Integrative Approach to Risk Factors in Simple Chronic Obstructive Airway Diseases of the Lung or Associated with Metabolic Syndrome—Analysis and Prediction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
- The Ethics and Deontology Committee within the University of Medicine and Pharmacy from Craiova (number 143/04.07.2022),
- The Clinical Emergency Country Hospital from Craiova (number 3000/17.01.2023) and
- The Clinical Hospital of Infectious Diseases and Pneumophtisiology‚ Victor Babeș’ from Craiova (number 7488/22.05.2023).
- -
- Elevated waist circumference (≥88 cm for women, ≥102 cm for men).
- -
- Elevated triglycerides (≥150 mg/dL) or drug treatment for high triglycerides levels.
- -
- High-density-lipoprotein (HDL) cholesterol (<40 mg/dL for men, <50 mg/dL for women) or drug treatment for low HDL cholesterol levels.
- -
- Elevated blood pressure (systolic ≥ 130 mmHg or diastolic ≥ 85 mmHg) or treatment for hypertension.
- -
- Elevated fasting glucose (≥100 mg/dL) or treatment for elevated glucose.
- -
- Conditions and symptoms that overlapped contraindications for performing spirometry;
- -
- Clinical conditions, physiological or pathological, that can result in abdominal volume expansion and subsequently impact the measurement of abdominal circumference and/or body weight;
- -
- The inability to maintain an upright position without support to be weighted;
- -
- Severely altered clinical condition incompatible with the patient’s ability to comply with the required evaluation steps. This decision was prompted by the need for patients to undergo spirometry (a demanding test), be mobilized for anthropometric measurements, and be able to communicate effectively to complete questionnaires;
- -
- Patients experiencing episodes of respiratory pathology exacerbation or diabetic ketoacidosis. This choice is justified by the observation that exacerbations of lung diseases are generally associated with a decrease in lung volumes measured by spirometry and increased severity levels of respiratory dysfunction in comparison to the baseline values of our patients. Furthermore, periods of diabetes decompensation correlate with substantial changes in biological markers.
2.2. Medical History, Anthropometric, and Blood Pressure Measurements
2.3. Blood Samples—Biochemical Parameters
2.4. Pulmonary Function Test—Spirometry
2.5. Assessment of Lifestyle and Diet
2.6. Statistical Analysis
3. Results
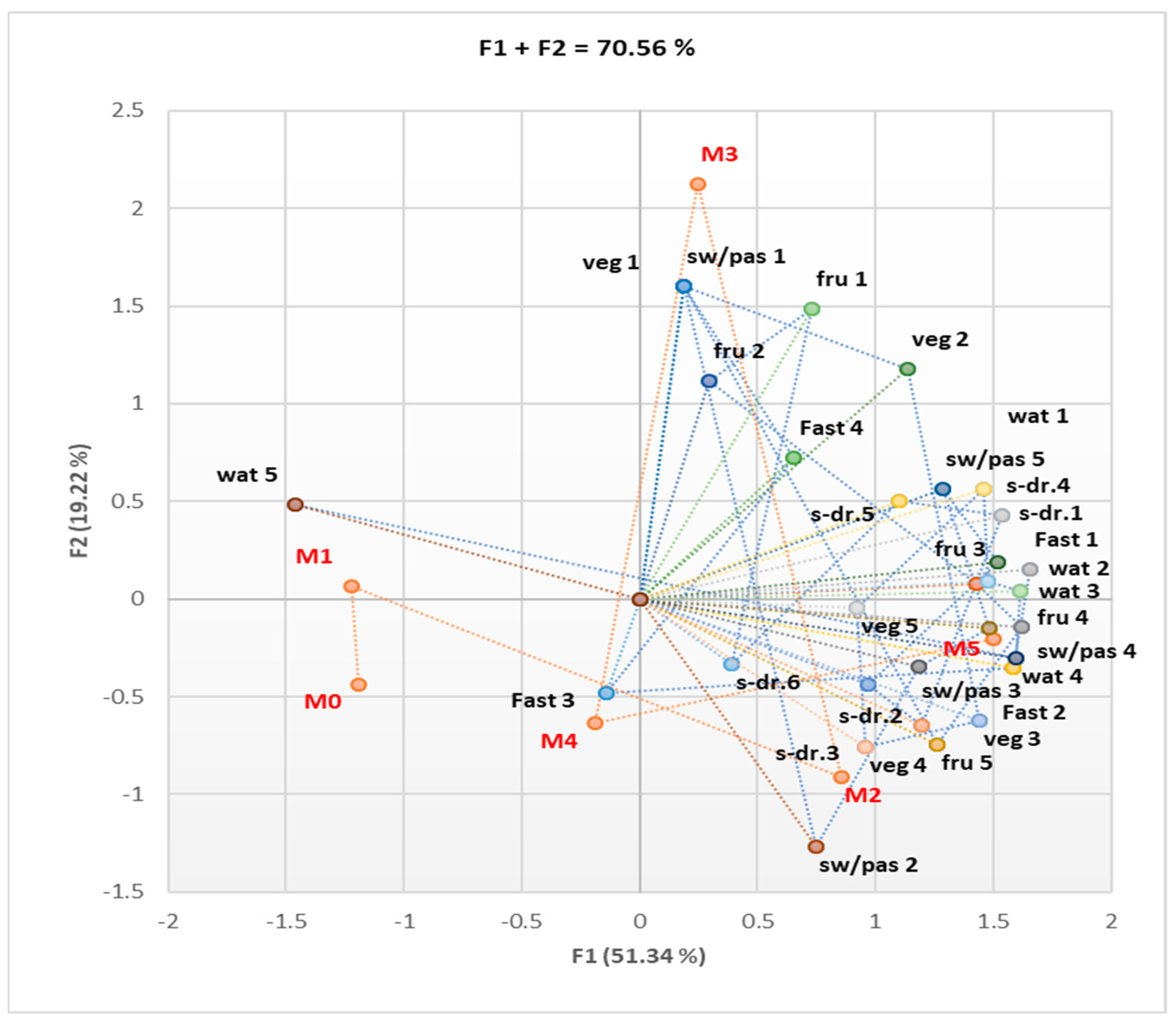
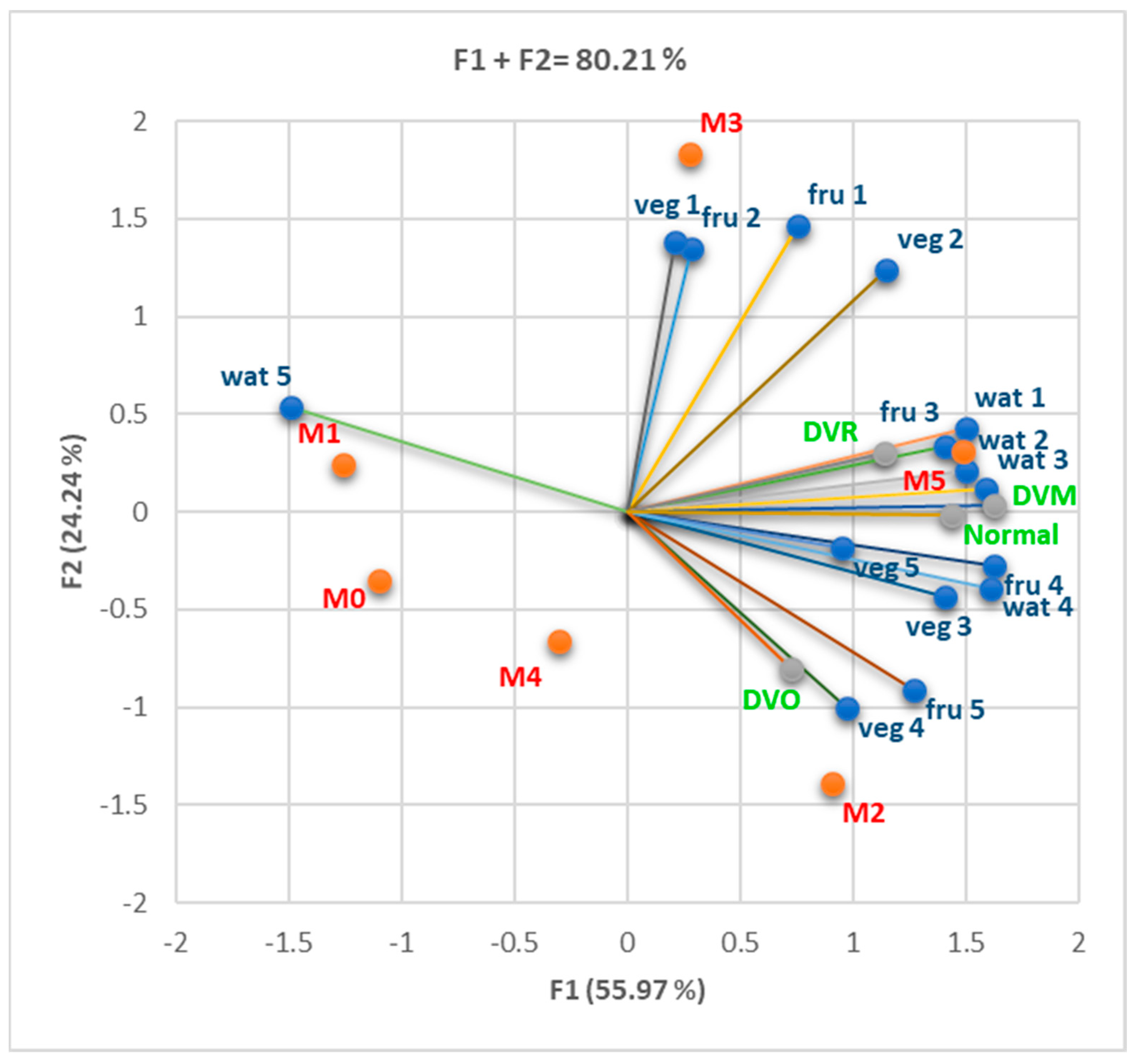
3.1. Assessment of Behavioral Risk Factors (Diet and Lifestyle)
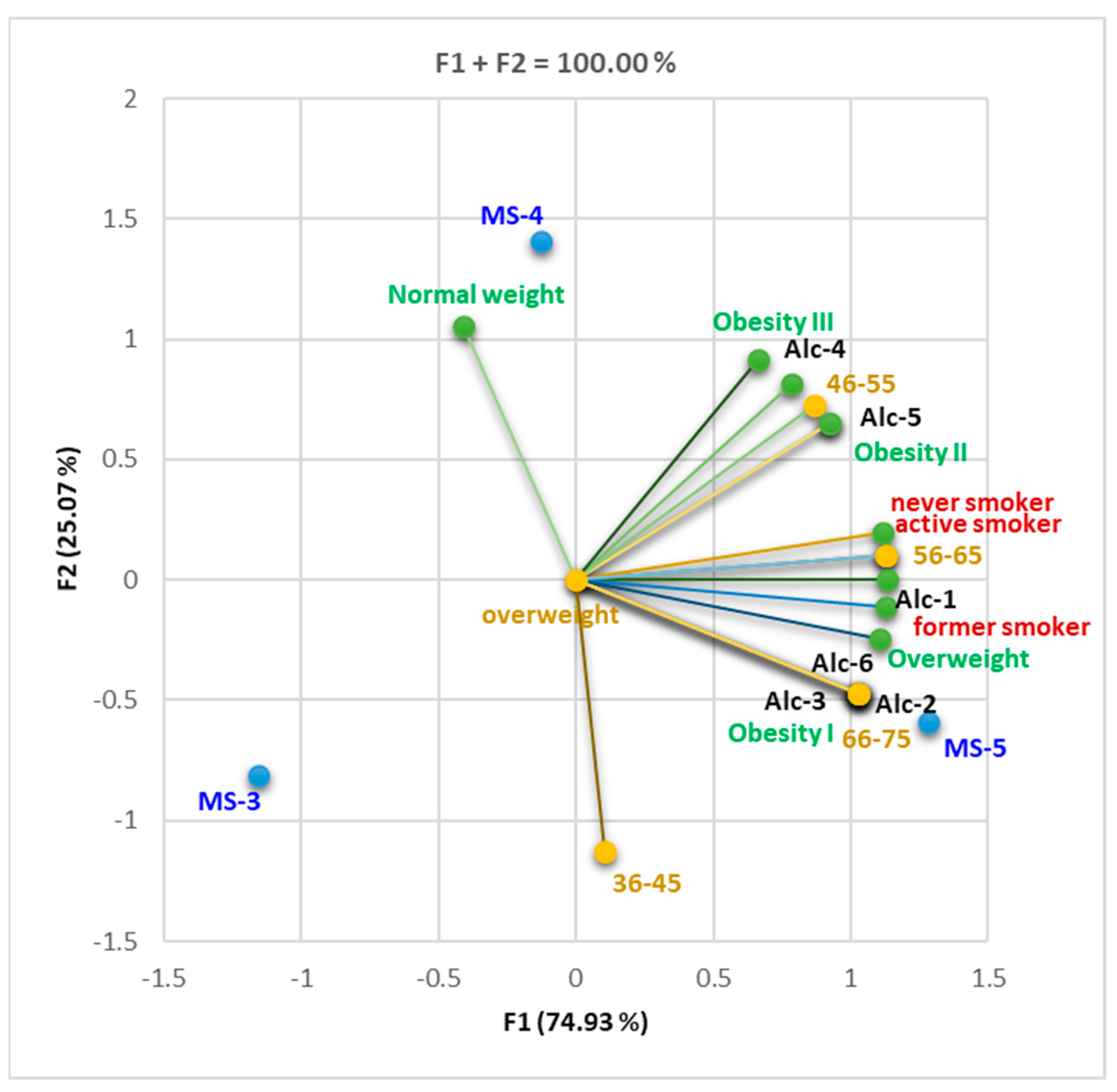
3.1.1. Cohort 1
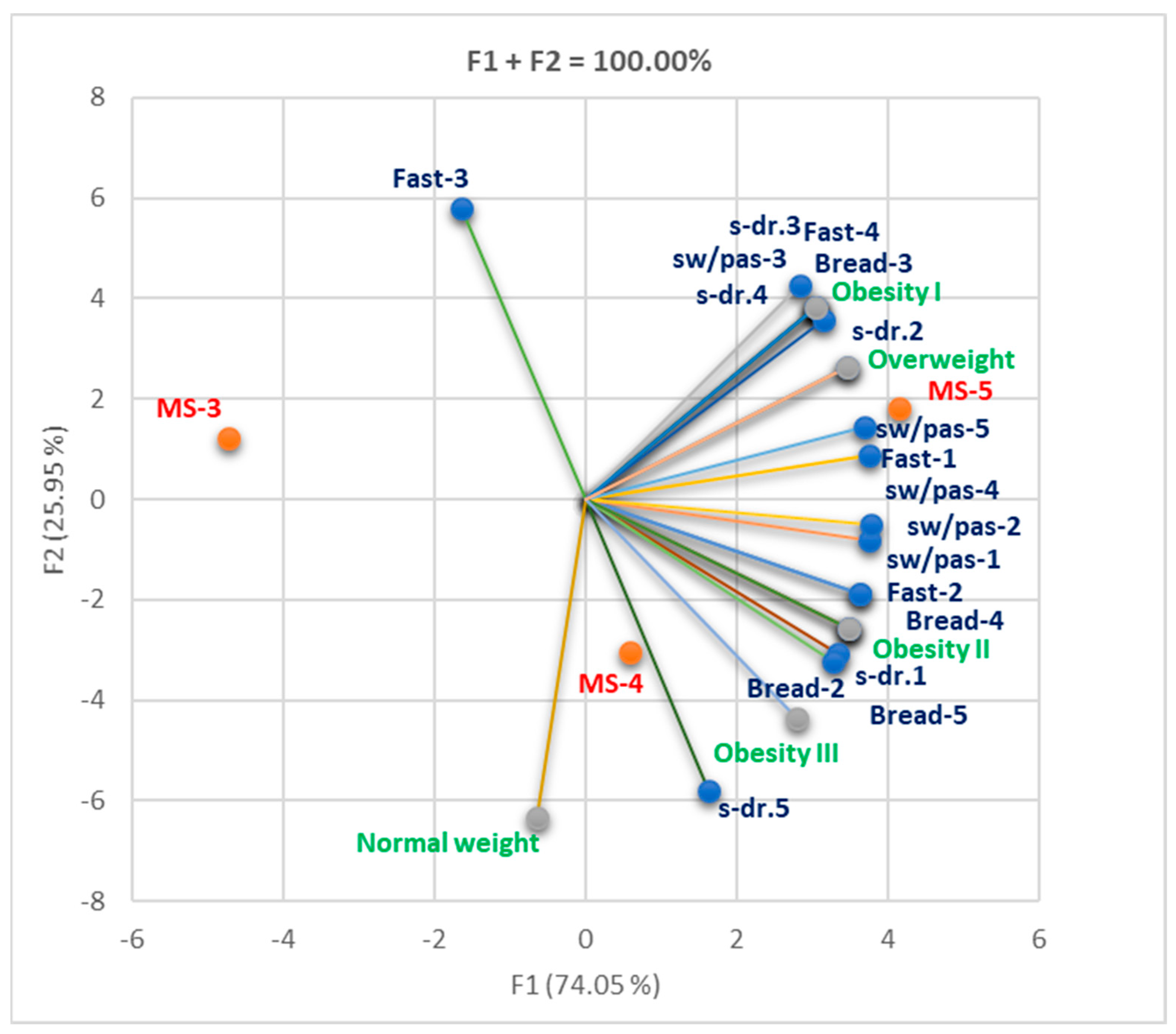
3.1.2. Cohort 2
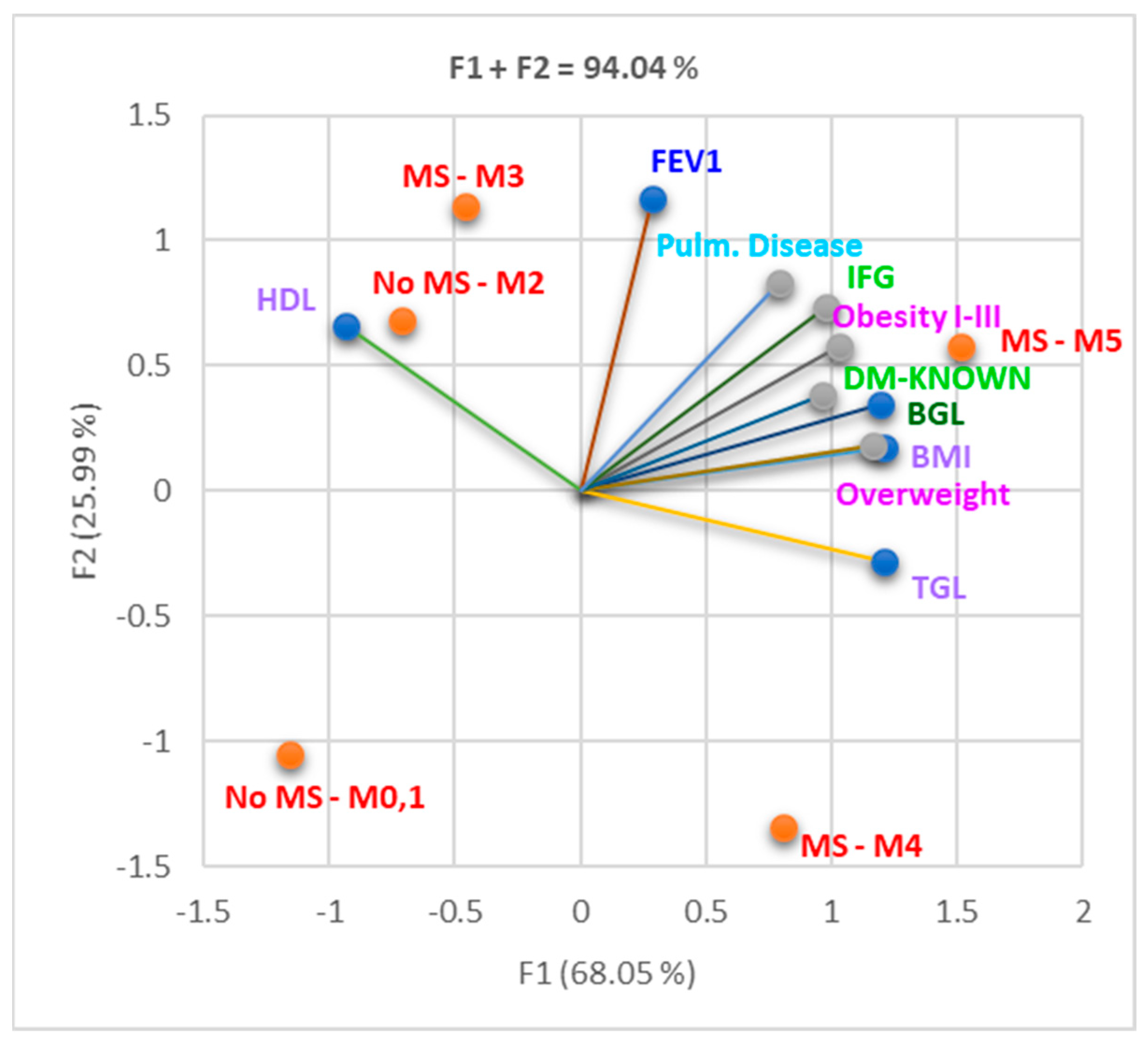
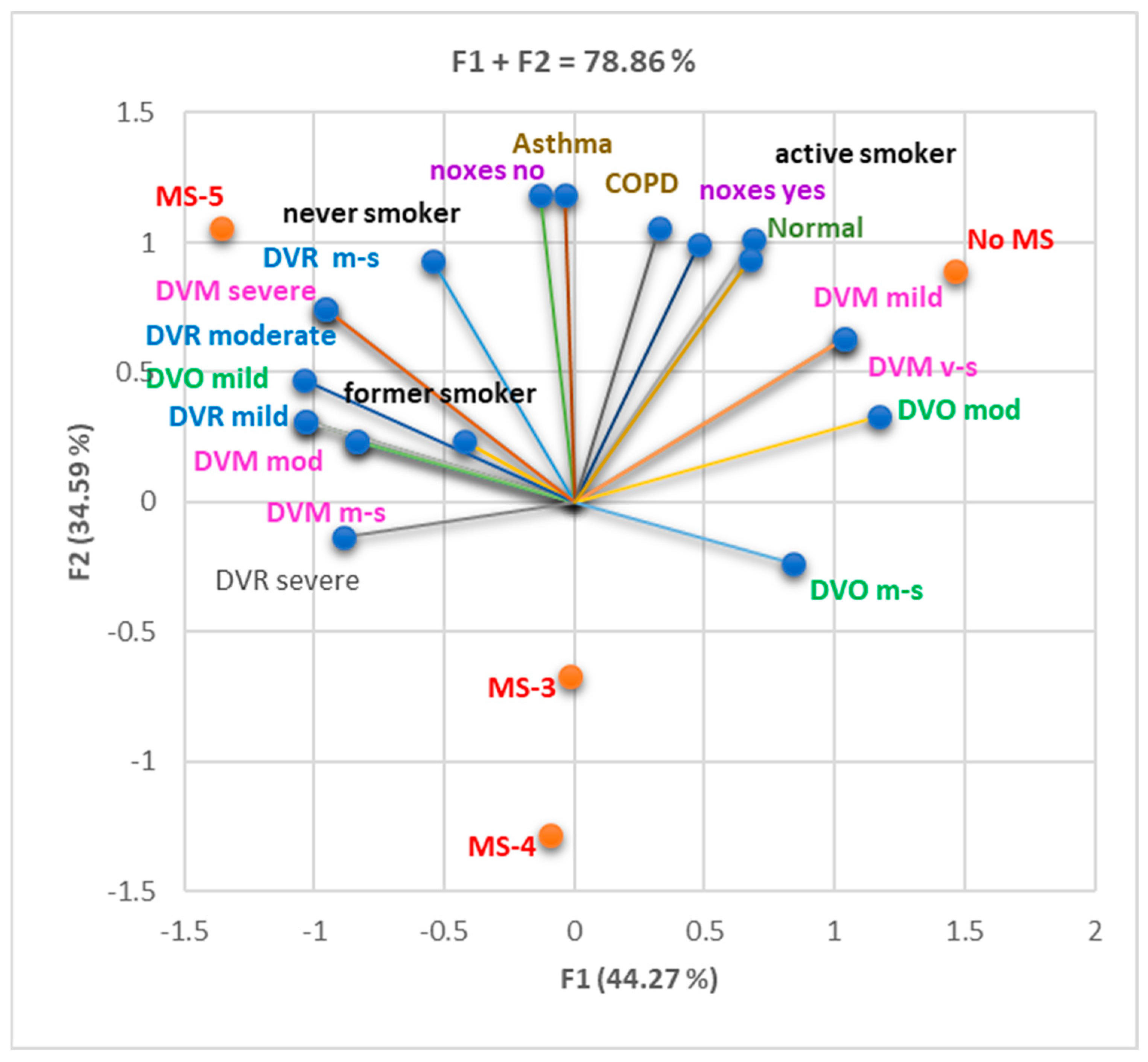
3.2. Evaluation of the Impact of MS on Pulmonary Function
3.2.1. Cohort 1
3.2.2. Cohort 2
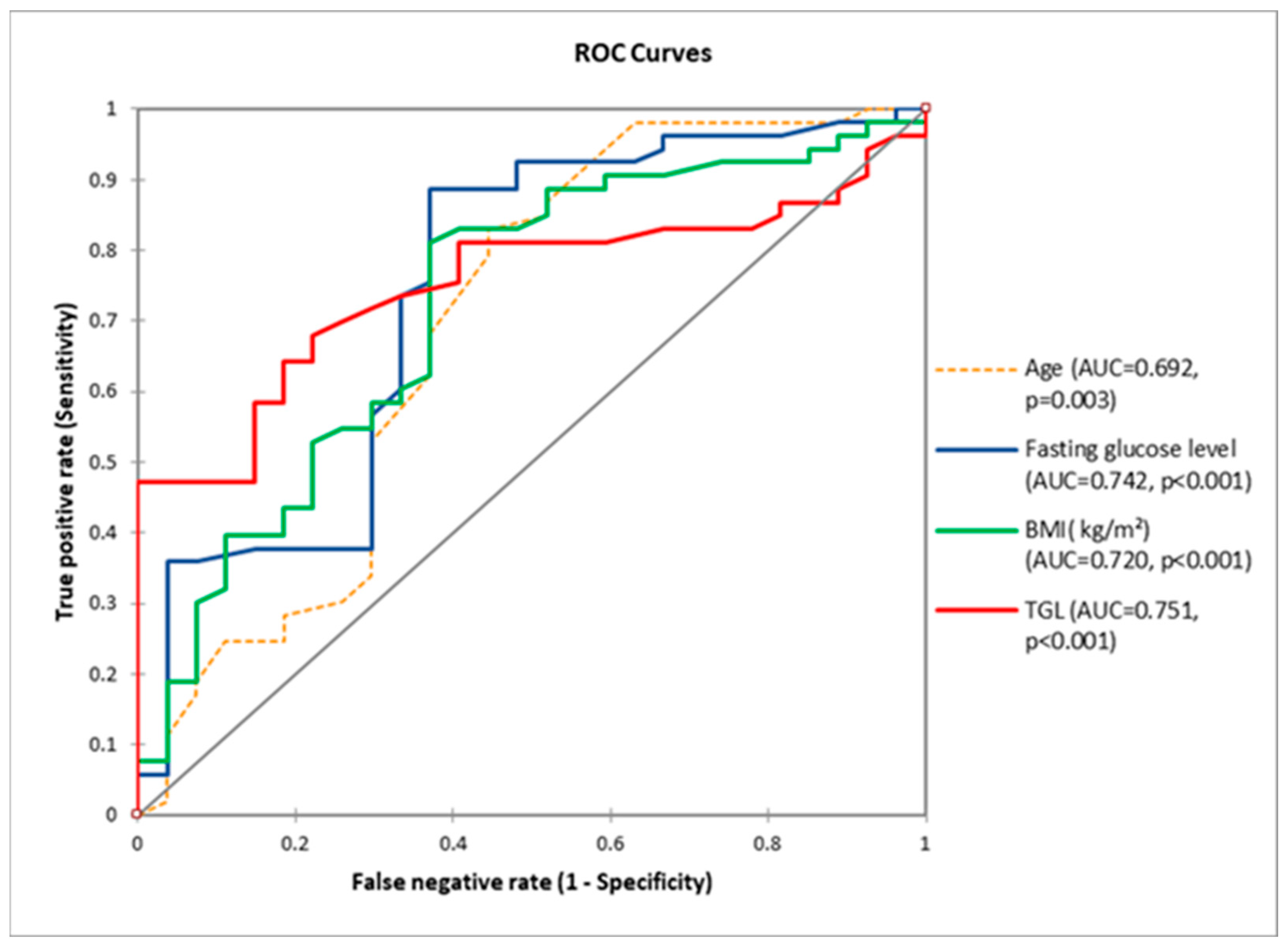
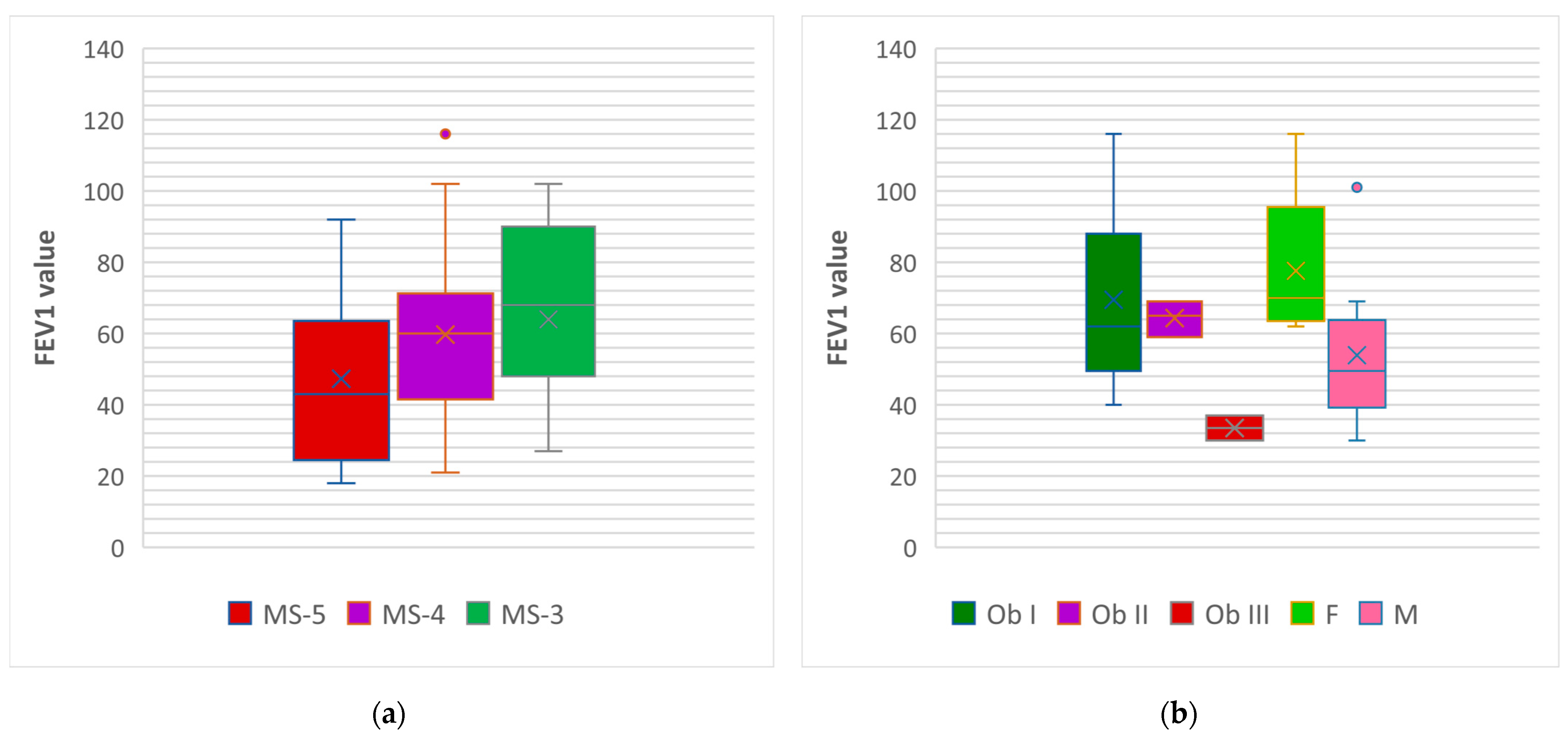
3.2.3. Both Cohorts Analyzed Together
4. Discussion
4.1. Involvement of Weight, Diet, and Metabolic Factors in the Two Cohorts
4.2. Involvement of Toxic Exposure and Harmful Habits in the Two Cohorts
4.3. Intervention on Modifiable Risk Factors of MS in Asthma or COPD Patients
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wisnivesky, J.; de-Torres, J.P. The Global Burden of Pulmonary Diseases: Most Prevalent Problems and Opportunities for Improvement. Ann. Glob. Health 2019, 85, 1. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Chronic Respiratory Diseases. Available online: https://www.who.int/health-topics/chronic-respiratory-diseases#tab=tab_1 (accessed on 15 March 2024).
- World Health Organization. Chronic Obstructive Pulmonary Disease (COPD). 16 March 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 15 March 2024).
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2023 GINA Main Report. Available online: https://ginasthma.org/2023-gina-main-report/ (accessed on 15 March 2024).
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Prevention, Diagnosis, and Management of COPD: 2023 Report. Available online: https://goldcopd.org/2023-gold-report-2/ (accessed on 15 March 2024).
- World Health Organization. Asthma. 4 May 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/asthma (accessed on 15 March 2024).
- Ruvuna, L.; Sood, A. Epidemiology of Chronic Obstructive Pulmonary Disease. Clin. Chest Med. 2020, 41, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Garrison, D.M.; Pendela, V.S.; Memon, J. Cor Pulmonale. [Updated 8 August 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430739/ (accessed on 15 March 2024).
- Klinger, J.R.; Hill, N.S. Right ventricular dysfunction in chronic obstructive pulmonary disease. Evaluation and management. Chest 1991, 99, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Beilby, J. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Clin. Biochem. Rev. 2004, 25, 195–198. [Google Scholar]
- Swarup, S.; Goyal, A.; Grigorova, Y.; Zeltser, R. Metabolic syndrome. [Updated 24 October 2022]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459248/ (accessed on 15 March 2024).
- Uppalakal, B.; Karanayil, L.S. Incidence of metabolic syndrome in Patients Admitted to Medical Wards with ST Elevation Myocardial Infarction. J. Clin. Diagn. Res. 2017, 11, OC17–OC20. [Google Scholar] [CrossRef]
- Divo, M.; Celli, B.R. Multimorbidity in Patients with Chronic Obstructive Pulmonary Disease. Clin. Chest Med. 2020, 41, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Varkonyi-Sepp, J.; Freeman, A.; Ainsworth, B.; Kadalayil, L.P.; Haitchi, H.M.; Kurukulaaratchy, R.J. Multimorbidity in Difficult Asthma: The Need for Personalised and Non-Pharmacological Approaches to Address a Difficult Breathing Syndrome. J. Pers. Med. 2022, 12, 1435. [Google Scholar] [CrossRef] [PubMed]
- Naik, D.; Josh, A.; Paul, T.V.; Thomas, N. Chronic obstructive pulmonary disease and the metabolic syndrome: Consequences of a dual threat. Indian J. Endocrinol. Metab. 2014, 18, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Díez-Manglano, J.; Barquero-Romero, J.; Almagro, P.; Cabrera, F.J.; López García, F.; Montero, L.; Soriano, J.B.; Working Group on COPD; Spanish Society of Internal Medicine. COPD patients with and without metabolic syndrome: Clinical and functional differences. Intern. Emerg. Med. 2014, 9, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Baffi, C.W.; Wood, L.; Winnica, D.; Strollo, P.J., Jr.; Gladwin, M.T.; Que, L.G.; Holguin, F. Metabolic syndrome and the Lung. Chest 2016, 149, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Leone, N.; Courbon, D.; Thomas, F.; Bean, K.; Jégo, B.; Leynaert, B.; Guize, L.; Zureik, M. Lung function impairment and metabolic syndrome: The critical role of abdominal obesity. Am. J. Respir. Crit. Care Med. 2009, 179, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Mook, S.; Halkes, C.J.C.; Bilecen, S.; Cabezas, M.C. In vivo regulation of plasma free fatty acids in insulin resistance. Metabolism 2004, 53, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Soutar, A.; Seaton, A.; Brown, K. Bronchial reactivity and dietary antioxidants. Thorax 1997, 52, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Pace, E.; Chanez, P.; Gras, D.; Vachier, I.; Chiappara, G.; La Guardia, M.; Gerbino, S.; Profita, M.; Gjomarkaj, M. Leptin and leptin receptor expression in asthma. J. Allergy Clin. Immunol. 2009, 124, 230–237.e4. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P.; Canetti, C.; Gottschalk, A.; Tithof, P.K.; Peters-Golden, M. Leptin augments alveolar macrophage leukotriene synthesis by increasing phospholipase activity and enhancing group IVC iPLA2 (cPLA2gamma) protein expression. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 287, L497–L502. [Google Scholar] [CrossRef] [PubMed]
- Sideleva, O.; Suratt, B.T.; Black, K.E. Obesity and asthma: An inflammatory disease of adipose tissue not the airway. Am. J. Respir. Crit. Care Med. 2012, 186, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Cho, J.Y.; Pham, A.; Ramsdell, J.; Broide, D.H. Adiponectin and functional adiponectin receptor 1 are expressed by airway epithelial cells in chronic obstructive pulmonary disease. J. Immunol. 2009, 182, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Berryman, D.E.; Glad, C.A.; List, E.O.; Johannsson, G. The GH/IGF-1 axis in obesity: Pathophysiology and therapeutic considerations. Nat. Rev. Endocrinol. 2013, 9, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, S.R.; Oh, Y.; Cho, S.H.; Schleimer, R.P.; Lee, Y.C. Targeting insulin-like growth factor-I and insulin-like growth factor-binding protein-3 signaling pathways. A novel therapeutic approach for asthma. Am. J. Respir. Cell Mol. Biol. 2014, 50, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Rakash, Y.S.; Linneberg, A.; Agrawal, A. Insulin and the lung: Connecting asthma and metabolic syndrome. J. Allergy 2013, 2013, 627384. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, B.G.; Schaafsma, D.; Tran, T.; Zaagsma, J.; Meurs, H. Insulin-induced laminin expression promotes a hypercontractile airway smooth muscle phenotype. Am. J. Respir. Cell Mol. Biol. 2009, 41, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Sodoyez-Goffaux, F.R.; Sodoyez, J.C.; De Vos, C.J. Insulin receptors in the fetal rat lung. A transient characteristic of fetal cells? Pediatr. Res. 1981, 15, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Sircar, K.; Hnizdo, E.; Petsonk, E.; Attfield, M. Decline in lung function and mortality: Implications for medical monitoring. Occup. Environ. Med. 2007, 64, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Massaro, M.; Garbarino, S.; Toraldo, D.M. Role of Diet in Chronic Obstructive Pulmonary Disease Prevention and Treatment. Nutrients 2019, 11, 1357. [Google Scholar] [CrossRef] [PubMed]
- Pleasants, R.A.; Riley, I.L.; Mannino, D.M. Defining and targeting health disparities in chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2475–2496. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; Montes de Oca, M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2023, 207, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and Management of the metabolic syndrome, An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement: Executive Summary. Circulation 2005, 112, e285–e290. [Google Scholar]
- Năstăsescu, V.; Mititelu, M.; Stanciu, T.I.; Drăgănescu, D.; Grigore, N.D.; Udeanu, D.I.; Stanciu, G.; Neacșu, S.M.; Dinu-Pîrvu, C.E.; Oprea, E. Food Habits and Lifestyle of Romanians in the Context of the COVID-19 Pandemic. Nutrients 2022, 14, 504. [Google Scholar] [CrossRef] [PubMed]
- Ioniță-Mîndrican, C.-B.; Mititelu, M.; Musuc, A.M.; Oprea, E.; Ziani, K.; Neacșu, S.M.; Grigore, N.D.; Negrei, C.; Dumitrescu, D.-E.; Mireșan, H.; et al. Honey and Other Beekeeping Products Intake among the Romanian Population and Their Therapeutic Use. Appl. Sci. 2022, 12, 9649. [Google Scholar] [CrossRef]
- Ashwell, M.; Gibson, S. Waist-to-height ratio as an indicator of ‘early health risk’: Simpler and more predictive than using a ‘matrix’ based on BMI and waist circumference. BMJ Open 2016, 6, e010159. [Google Scholar] [CrossRef] [PubMed]
- Moore, V.C. Spirometry: Step by step. Breathe Mar. 2012, 8, 232–240. [Google Scholar] [CrossRef]
- Mititelu, M.; Oancea, C.N.; Neacșu, S.M.; Musuc, A.M.; Gheonea, T.C.; Stanciu, T.I.; Rogoveanu, I.; Hashemi, F.; Stanciu, G.; Ioniță-Mîndrican, C.B.; et al. Evaluation of Junk Food Consumption and the Risk Related to Consumer Health among the Romanian Population. Nutrients 2023, 15, 3591. [Google Scholar] [CrossRef] [PubMed]
- Mititelu, M.; Oancea, C.-N.; Neacșu, S.M.; Olteanu, G.; Cîrțu, A.-T.; Hîncu, L.; Gheonea, T.C.; Stanciu, T.I.; Rogoveanu, I.; Hashemi, F.; et al. Evaluation of Non-Alcoholic Beverages and the Risk Related to Consumer Health among the Romanian Population. Nutrients 2023, 15, 3841. [Google Scholar] [CrossRef] [PubMed]
- Mititelu, M.; Neacsu, S.M.; Oprea, E.; Dumitrescu, D.-E.; Nedelescu, M.; Drăgănescu, D.; Nicolescu, T.O.; Rosca, A.C.; Ghica, M. Black Sea Mussels Qualitative and Quantitative Chemical Analysis: Nutritional Benefits and Possible Risks through Consumption. Nutrients 2022, 14, 964. [Google Scholar] [CrossRef] [PubMed]
- Leahu, A.; Lupu, E.C. Statistical simulation and prediction in software reliability. Analele Univ. Ovidius Constanta Ser. Mat. 2008, 16, 81–90. [Google Scholar]
- Eckhardt, C.M.; Wu, H. Environmental Exposures and Lung Aging: Molecular Mechanisms and Implications for Improving Respiratory Health. Curr. Environ. Health Rep. 2021, 8, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Olloquequi, J.; Jaime, S.; Parra, V.; Cornejo-Córdova, E.; Valdivia, G.; Agustí, À.; Silva, O.R. Comparative analysis of COPD associated with tobacco smoking, biomass smoke exposure or both. Respir. Res. 2018, 19, 13, Erratum in Respir. Res. 2018, 19, 77. [Google Scholar] [CrossRef] [PubMed]
- Omer, T.D.; Sahande, E.; Sefa, L.O.; Kursat, E.; Ersin, T.; Ibrahim, A. Pulmonary toxicity of chronic exposure to tobacco and biomass smoke in rats. Clinics 2011, 66, 1081–1087. [Google Scholar]
- Tian, T.; Jiang, X.; Qin, R.; Ding, Y.; Yu, C.; Xu, X.; Song, C. Effect of Smoking on Lung Function Decline in a Retrospective Study of a Health Examination Population in Chinese Males. Front. Med. 2023, 9, 843162. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. W.H.O. Report on the Global Tobacco Epidemic; World Health Organization: Geneva, Switzerland, 2019. Available online: https://www.who.int/tobacco/global_report/en/ (accessed on 15 March 2024).
- Doo, J.H.; Kim, S.M.; Park, Y.J.; Kim, K.H.; Oh, Y.H.; Kim, J.S.; Park, S.M. Smoking cessation after diagnosis of COPD is associated with lower all-cause and cause-specific mortality: A nationwide population-based cohort study of South Korean men. BMC Pulm. Med. 2023, 23, 237. [Google Scholar] [CrossRef]
- Neeraj, M.; Shah, G.K. Respiratory complications of obesity: From early changes to respiratory failure. Breathe Mar. 2023, 19, 220263. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.C.; Silva, M.A.; Calles, A.C. Obesity and lung function: A systematic review. Einstein (Sao Paulo) 2014, 12, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.E.; Holguin, F.; Sood, A.; Salome, C.M.; Pratley, R.E.; Beuther, D.A.; Celedón, J.C.; Shore, S.A. American Thoracic Society Ad Hoc Subcommittee on Obesity and Lung Disease. An official American Thoracic Society Workshop report: Obesity and asthma. Proc. Am. Thorac. Soc. 2010, 7, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Juel, C.T.; Ali, Z.; Nilas, L.; Ulrik, C.S. Asthma and obesity: Does weight loss improve asthma control? a systematic review. J. Asthma Allergy 2012, 5, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, S.F.; Quesenberry, C.P., Jr.; Van Den Eeden, S.K.; Shan, J.; Ferrara, A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care 2010, 33, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Karamzad, N.; Izadi, N.; Sanaie, S.; Ahmadian, E.; Eftekhari, A.; Sullman, M.J.M.; Safiri, S. Asthma and metabolic syndrome: A comprehensive systematic review and meta-analysis of observational studies. J. Cardiovasc. Thorac. Res. 2020, 12, 120–128. [Google Scholar] [CrossRef]
- Strasser, B.; Siebert, U.; Schobersberger, W. Effects of resistance training on respiratory function in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Sleep Breath. 2013, 17, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Osterling, K.; MacFadyen, K.; Gilbert, R.; Dechman, G. The effects of high intensity exercise during pulmonary rehabilitation on ventilatory parameters in people with moderate to severe stable COPD: A systematic review. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Good, J.; Gardiner, P.A.; Copeland, J.L.; Stickland, M.K.; Rudolerd, D.; Buman, M.P. Effects of replacing sitting time with physical activity on lung function: An analysis of the Canadian Longitudinal Study on Aging. Health Rep. 2019, 30, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Furlanetto, K.C.; Donária, L.; Schneider, L.P.; Lopes, J.R.; Ribeiro, M.; Fernandes, K.B.; Hernandes, N.A.; Pitta, F. Sedentary Behavior Is an Independent Predictor of Mortality in Subjects With COPD. Respir. Care 2017, 62, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Nkosi, V.; Rathogwa-Takalani, F.; Voyi, K. The Frequency of Fast Food Consumption in Relation to Wheeze and Asthma Among Adolescents in Gauteng and North West Provinces, South Africa. Int. J. Environ. Res. Public Health 2020, 17, 1994. [Google Scholar] [CrossRef] [PubMed]
- Varraso, R.; Fung, T.T.; HU, F.B.; Willett, W.; Camargo, C.A. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US men. Thorax 2007, 62, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Orea-Tejeda, A.; Gómez-Martínez, M.; González-Islas, D.; Flores-Cisneros, L.; Keirns-Davis, C.; Sánchez-Santillán, R.; Pérez-García, I.; Martínez-Luna, N.; Robles-Hernández, R.; Sánchez-Moreno, C.; et al. The impact of hydration status and fluid distribution on pulmonary function in COPD patients. Sci. Rep. 2022, 12, 1216. [Google Scholar] [CrossRef] [PubMed]
- Nseir, W.; Nassar, F.; Assy, N. Soft drinks consumption and nonalcoholic fatty liver disease. World J. Gastroenterol. 2010, 16, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Tzima, N.; Pitsavos, C.; Chrysohoou, C.; Papakonstantinou, E.; Zampelas, A.; Stefanadis, C. The relationship between dietary habits, blood glucose and insulin levels among people without cardiovascular disease and type 2 diabetes; the ATTICA study. Rev. Diabet. Stud. 2005, 2, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Perez Perez, J.L.; Perez Gandara, B.; Agudelo, C.W.; Rodriguez Ortega, R.; Ahmed, H.; Garcia-Arcos, I.; McCarthy, C.; Geraghty, P. Mechanisms Linking COPD to Type 1 and 2 Diabetes Mellitus: Is There a Relationship between Diabetes and COPD? Medicina 2022, 58, 1030. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Hui, K.; Farne, H.; Garnett, J.P.; Baines, D.L.; Moore, L.S.; Holmes, A.H.; Filloux, A.; Tregoning, J.S. Increased airway glucose increases airway bacterial load in hyperglycaemia. Sci. Rep. 2016, 6, 27636. [Google Scholar] [CrossRef] [PubMed]






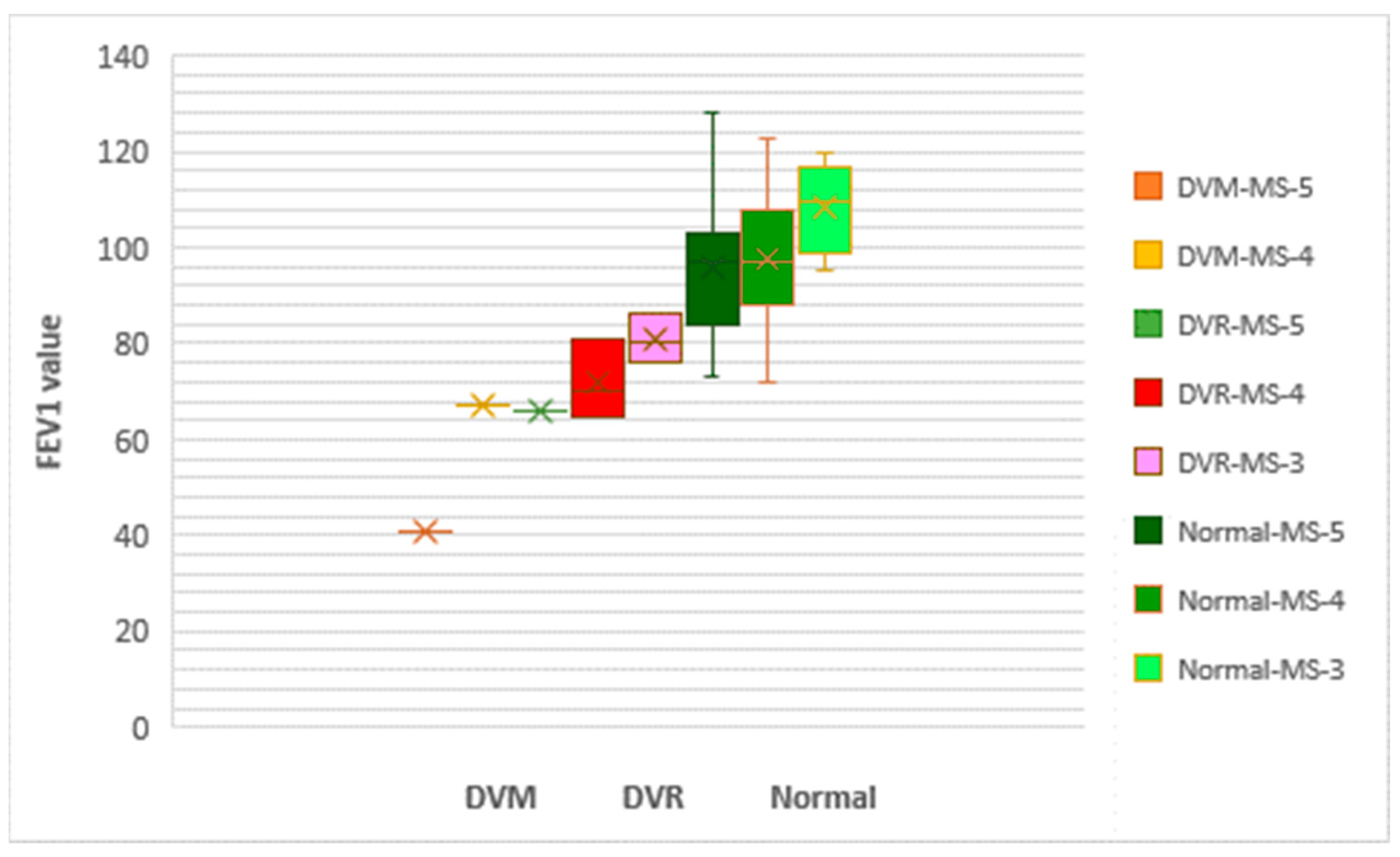

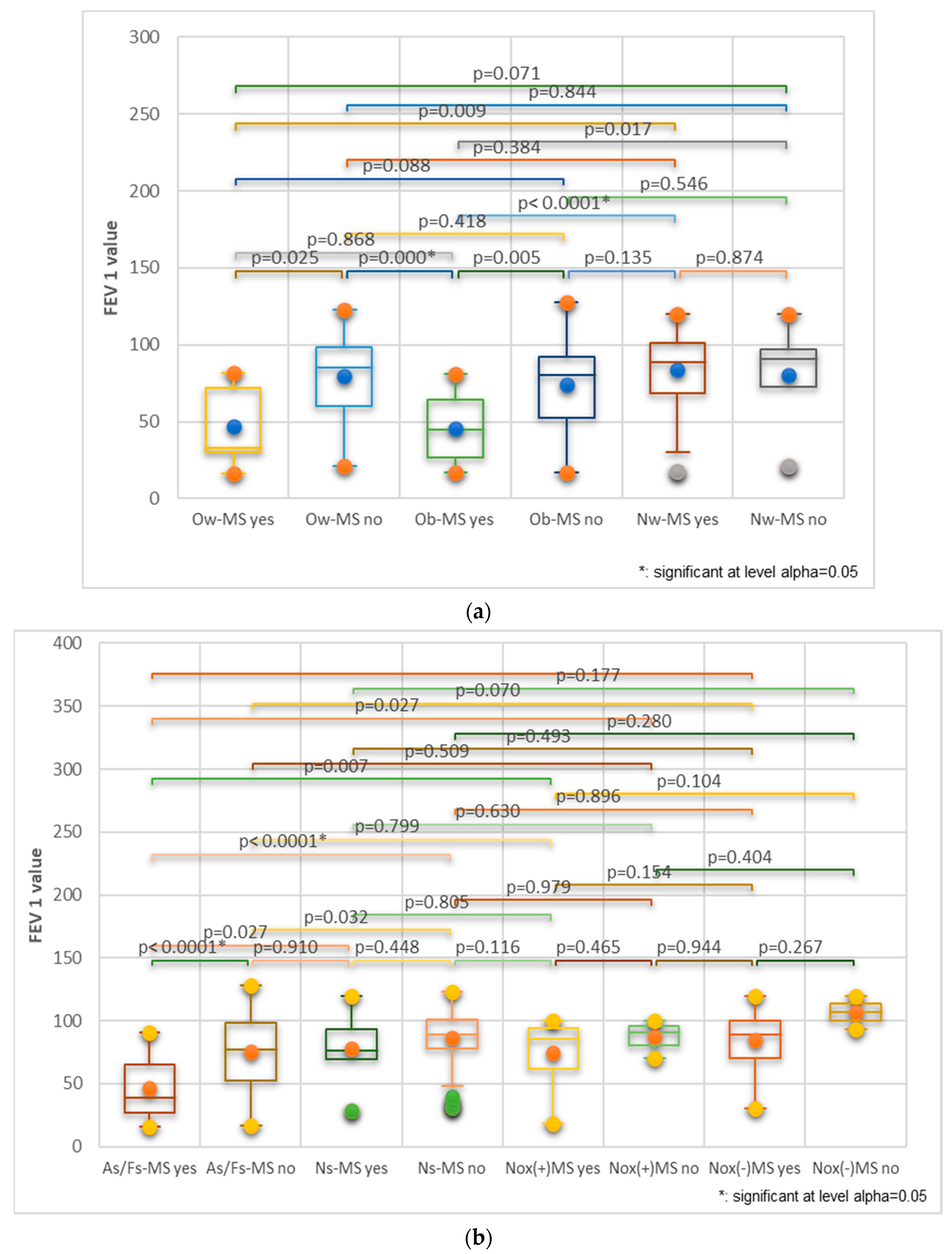
| Variable | Categories | Total | M | F | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | T/M | M/F | T/F | ||
| Age group | 66–75 | 25.00 | 31.25 | 16.00 | 31.37 | 9.00 | 31.03 | 0.30 | 0.22 | 0.05 |
| 56–65 | 27.00 | 33.75 | 18.00 | 35.29 | 9.00 | 31.03 | ||||
| 46–55 | 14.00 | 17.50 | 9.00 | 17.65 | 5.00 | 17.24 | ||||
| <45 | 7.00 | 8.75 | 6.00 | 11.76 | 1.00 | 3.45 | ||||
| >75 | 7.00 | 8.75 | 2.00 | 3.92 | 5.00 | 17.24 | ||||
| BMI | Normal weight | 25.00 | 31.25 | 16.00 | 31.37 | 9.00 | 31.03 | 0.36 | 0.27 | 0.09 |
| Overweight | 25.00 | 31.25 | 15.00 | 29.41 | 10.00 | 34.48 | ||||
| Underweight | 3.00 | 3.75 | 2.00 | 3.92 | 1.00 | 3.45 | ||||
| Obesity I | 18.00 | 22.50 | 12.00 | 23.53 | 6.00 | 20.69 | ||||
| Obesity II | 5.00 | 6.25 | 3.00 | 5.88 | 2.00 | 6.90 | ||||
| Obesity III | 4.00 | 5.00 | 3.00 | 5.88 | 1.00 | 3.45 | ||||
| Smoking status | former smoker | 29.00 | 36.25 | 25.00 | 49.02 | 4.00 | 13.79 | 0.17 | 0.37 | 0.02 * |
| active smoker | 26.00 | 32.50 | 20.00 | 39.22 | 6.00 | 20.69 | ||||
| never smoker | 25.00 | 31.25 | 6.00 | 11.76 | 19.00 | 65.52 | ||||
| Exposure to respiratory noxious | no | 45.00 | 56.25 | 25.00 | 49.02 | 20.00 | 68.97 | 0.10 | 0.18 | 0.07 |
| yes | 35.00 | 43.75 | 26.00 | 50.98 | 9.00 | 31.03 | ||||
| MS status | no | 27.00 | 33.75 | 19.00 | 37.25 | 8.00 | 27.59 | 0.58 | 0.24 | 0.16 |
| MS conditions | MS = 0 | 3.00 | 3.75 | 2.00 | 3.92 | 1.00 | 3.45 | |||
| MS = 1 | 5.00 | 6.25 | 5.00 | 9.80 | 0.00 | 0.00 | ||||
| MS = 2 | 19.00 | 23.75 | 12.00 | 23.53 | 7.00 | 24.14 | ||||
| MS status | yes | 53.00 | 66.25 | 32.00 | 62.75 | 21.00 | 72.41 | 0.37 | 0.44 | 0.16 |
| MS conditions | MS = 3 | 15.00 | 18.75 | 8.00 | 15.69 | 7.00 | 24.14 | |||
| MS = 4 | 12.00 | 15.00 | 9.00 | 17.65 | 3.00 | 10.34 | ||||
| MS = 5 | 26.00 | 32.50 | 15.00 | 29.41 | 11.00 | 37.93 | ||||
| Residence | Rural | 45.00 | 56.25 | 30.00 | 58.82 | 15.00 | 51.72 | 0.16 | 0.13 | 0.03 * |
| Urban | 35.00 | 43.75 | 21.00 | 41.18 | 14.00 | 48.28 | ||||
| Level of education | General/primary education (without a baccalaureate degree) | 42.00 | 52.50 | 27.00 | 52.94 | 15.00 | 51.72 | 0.53 | 0.44 | 0.23 |
| Secondary education (baccalaureate diploma) | 13.00 | 16.25 | 9.00 | 17.65 | 4.00 | 13.79 | ||||
| Post-secondary studies | 21.00 | 26.25 | 13.00 | 25.49 | 8.00 | 27.59 | ||||
| Postgraduate studies (master, residency, doctorate, other specialization) | 2.00 | 2.50 | 1.00 | 1.96 | 1.00 | 3.45 | ||||
| Higher education (bachelor’s degree) | 2.00 | 2.50 | 1.00 | 1.96 | 1.00 | 3.45 | ||||
| Occupation Status | Retired | 4.00 | 5.00 | 2.00 | 3.92 | 2.00 | 6.90 | 0.62 | 0.54 | 0.34 |
| Householder | 8.00 | 10.00 | 5.00 | 9.80 | 3.00 | 10.34 | ||||
| Employed/I go to work every day | 14.00 | 17.50 | 9.00 | 17.65 | 5.00 | 17.24 | ||||
| Socially assisted | 53.00 | 66.25 | 34.00 | 66.67 | 19.00 | 65.52 | ||||
| Unemployed | 1.00 | 1.25 | 1.00 | 1.96 | 0.00 | 0.00 | ||||
| Alcohol consumption | No | 51 | 63.75 | 26 | 50.98 | 25 | 86.20 | 0.50 | 0.46 | 0.31 |
| Yes, chronic | 10 | 12.50 | 10 | 19.60 | 0 | 0.00 | ||||
| Yes, occasionally | 19 | 23.75 | 15 | 29.41 | 4 | 13.79 | ||||
| MS Score | T | MS-0 | MS-1 | MS-2 | MS-3 | MS-4 | MS-5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency/Relative Frequency | N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||
| Median Age (years) | 62.5 | 61.3 | 56.8 | 56.0 | 66.4 | 63.3 | 66.0 | |||||||||
| Gender | F | 29.0 | 36.3 | 1.0 | 33.3 | 0.0 | 0.0 | 7.0 | 36.8 | 7.0 | 46.7 | 3.0 | 25.0 | 11.0 | 42.3 | |
| M | 51.0 | 63.8 | 2.0 | 66.7 | 5.0 | 100.0 | 12.0 | 63.2 | 8.0 | 53.3 | 9.0 | 75.0 | 15.0 | 57.7 | ||
| Body weight | normal weight | 25.0 | 31.3 | 3.0 | 100.0 | 3.0 | 60.0 | 9.0 | 47.4 | 8.0 | 53.3 | 2.0 | 16.7 | 0.0 | 0.0 | |
| obesity I | 18.0 | 22.5 | 0.0 | 0.0 | 0.0 | 0.0 | 4.0 | 21.1 | 2.0 | 13.3 | 3.0 | 25.0 | 9.0 | 34.6 | ||
| obesity II | 5.0 | 6.3 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 5.3 | 2.0 | 13.3 | 0.0 | 0.0 | 2.0 | 7.7 | ||
| obesity III | 4.0 | 5.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 8.3 | 3.0 | 11.5 | ||
| overweight | 25.0 | 31.3 | 0.0 | 0.0 | 1.0 | 20.0 | 4.0 | 21.1 | 2.0 | 13.3 | 6.0 | 50.0 | 12.0 | 46.2 | ||
| underweight | 3.0 | 3.8 | 0.0 | 0.0 | 1.0 | 20.0 | 1.0 | 5.3 | 1.0 | 6.7 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Spirometry | DVM | mild | 1.0 | 1.3 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 5.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| moderate | 4.0 | 5.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.0 | 13.3 | 0.0 | 0.0 | 2.0 | 7.7 | ||
| moderate-severe | 7.0 | 8.8 | 1.0 | 33.3 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 6.7 | 2.0 | 16.7 | 3.0 | 11.5 | ||
| severe | 14.0 | 17.5 | 0.0 | 0.0 | 0.0 | 0.0 | 3.0 | 15.8 | 3.0 | 20.0 | 3.0 | 25.0 | 5.0 | 19.2 | ||
| very severe | 19.0 | 23.8 | 0.0 | 0.0 | 3.0 | 60.0 | 7.0 | 36.8 | 3.0 | 20.0 | 3.0 | 25.0 | 3.0 | 11.5 | ||
| DVO | mild | 3.0 | 3.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 8.3 | 2.0 | 7.7 | |
| moderate | 5.0 | 6.3 | 2.0 | 66.7 | 1.0 | 20.0 | 1.0 | 5.3 | 1.0 | 6.7 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| moderate-severe | 1.0 | 1.3 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 5.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| DVR | mild | 2.0 | 2.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 6.7 | 0.0 | 0.0 | 1.0 | 3.8 | |
| moderate | 4.0 | 5.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 8.3 | 3.0 | 11.5 | ||
| moderate-severe | 2.0 | 2.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.0 | 7.7 | ||
| severe | 2.0 | 2.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 8.3 | 1.0 | 3.8 | ||
| Normal | 16.0 | 20.0 | 0.0 | 0.0 | 1.0 | 20.0 | 6.0 | 31.6 | 4.0 | 26.7 | 1.0 | 8.3 | 4.0 | 15.4 | ||
| Variable | Categories | Total | F | M | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | T/F | F/M | M/T | ||
| Age group | 36–45 | 5 | 6.25 | 2 | 4.55 | 3 | 8.33 | 0.25 | 0.68 | 0.16 |
| 46–55 | 21 | 26.25 | 9 | 20.45 | 12 | 33.33 | ||||
| 56–65 | 30 | 37.50 | 16 | 36.36 | 14 | 38.89 | ||||
| 66–75 | 21 | 26.25 | 14 | 31.82 | 7 | 19.44 | ||||
| 76–85 | 3 | 3.75 | 3 | 6.82 | 0 | 0 | ||||
| Total | - | 80 | - | 44 | 55 | 36 | 45 | |||
| BMI status | Normal weight | 12 | 15.00 | 9 | 20.45 | 3 | 8.33 | 0.22 | 0.70 | 0.16 |
| Obesity I | 23 | 28.75 | 17 | 38.64 | 6 | 16.67 | ||||
| Obesity II | 9 | 11.25 | 4 | 9.09 | 5 | 13.89 | ||||
| Obesity III | 5 | 6.25 | 3 | 6.2 | 2 | 5.56 | ||||
| Overweight | 31 | 38.75 | 11 | 25.00 | 20 | 55.56 | ||||
| Smoking status | active smoker | 18 | 22.50 | 6 | 13.64 | 12 | 33.33 | 0.30 | 0.74 | 0.09 |
| former smoker | 22 | 27.50 | 8 | 18.18 | 14 | 38.89 | ||||
| never smoker | 40 | 50.00 | 30 | 68.18 | 10 | 27.78 | ||||
| Exposure to respiratory noxious | No | 65 | 81.25 | 38 | 86.36 | 27 | 75.00 | 0.30 | 0.74 | 0.09 |
| Yes | 15 | 18.75 | 6 | 13.64 | 9 | 25.00 | ||||
| MS conditions | MS-3 | 11 | 13.75 | 5 | 45.45 | 6 | 54.55 | 0.34 | 0.72 | 0.20 |
| MS-4 | 26 | 32.50 | 12 | 46.15 | 14 | 53.85 | ||||
| MS-5 | 43 | 53.75 | 27 | 62.79 | 16 | 37.21 | ||||
| Level of education | General/primary education (without a baccalaureate degree) | 39 | 48.75 | 22 | 50.00 | 17 | 47.22 | 0.22 | 0.70 | 0.16 |
| Secondary education (baccalaureate diploma) | 8 | 10.00 | 5 | 11.36 | 3 | 8.33 | ||||
| Post-secondary studies | 19 | 23.75 | 10 | 22.72 | 9 | 25.00 | ||||
| Postgraduate studies (master, residency, doctorate, other specialization) | 3 | 3.75 | 1 | 2.27 | 2 | 5.56 | ||||
| Higher education (bachelor’s degree) | 11 | 13.75 | 6 | 13.63 | 5 | 13.89 | ||||
| Occupation status | Retired | 50 | 62.5 | 32 | 72.72 | 18 | 50.00 | 0.25 | 0.68 | 0.16 |
| Employed/I go to work every day | 14 | 17.5 | 6 | 13.63 | 8 | 22.22 | ||||
| Householder | 12 | 15.00 | 4 | 9.03 | 8 | 22.22 | ||||
| Socially assisted | 3 | 3.75 | 1 | 2.27 | 2 | 5.56 | ||||
| Unemployed | 1 | 1.25 | 1 | 2.27 | 0 | 0 | ||||
| Residence | Rural | 34 | 42.5 | 19 | 43.18 | 15 | 41,67 | 0.30 | 0.74 | 0.09 |
| Urban | 46 | 57.5 | 25 | 56.81 | 21 | 58.33 | ||||
| Alcohol consumption | No | 46 | 57.5 | 36 | 81.81 | 10 | 27.78 | 0.34 | 0.72 | 0.20 |
| Yes, chronic | 10 | 12.5 | 0 | 0 | 10 | 27.78 | ||||
| Yes, occasionally | 24 | 30 | 8 | 18.18 | 16 | 44.44 | ||||
| MS Score | T | MS-3 | MS-4 | MS-5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency/Relative Frequency | N | % | N | % | N | % | N | % | ||
| Median Age (years) | 60.1 | 61.5 | 57.8 | 61.2 | ||||||
| Gender | F | 44.0 | 55.0 | 5.0 | 45.5 | 12.0 | 46.2 | 27.0 | 62.8 | |
| M | 36.0 | 45.0 | 6.0 | 54.5 | 14.0 | 53.8 | 16.0 | 37.2 | ||
| Body weight | normal weight | 12.0 | 15.0 | 3.0 | 27.3 | 7.0 | 26.9 | 2.0 | 4.7 | |
| obesity I | 23.0 | 28.8 | 3.0 | 27.3 | 4.0 | 15.4 | 16.0 | 37.2 | ||
| obesity II | 8.0 | 10.0 | 1.0 | 9.1 | 3.0 | 11.5 | 4.0 | 9.3 | ||
| obesity II | 1.0 | 1.3 | 0.0 | 0.0 | 1.0 | 3.8 | 0.0 | 0.0 | ||
| obesity III | 5.0 | 6.3 | 1.0 | 9.1 | 1.0 | 3.8 | 3.0 | 7.0 | ||
| overweight | 31.0 | 38.8 | 3.0 | 27.3 | 10.0 | 38.5 | 18.0 | 41.9 | ||
| Spirometry | DVM | moderate | 1.0 | 1.3 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 2.3 |
| severe | 1.0 | 1.3 | 0.0 | 0.0 | 1.0 | 3.8 | 0.0 | 0.0 | ||
| DVR | mild | 4.0 | 5.0 | 0.0 | 0.0 | 3.0 | 11.5 | 1.0 | 2.3 | |
| moderate | 3.0 | 3.8 | 1.0 | 9.1 | 0.0 | 0.0 | 2.0 | 4.7 | ||
| Normal | 71.0 | 88.8 | 10.0 | 90.9 | 22.0 | 84.6 | 39.0 | 90.7 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Streba, L.; Popovici, V.; Mihai, A.; Mititelu, M.; Lupu, C.E.; Matei, M.; Vladu, I.M.; Iovănescu, M.L.; Cioboată, R.; Călărașu, C.; et al. Integrative Approach to Risk Factors in Simple Chronic Obstructive Airway Diseases of the Lung or Associated with Metabolic Syndrome—Analysis and Prediction. Nutrients 2024, 16, 1851. https://doi.org/10.3390/nu16121851
Streba L, Popovici V, Mihai A, Mititelu M, Lupu CE, Matei M, Vladu IM, Iovănescu ML, Cioboată R, Călărașu C, et al. Integrative Approach to Risk Factors in Simple Chronic Obstructive Airway Diseases of the Lung or Associated with Metabolic Syndrome—Analysis and Prediction. Nutrients. 2024; 16(12):1851. https://doi.org/10.3390/nu16121851
Chicago/Turabian StyleStreba, Liliana, Violeta Popovici, Andreea Mihai, Magdalena Mititelu, Carmen Elena Lupu, Marius Matei, Ionela Mihaela Vladu, Maria Livia Iovănescu, Ramona Cioboată, Cristina Călărașu, and et al. 2024. "Integrative Approach to Risk Factors in Simple Chronic Obstructive Airway Diseases of the Lung or Associated with Metabolic Syndrome—Analysis and Prediction" Nutrients 16, no. 12: 1851. https://doi.org/10.3390/nu16121851
APA StyleStreba, L., Popovici, V., Mihai, A., Mititelu, M., Lupu, C. E., Matei, M., Vladu, I. M., Iovănescu, M. L., Cioboată, R., Călărașu, C., Busnatu, Ș. S., & Streba, C.-T. (2024). Integrative Approach to Risk Factors in Simple Chronic Obstructive Airway Diseases of the Lung or Associated with Metabolic Syndrome—Analysis and Prediction. Nutrients, 16(12), 1851. https://doi.org/10.3390/nu16121851










