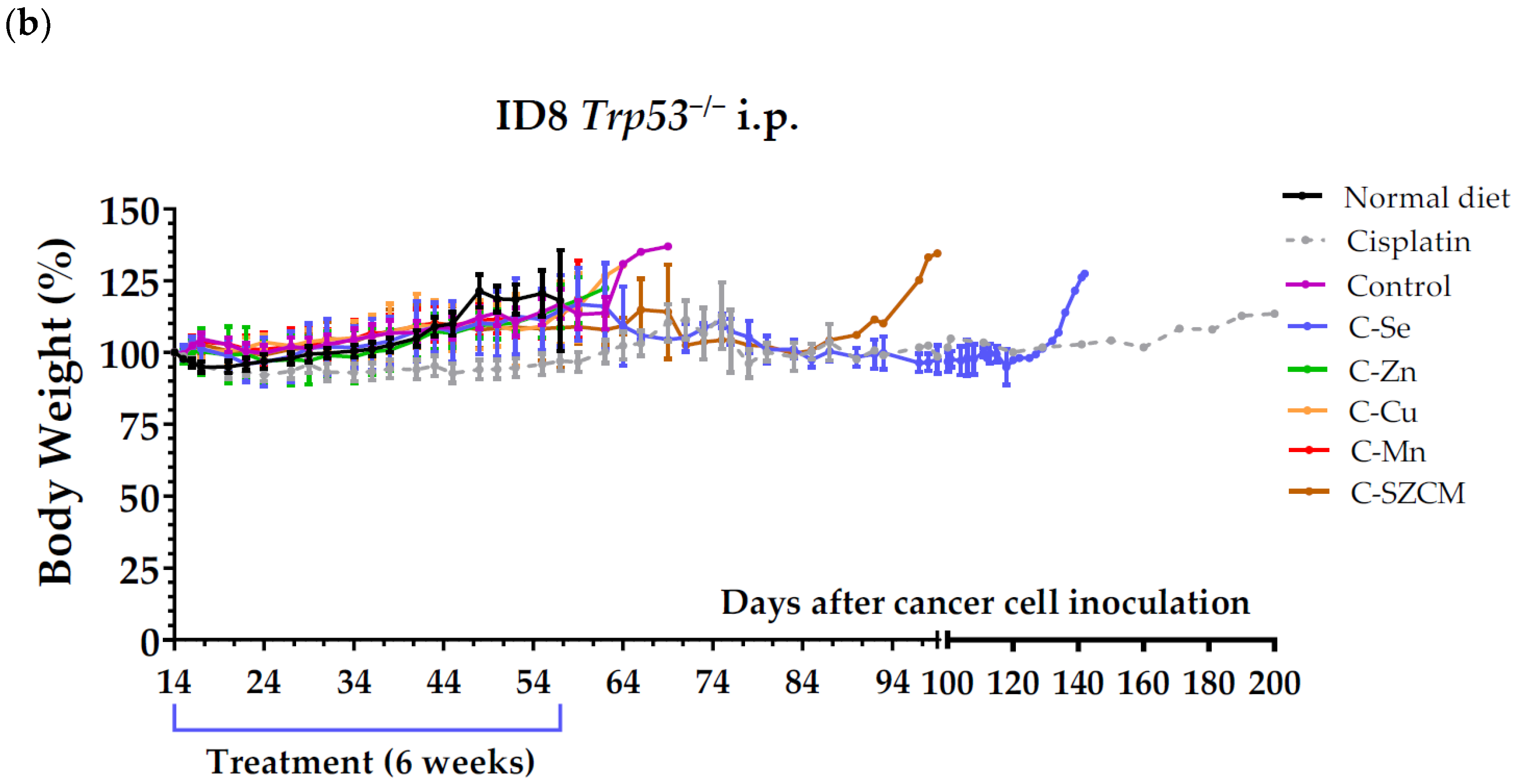
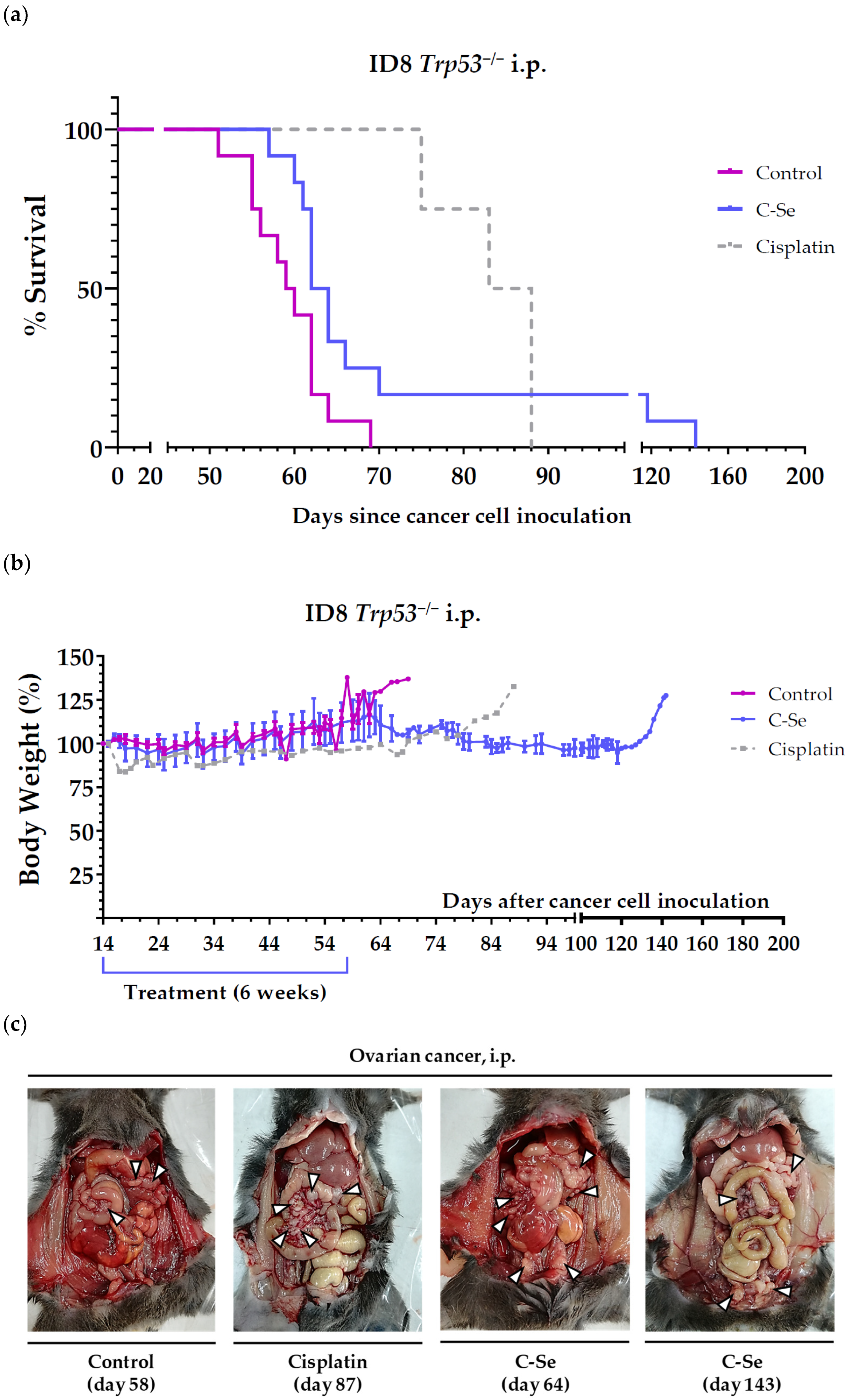
3.1. A Diet Lacking Selenium, but Not Zinc, Copper, or Manganese, Induces Anticancer Activity in Mice with Metastatic Ovarian Cancer
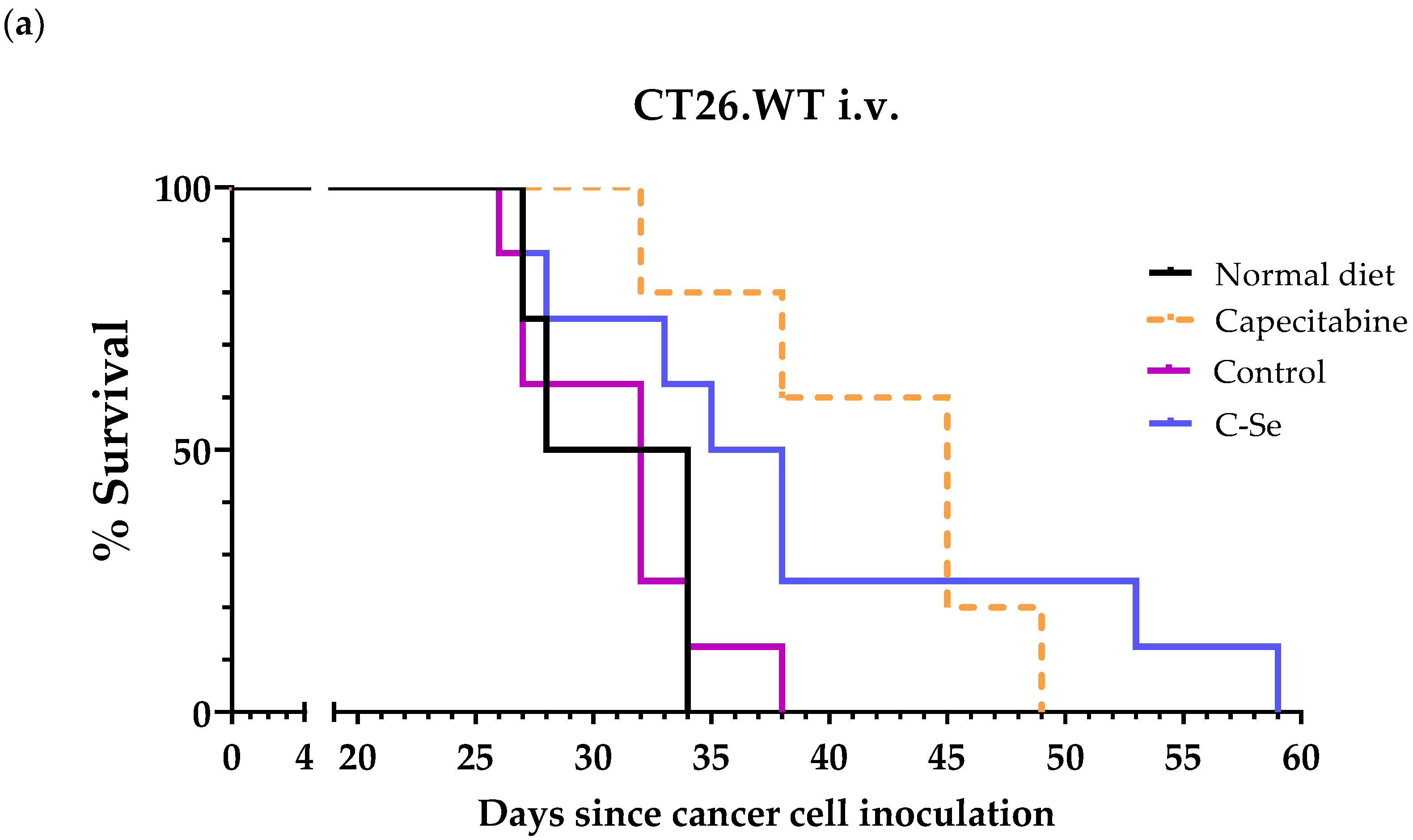
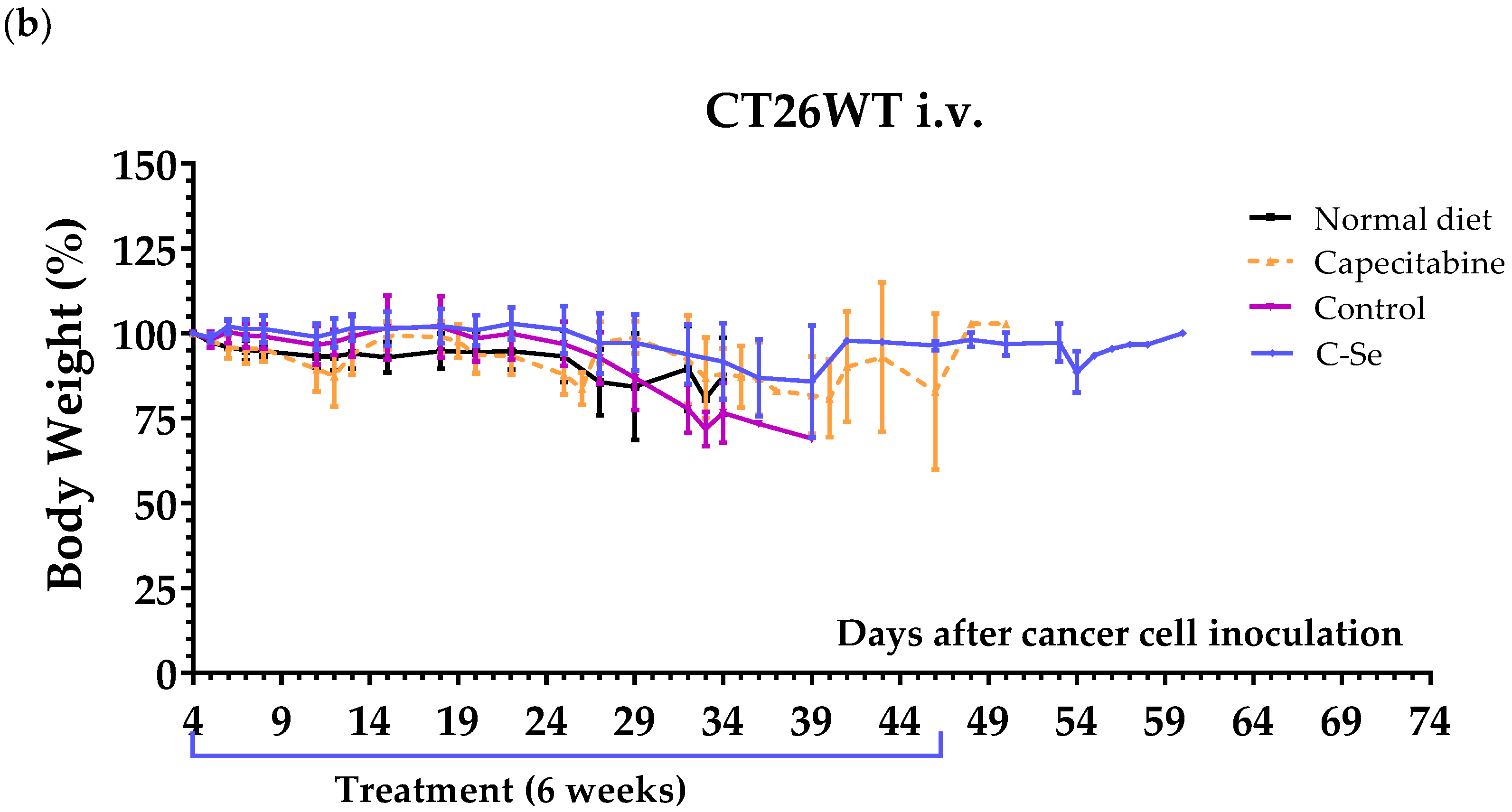
3.2. A Diet Lacking Selenium Induces Anticancer Activity in Mice with Metastasic Colon Cancer
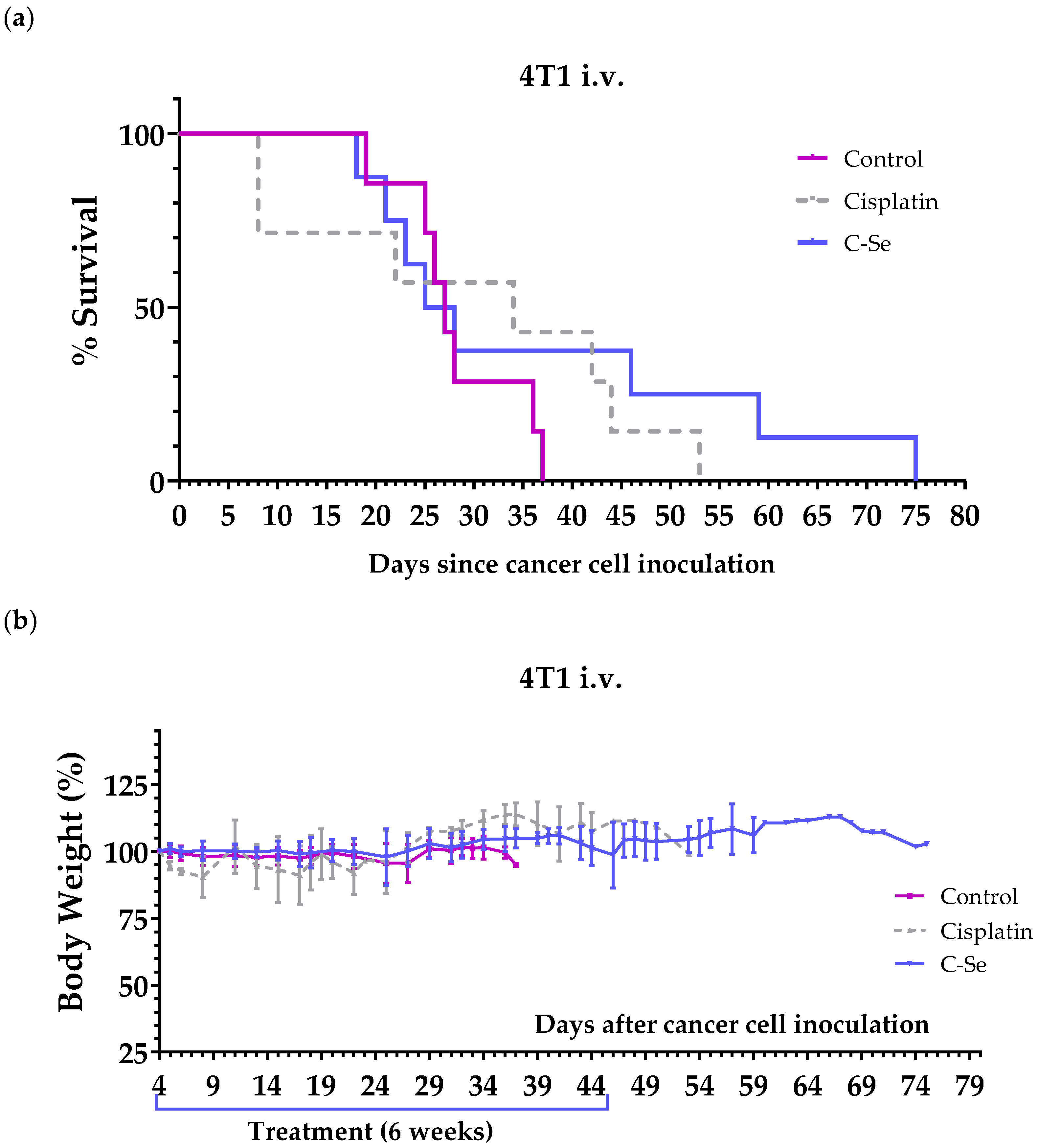
3.3. A Diet Lacking Selenium Induces Anticancer Activity in Mice with Metastasic Triple-Negative Breast Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. Selective Amino Acid Restriction Therapy (SAART): A Non-Pharmacological Strategy against All Types of Cancer Cells. Oncoscience 2015, 2, 857–866. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. Two Preclinical Tests to Evaluate Anticancer Activity and to Help Validate Drug Candidates for Clinical Trials. Oncoscience 2015, 2, 91–98. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of Large Amounts of Hydrogen Peroxide by Human Tumor Cells. Cancer Res. 1991, 51, 794–798. [Google Scholar] [PubMed]
- López-Lázaro, M. Dual Role of Hydrogen Peroxide in Cancer: Possible Relevance to Cancer Chemoprevention and Therapy. Cancer Lett. 2007, 252, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martin-Cordero, C.; Leon-Gonzalez, A.J.; Calderon-Montano, J.M.; Burgos-Moron, E.; Lopez-Lazaro, M. Pro-Oxidant Natural Products as Anticancer Agents. Curr. Drug Targets 2012, 13, 1006–1028. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. Excessive Superoxide Anion Generation Plays a Key Role in Carcinogenesis. Int. J. Cancer 2007, 120, 1378–1380. [Google Scholar] [CrossRef] [PubMed]
- Ganichkin, O.M.; Xu, X.-M.; Carlson, B.A.; Mix, H.; Hatfield, D.L.; Gladyshev, V.N.; Wahl, M.C. Structure and Catalytic Mechanism of Eukaryotic Selenocysteine Synthase. J. Biol. Chem. 2008, 283, 5849–5865. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The Importance of Selenium to Human Health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical Role as a Component of Glutathione Peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and Selenocysteine: Roles in Cancer, Health, and Development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Toppo, S.; Orian, L. The Glutathione Peroxidase Family: Discoveries and Mechanism. Free Radic. Biol. Med. 2022, 187, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Terrin, G.; Berni Canani, R.; Di Chiara, M.; Pietravalle, A.; Aleandri, V.; Conte, F.; De Curtis, M. Zinc in Early Life: A Key Element in the Fetus and Preterm Neonate. Nutrients 2015, 7, 10427–10446. [Google Scholar] [CrossRef] [PubMed]
- King, J.C. Zinc: An Essential but Elusive Nutrient. Am. J. Clin. Nutr. 2011, 94, 679S–684S. [Google Scholar] [CrossRef] [PubMed]
- Chasapis, C.T.; Ntoupa, P.-S.A.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent Aspects of the Effects of Zinc on Human Health. Arch. Toxicol. 2020, 94, 1443–1460. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.C.; Hazegh-Azam, M. Copper Biochemistry and Molecular Biology. Am. J. Clin. Nutr. 1996, 63, 797S–811S. [Google Scholar] [CrossRef] [PubMed]
- Prohaska, J.R. Impact of Copper Limitation on Expression and Function of Multicopper Oxidases (Ferroxidases). Adv. Nutr. 2011, 2, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Br, S. Ferroptosis Turns 10: Emerging Mechanisms, Physiological Functions, and Therapeutic Applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- Tang, D.; Kroemer, G.; Kang, R. Targeting Cuproplasia and Cuproptosis in Cancer. Nat. Rev. Clin. Oncol. 2024, 21, 370–388. [Google Scholar] [CrossRef] [PubMed]
- Aschner, J.L.; Aschner, M. Nutritional Aspects of Manganese Homeostasis. Mol. Aspects Med. 2005, 26, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Minerals. Available online: https://lpi.oregonstate.edu/mic/minerals (accessed on 2 July 2024).
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawska, A.; Bielawski, K. Selenium as a Bioactive Micronutrient in the Human Diet and Its Cancer Chemopreventive Activity. Nutrients 2021, 13, 1649. [Google Scholar] [CrossRef] [PubMed]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Björnstedt, M. Selenium Stimulates the Antitumour Immunity: Insights to Future Research. Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Golara, A.; Kozłowski, M.; Guzik, P.; Kwiatkowski, S.; Cymbaluk-Płoska, A. The Role of Selenium and Manganese in the Formation, Diagnosis and Treatment of Cervical, Endometrial and Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 10887. [Google Scholar] [CrossRef] [PubMed]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef] [PubMed]
- Bendellaa, M.; Lelièvre, P.; Coll, J.-L.; Sancey, L.; Deniaud, A.; Busser, B. Roles of Zinc in Cancers: From Altered Metabolism to Therapeutic Applications. Int. J. Cancer 2024, 154, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Ge, E.J.; Bush, A.I.; Casini, A.; Cobine, P.A.; Cross, J.R.; DeNicola, G.M.; Dou, Q.P.; Franz, K.J.; Gohil, V.M.; Gupta, S.; et al. Connecting Copper and Cancer: From Transition Metal Signalling to Metalloplasia. Nat. Rev. Cancer 2022, 22, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, V.C.; Gudekar, N.; Jasmer, K.; Papageorgiou, C.; Singh, K.; Petris, M.J. Copper Metabolism as a Unique Vulnerability in Cancer. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118893. [Google Scholar] [CrossRef] [PubMed]
- Lelièvre, P.; Sancey, L.; Coll, J.-L.; Deniaud, A.; Busser, B. The Multifaceted Roles of Copper in Cancer: A Trace Metal Element with Dysregulated Metabolism, but Also a Target or a Bullet for Therapy. Cancers 2020, 12, 3594. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Chen, M.; Zhang, R.; Zhang, W.; Wang, C.; Zhang, Y.; Wei, X.; Guan, Y.; Liu, J.; Feng, K.; et al. Manganese Is Critical for Antitumor Immune Responses via cGAS-STING and Improves the Efficacy of Clinical Immunotherapy. Cell Res. 2020, 30, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.A.K.; Adly, H.M.; Abdelkhaliq, A.A.; Nassir, A.M. Serum Levels of Selenium, Zinc, Copper, Manganese, and Iron in Prostate Cancer Patients. Curr. Urol. 2020, 14, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Szwiec, M.; Marciniak, W.; Derkacz, R.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Dębniak, T.; Jakubowska, A.; Lener, M.R.; Falco, M.; et al. Serum Levels of Copper and Zinc and Survival in Breast Cancer Patients. Nutrients 2024, 16, 1000. [Google Scholar] [CrossRef] [PubMed]
- Szwiec, M.; Marciniak, W.; Derkacz, R.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Dębniak, T.; Jakubowska, A.; Lener, M.; Falco, M.; et al. Serum Selenium Level Predicts 10-Year Survival after Breast Cancer. Nutrients 2021, 13, 953. [Google Scholar] [CrossRef] [PubMed]
- Charalabopoulos, K.; Kotsalos, A.; Batistatou, A.; Charalabopoulos, A.; Vezyraki, P.; Peschos, D.; Kalfakakou, V.; Evangelou, A. Selenium in Serum and Neoplastic Tissue in Breast Cancer: Correlation with CEA. Br. J. Cancer 2006, 95, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Walton, J.B.; Farquharson, M.; Mason, S.; Port, J.; Kruspig, B.; Dowson, S.; Stevenson, D.; Murphy, D.; Matzuk, M.; Kim, J.; et al. CRISPR/Cas9-Derived Models of Ovarian High Grade Serous Carcinoma Targeting Brca1, Pten and Nf1, and Correlation with Platinum Sensitivity. Sci. Rep. 2017, 7, 16827. [Google Scholar] [CrossRef] [PubMed]
- Corbett, T.H.; Griswold, D.P.; Roberts, B.J.; Peckham, J.C.; Schabel, F.M. Tumor Induction Relationships in Development of Transplantable Cancers of the Colon in Mice for Chemotherapy Assays, with a Note on Carcinogen Structure. Cancer Res. 1975, 35, 2434–2439. [Google Scholar] [PubMed]
- Wang, L.; Hu, X.; Xu, Y.; Liu, Z. Arsenic Trioxide Inhibits Lung Metastasis of Mouse Colon Cancer via Reducing the Infiltration of Regulatory T Cells. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 15165–15173. [Google Scholar] [CrossRef] [PubMed]
- Kolinsky, K.; Shen, B.-Q.; Zhang, Y.-E.; Kohles, J.; Dugan, U.; Zioncheck, T.F.; Heimbrook, D.; Packman, K.; Higgins, B. In Vivo Activity of Novel Capecitabine Regimens Alone and with Bevacizumab and Oxaliplatin in Colorectal Cancer Xenograft Models. Mol. Cancer Ther. 2009, 8, 75–82. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aslakson, C.J.; Miller, F.R. Selective Events in the Metastatic Process Defined by Analysis of the Sequential Dissemination of Subpopulations of a Mouse Mammary Tumor. Cancer Res. 1992, 52, 1399–1405. [Google Scholar] [PubMed]
- Liu, Y.; Wang, L.; Liu, J.; Xie, X.; Hu, H.; Luo, F. Anticancer Effects of ACT001 via NF-κB Suppression in Murine Triple-Negative Breast Cancer Cell Line 4T1. Cancer Manag. Res. 2020, 12, 5131–5139. [Google Scholar] [CrossRef] [PubMed]
- Guillén-Mancina, E.; Jiménez-Alonso, J.J.; Calderón-Montaño, J.M.; Jiménez-González, V.; Díaz-Ortega, P.; Burgos-Morón, E.; López-Lázaro, M. Artificial Diets with Selective Restriction of Amino Acids and Very Low Levels of Lipids Induce Anticancer Activity in Mice with Metastatic Triple-Negative Breast Cancer. Cancers 2023, 15, 1540. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Alonso, J.J.; Guillén-Mancina, E.; Calderón-Montaño, J.M.; Jiménez-González, V.; Díaz-Ortega, P.; Burgos-Morón, E.; López-Lázaro, M. Artificial Diets with Altered Levels of Sulfur Amino Acids Induce Anticancer Activity in Mice with Metastatic Colon Cancer, Ovarian Cancer and Renal Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 4587. [Google Scholar] [CrossRef] [PubMed]
- Prohaska, J.R.; Brokate, B. Lower Copper, Zinc-Superoxide Dismutase Protein but Not mRNA in Organs of Copper-Deficient Rats. Arch. Biochem. Biophys. 2001, 393, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.S.; Song, Y.; Leonard, S.W.; Mustacich, D.J.; Taylor, A.W.; Traber, M.G.; Ho, E. Dietary Zinc Restriction in Rats Alters Antioxidant Status and Increases Plasma F2 Isoprostanes. J. Nutr. Biochem. 2007, 18, 509–518. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M.; Calderón-Montaño, J.M.; Burgos-Morón, E.; Austin, C.A. Green Tea Constituents (-)-Epigallocatechin-3-Gallate (EGCG) and Gallic Acid Induce Topoisomerase I- and Topoisomerase II-DNA Complexes in Cells Mediated by Pyrogallol-Induced Hydrogen Peroxide. Mutagenesis 2011, 26, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, C.S.R.; Rabinowitz, J.D. Selenium Modulates Cancer Cell Response to Pharmacologic Ascorbate. Cancer Res. 2022, 82, 3486–3498. [Google Scholar] [CrossRef]
- Felix, K.; Gerstmeier, S.; Kyriakopoulos, A.; Howard, O.M.Z.; Dong, H.-F.; Eckhaus, M.; Behne, D.; Bornkamm, G.W.; Janz, S. Selenium Deficiency Abrogates Inflammation-Dependent Plasma Cell Tumors in Mice. Cancer Res. 2004, 64, 2910–2917. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, S.V.; Calvisi, D.F.; Labunskyy, V.M.; Factor, V.M.; Carlson, B.A.; Fomenko, D.E.; Moustafa, M.E.; Hatfield, D.L.; Gladyshev, V.N. Selenoprotein Deficiency and High Levels of Selenium Compounds Can Effectively Inhibit Hepatocarcinogenesis in Transgenic Mice. Oncogene 2005, 24, 8003–8011. [Google Scholar] [CrossRef] [PubMed]
- Eagle, K.; Jiang, Y.; Shi, X.; Li, M.; Obholzer, N.P.; Hu, T.; Perez, M.W.; Koren, J.V.; Kitano, A.; Yi, J.S.; et al. An Oncogenic Enhancer Encodes Selective Selenium Dependency in AML. Cell Stem Cell 2022, 29, 386–399.e7. [Google Scholar] [CrossRef]
- Schuschke, D.A.; Reed, M.W.; Saari, J.T.; Olson, M.D.; Ackermann, D.M.; Miller, F.N. Short-Term Dietary Copper Deficiency Does Not Inhibit Angiogenesis in Tumours Implanted in Striated Muscle. Br. J. Cancer 1992, 66, 1059–1064. [Google Scholar] [CrossRef][Green Version]
- Fong, L.Y.; Sivak, A.; Newberne, P.M. Zinc Deficiency and Methylbenzylnitrosamine-Induced Esophageal Cancer in Rats. J. Natl. Cancer Inst. 1978, 61, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, M.; Liu, Y.; Si, Z. Cope with Copper: From Copper Linked Mechanisms to Copper-Based Clinical Cancer Therapies. Cancer Lett. 2023, 561, 216157. [Google Scholar] [CrossRef] [PubMed]
- Baldari, S.; Di Rocco, G.; Toietta, G. Current Biomedical Use of Copper Chelation Therapy. Int. J. Mol. Sci. 2020, 21, 1069. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-Negative Breast Cancer: Challenges and Opportunities of a Heterogeneous Disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Yim, S.H.; Clish, C.B.; Gladyshev, V.N. Selenium Deficiency Is Associated with Pro-Longevity Mechanisms. Cell Rep. 2019, 27, 2785–2797.e3. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, W.C.; Kelley, D.S.; Taylor, P.C. The Effects of Dietary Selenium on the Immune System in Healthy Men. Biol. Trace Elem. Res. 2001, 81, 189–213. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, R.R.; Scott, K.G.; Sairenji, E. Selenite (75Se) as a Tumor-Localizing Agent in Man. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1966, 7, 197–208. [Google Scholar]
- Cavalieri, R.R.; Scott, K.G. Sodium Selenite Se 75. A More Specific Agent for Scanning Tumors. JAMA 1968, 206, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, A.E.; Lee, N.; Matthew-Onabanjo, A.N.; Spears, M.E.; Park, S.J.; Youkana, D.; Doshi, M.B.; Peppers, A.; Li, R.; Joseph, A.B.; et al. Selenium Detoxification Is Required for Cancer-Cell Survival. Nat. Metab. 2020, 2, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Alonso, J.J.; López-Lázaro, M. Dietary Manipulation of Amino Acids for Cancer Therapy. Nutrients 2023, 15, 2879. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Alonso, J.J.; Guillén-Mancina, E.; Calderón-Montaño, J.M.; Jiménez-González, V.; Díaz-Ortega, P.; Burgos-Morón, E.; López-Lázaro, M. Artificial Diets Based on Selective Amino Acid Restriction versus Capecitabine in Mice with Metastatic Colon Cancer. Nutrients 2022, 14, 3378. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Montaño, J.M.; Guillén-Mancina, E.; Jiménez-Alonso, J.J.; Jiménez-González, V.; Burgos-Morón, E.; Mate, A.; Pérez-Guerrero, M.C.; López-Lázaro, M. Manipulation of Amino Acid Levels with Artificial Diets Induces a Marked Anticancer Activity in Mice with Renal Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 16132. [Google Scholar] [CrossRef] [PubMed]
| Cancer Model | Metastatic Localization | Cell Line Inoculation | Mice (Sex, Train) | Positive Control Drug | Treatment Start Day |
|---|---|---|---|---|---|
| Ovarian cancer | Peritoneal dissemination | 5,000,000 ID8 Trp53−/− cells into the peritoneal cavity | Female C57BL/6JRj | Cisplatin 5 mg/kg i.p. once a week for 4 weeks | 15 |
| Colon cancer | Pulmonary metastases | 100,000 CT26.WT cells into the tail vein | Female BALB/cAnNRj | Capecitabine 450 mg/kg/day in the diet 7/7 on/off schedule | 4 |
| Triple-negative breast cancer | Pulmonary metastases | 100,000 4T1 cells into the tail vein | Female BALB/cAnNRj | Cisplatin 5 mg/kg i.p. once a week for 4 weeks | 4 |
| DIET | C | C-Se | C-Zn | C-Cu | C-Mn | C-SZCM |
|---|---|---|---|---|---|---|
| Casein | 20 | 20 | 20 | 20 | 20 | 20 |
| Corn oil | 7 | 7 | 7 | 7 | 7 | 7 |
| Corn starch | 48.498 | 48.498 | 48.498 | 48.498 | 48.498 | 48.498 |
| Sucrose | 15 | 15 | 15 | 15 | 15 | 15 |
| Cellulose | 5 | 5 | 5 | 5 | 5 | 5 |
| Choline bitartrate | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Vitamin mix | 1 | 1 | 1 | 1 | 1 | 1 |
| Tert-butylhydroquinone | 0.0008 | 0.0008 | 0.0008 | 0.0008 | 0.0008 | 0.0008 |
| Calcium carbonate | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Potassium phosphate monobasic | 0.88 | 0.88 | 0.88 | 0.88 | 0.88 | 0.88 |
| Potassium citrate monohydrate | 0.098 | 0.098 | 0.098 | 0.098 | 0.098 | 0.098 |
| Sodium chloride | 0.26 | 0.26 | 0.26 | 0.26 | 0.26 | 0.26 |
| Potassium sulphate | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
| Iron (III) citrate | 0.028 | 0.028 | 0.028 | 0.028 | 0.028 | 0.028 |
| Potassium iodate | 0.000051 | 0.000051 | 0.000051 | 0.000051 | 0.000051 | 0.000051 |
| Ammonium molybdate | 0.000025 | 0.000025 | 0.000025 | 0.000025 | 0.000025 | 0.000025 |
| Silicon dioxide | 0.0011 | 0.0011 | 0.0011 | 0.0011 | 0.0011 | 0.0011 |
| Chromium (III) chloride hexahydrate | 0.00090 | 0.00090 | 0.00090 | 0.00090 | 0.00090 | 0.00090 |
| Boric acid | 0.00029 | 0.00029 | 0.00029 | 0.00029 | 0.00029 | 0.00029 |
| Magnesium oxide | 0.084 | 0.084 | 0.084 | 0.084 | 0.084 | 0.084 |
| Sodium fluoride | 0.00022 | 0.00022 | 0.00022 | 0.00022 | 0.00022 | 0.00022 |
| Nickel (II) carbonate hydroxide tetrahydrate | 0.00011 | 0.00011 | 0.00011 | 0.00011 | 0.00011 | 0.00011 |
| Ammonium metavanadate | 0.000023 | 0.000023 | 0.000023 | 0.000023 | 0.000023 | 0.000023 |
| Lithium chloride | 0.000061 | 0.000061 | 0.000061 | 0.000061 | 0.000061 | 0.000061 |
| Basic copper carbonate | 0.0011 | 0.0011 | 0.0011 | 0 | 0.0011 | 0 |
| Basic zinc carbonate | 0.0055 | 0.0055 | 0 | 0.0055 | 0.0055 | 0 |
| Manganese carbonate | 0.0024 | 0.0024 | 0.0024 | 0.0024 | 0 | 0 |
| Sodium selenate | 0.000036 | 0 | 0.000036 | 0.000036 | 0.000036 | 0 |
| Treatment | n | Survival Time (Mean ± SEM; Days) | Survival Improvement vs. Control (Days) | p Value vs. Control |
|---|---|---|---|---|
| Normal diet | 6 | 52.5 ± 1.6 | - | - |
| Cisplatin (a) | 6 | >96.8 ± 19.0 | >+44.3 | 0.0015 (**) |
| Control (C) | 6 | 60.7 ± 2.2 | - | - |
| C-Se | 6 | 84.3 ± 13.7 | +23.6 | 0.2466 |
| C-Zn | 6 | 58.5 ± 1.3 | −2.2 | 0.3659 |
| C-Cu | 6 | 57.7 ± 1.6 | −3.0 | 0.3311 |
| C-Mn | 6 | 58.7 ± 1.2 | −2.0 | 0.3311 |
| C-SZCM | 6 | 67.3 ± 6.0 | +6.6 | 0.6864 |
| Treatment | n | Survival Time (Mean ± SEM; Days) | Survival Improvement vs. Control (Days) | p Value vs. Control |
|---|---|---|---|---|
| Control (a) | 12 | 59.4 ± 1.3 | - | - |
| C-Se (a) | 12 | 74.1 ± 7.5 | 14.7 | 0.0207 (*) |
| Cisplatin (b) | 4 | 83.5 ± 2.7 | 24.1 | 0.0061 (**) |
| Treatment | n | Survival Time (Mean ± SEM; Days) | Survival Improvement vs. Control (Days) | p Value vs. Control |
|---|---|---|---|---|
| Normal diet | 5 | 30.0 ± 1.5 | - | - |
| Capecitabine (b) | 5 | 41.8 ± 2.7 | +11.8 | 0.0206 (*) |
| Control | 8 | 31.0 ± 1.4 | - | - |
| C-Se | 8 | 38.9 ± 3.8 | +7.9 | 0.0684 |
| Treatment | n | Survival Time (Mean ± SEM; Days) | Survival Improvement vs. Control (Days) | p Value vs. Control |
|---|---|---|---|---|
| Control | 7 | 28.3 ± 2.2 | - | - |
| Cisplatin | 5 | 39.0 ± 4.7 | +10.7 | 0.1258 |
| C-Se | 8 | 36.9 ± 6.9 | +8.6 | 0.9084 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Ortega, P.; Calderón-Montaño, J.M.; Jiménez-Alonso, J.J.; Guillén-Mancina, E.; Jiménez-González, V.; Burgos-Morón, E.; López-Lázaro, M. A Diet Lacking Selenium, but Not Zinc, Copper or Manganese, Induces Anticancer Activity in Mice with Metastatic Cancers. Nutrients 2024, 16, 2249. https://doi.org/10.3390/nu16142249
Díaz-Ortega P, Calderón-Montaño JM, Jiménez-Alonso JJ, Guillén-Mancina E, Jiménez-González V, Burgos-Morón E, López-Lázaro M. A Diet Lacking Selenium, but Not Zinc, Copper or Manganese, Induces Anticancer Activity in Mice with Metastatic Cancers. Nutrients. 2024; 16(14):2249. https://doi.org/10.3390/nu16142249
Chicago/Turabian StyleDíaz-Ortega, Patricia, José Manuel Calderón-Montaño, Julio José Jiménez-Alonso, Emilio Guillén-Mancina, Víctor Jiménez-González, Estefanía Burgos-Morón, and Miguel López-Lázaro. 2024. "A Diet Lacking Selenium, but Not Zinc, Copper or Manganese, Induces Anticancer Activity in Mice with Metastatic Cancers" Nutrients 16, no. 14: 2249. https://doi.org/10.3390/nu16142249
APA StyleDíaz-Ortega, P., Calderón-Montaño, J. M., Jiménez-Alonso, J. J., Guillén-Mancina, E., Jiménez-González, V., Burgos-Morón, E., & López-Lázaro, M. (2024). A Diet Lacking Selenium, but Not Zinc, Copper or Manganese, Induces Anticancer Activity in Mice with Metastatic Cancers. Nutrients, 16(14), 2249. https://doi.org/10.3390/nu16142249







