Marginal Zinc Deficiency Promotes Pancreatic Islet Enlargement While Zinc Supplementation Improves the Pancreatic Insulin Response in Zucker Diabetic Fatty Rats
Abstract
1. Introduction
2. Methods
2.1. Animals and Diets
2.2. Glycemic Control and Glucose Tolerance
2.3. Body Composition
2.4. Tissue Collection
2.5. Pancreatic Islet Insulin Immunostaining and Liver Lipid Concentration
2.6. Serum and Urine Biochemistry
2.7. Mineral Status
2.8. Western Immunoblotting
2.9. Statistical Analyses
3. Results
3.1. Feed Intake, Body Weight and Body Composition
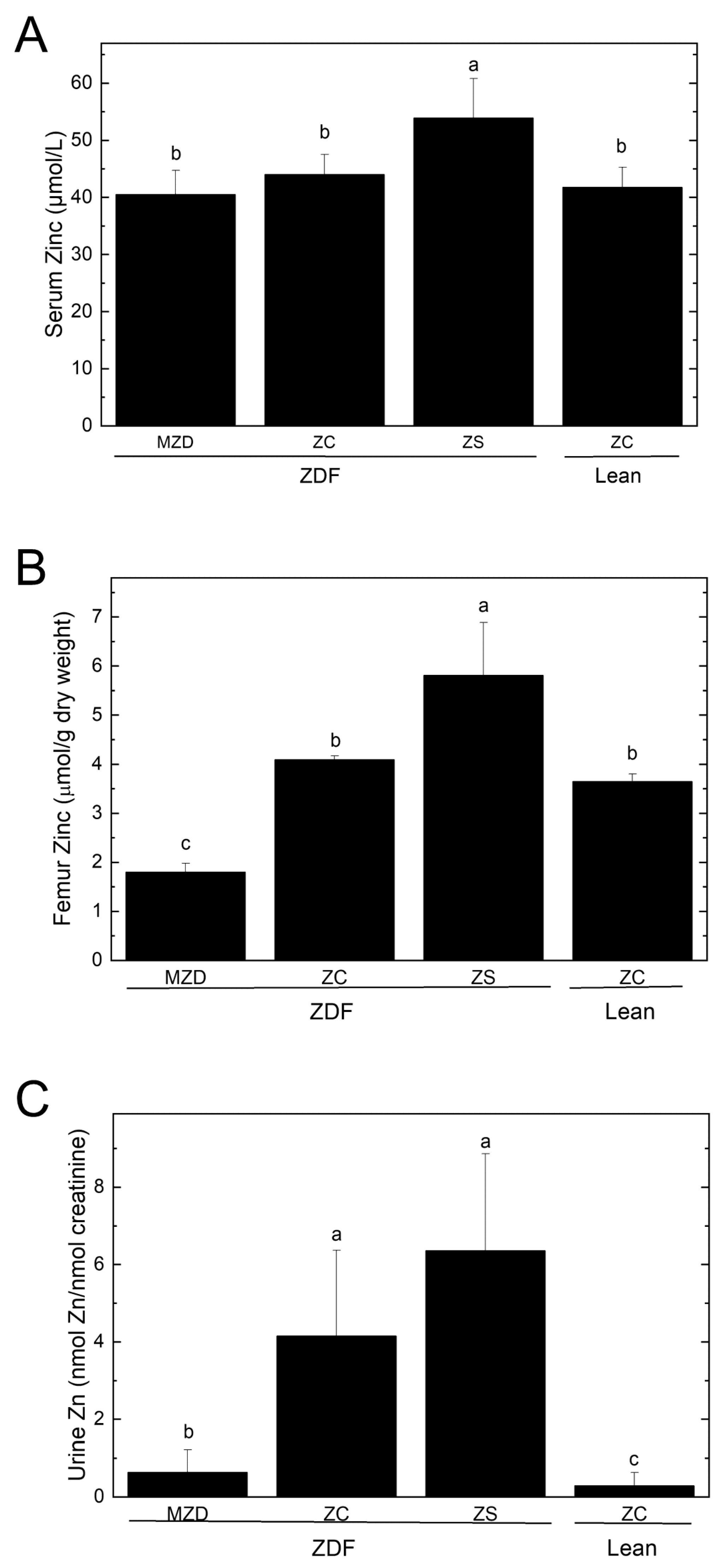
3.2. Zinc Status
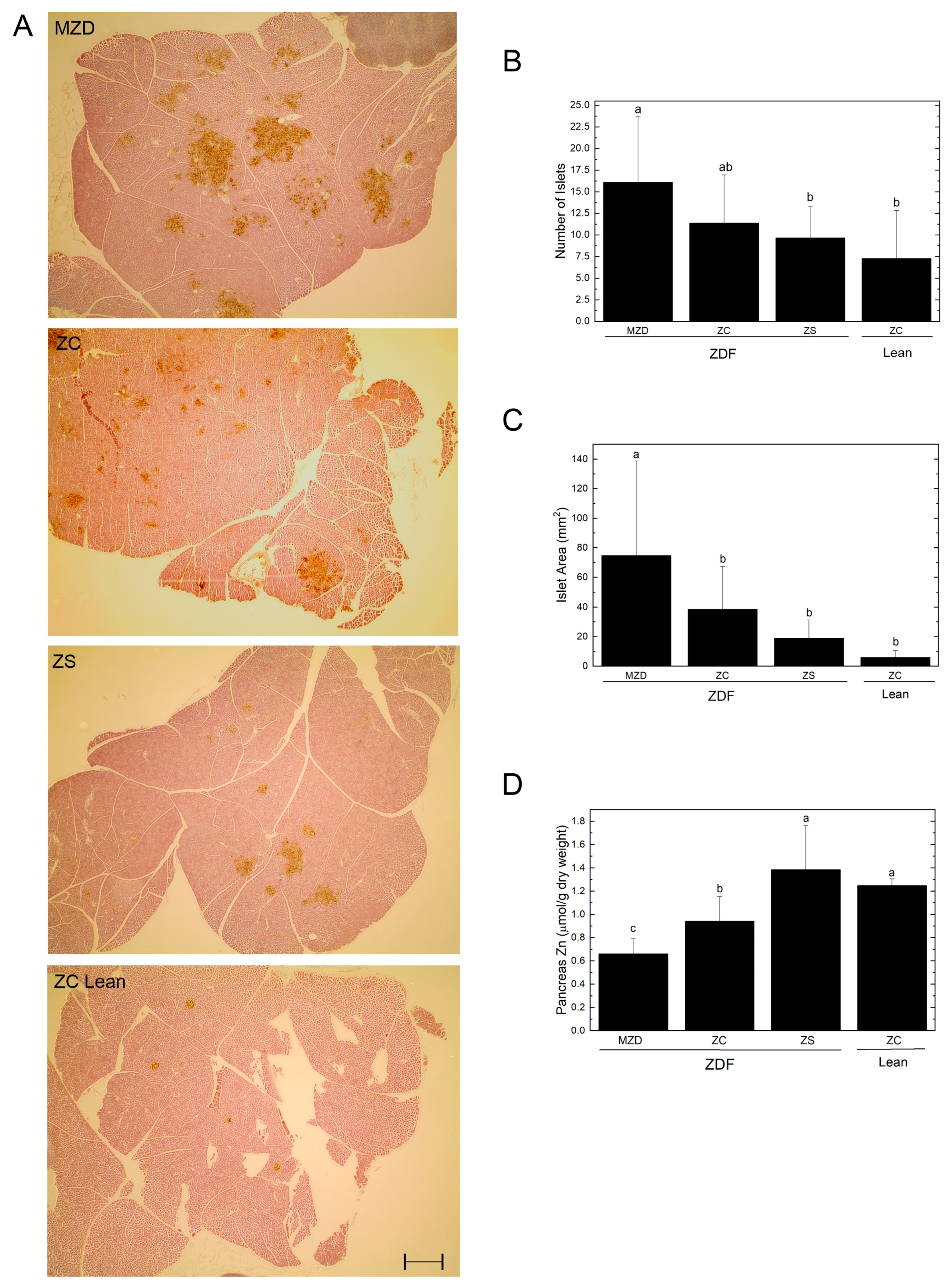
3.3. Pancreatic Islets and Zinc
3.4. Glycemic Control
3.5. Hepatic Steatosis and Circulating Lipids
3.6. Renal Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lovic, D.; Piperidou, A.; Zografou, I.; Grassos, H.; Pittaras, A.; Manolis, A. The Growing Epidemic of Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 104–109. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Dominguez, M.; Golestaneh, L. Diabetic Kidney Disease: An Update. Med. Clin. N. Am. 2023, 107, 689–705. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Maechler, P.; Gjinovci, A.; Herrera, P.L.; Wollheim, C.B. Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat. Cell Biol. 2003, 5, 330–335. [Google Scholar] [CrossRef]
- Li, Y.V. Zinc and insulin in pancreatic beta-cells. Endocrine 2014, 45, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.G. Zinc, the pancreas, and diabetes: Insights from rodent studies and future directions. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2005, 18, 305–312. [Google Scholar] [CrossRef]
- Jansen, J.; Karges, W.; Rink, L. Zinc and diabetes—Clinical links and molecular mechanisms. J. Nutr. Biochem. 2009, 20, 399–417. [Google Scholar] [CrossRef]
- Chen, B.; Yu, P.; Chan, W.N.; Xie, F.; Zhang, Y.; Liang, L.; Leung, K.T.; Lo, K.W.; Yu, J.; Tse, G.M.K.; et al. Cellular zinc metabolism and zinc signaling: From biological functions to diseases and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 6. [Google Scholar] [CrossRef]
- Chausmer, A.B. Zinc, insulin and diabetes. J. Am. Coll. Nutr. 1998, 17, 109–115. [Google Scholar] [CrossRef]
- Tamura, Y. The Role of Zinc Homeostasis in the Prevention of Diabetes Mellitus and Cardiovascular Diseases. J. Atheroscler. Thromb. 2021, 28, 1109–1122. [Google Scholar] [CrossRef]
- Ghaedi, K.; Ghasempour, D.; Jowshan, M.; Zheng, M.; Ghobadi, S.; Jafari, A. Effect of zinc supplementation in the management of type 2 diabetes: A grading of recommendations assessment, development, and evaluation-assessed, dose-response meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; van Dam, R.M.; Willett, W.C.; Hu, F.B. Prospective study of zinc intake and risk of type 2 diabetes in women. Diabetes Care 2009, 32, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, W.; Zheng, W.; Fang, X.; Chen, L.; Rink, L.; Min, J.; Wang, F. Zinc supplementation improves glycemic control for diabetes prevention and management: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2019, 110, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.L.; Watson, R.N.; Loomes, K.M.; Phillips, A.R.; Cooper, G.J. Insulin resistance in the Zucker diabetic fatty rat: A metabolic characterisation of obese and lean phenotypes. Acta Diabetol. 2005, 42, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Noto, A.; Zahradka, P.; Ryz, N.R.; Yurkova, N.; Xie, X.; Taylor, C.G. Dietary conjugated linoleic acid preserves pancreatic function and reduces inflammatory markers in obese, insulin-resistant rats. Metab. Clin. Exp. 2007, 56, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Stringer, D.M.; Zahradka, P.; Declercq, V.C.; Ryz, N.R.; Diakiw, R.; Burr, L.L.; Xie, X.; Taylor, C.G. Modulation of lipid droplet size and lipid droplet proteins by trans-10,cis-12 conjugated linoleic acid parallels improvements in hepatic steatosis in obese, insulin-resistant rats. Biochim. Biophys. Acta 2010, 1801, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Charles River Laboratories. Available online: https://www.criver.com/products-services/find-model/zdf-rat-obese?region=24 (accessed on 24 April 2024).
- Brouns, F.; Bjorck, I.; Frayn, K.N.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T.M. Glycaemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.N. Does the Lee obesity index measure general obesity? Physiol. Behav. 1980, 25, 313–315. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Lepage, L.M.; Giesbrecht, J.A.; Taylor, C.G. Expression of T lymphocyte p56(lck), a zinc-finger signal transduction protein, is elevated by dietary zinc deficiency and diet restriction in mice. J. Nutr. 1999, 129, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh, A.; Zahradka, P.; Taylor, C.G. Trans-10,cis-12 conjugated linoleic acid (t10-c12 CLA) treatment and caloric restriction differentially affect adipocyte cell turnover in obese and lean mice. J. Nutr. Biochem. 2017, 49, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Rutter, G.A.; Chabosseau, P.; Bellomo, E.A.; Maret, W.; Mitchell, R.K.; Hodson, D.J.; Solomou, A.; Hu, M. Intracellular zinc in insulin secretion and action: A determinant of diabetes risk? Proc. Nutr. Soc. 2016, 75, 61–72. [Google Scholar] [CrossRef]
- Maret, W. Zinc in Pancreatic Islet Biology, Insulin Sensitivity, and Diabetes. Prev. Nutr. Food Sci. 2017, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chabosseau, P.; Rutter, G.A. Zinc and diabetes. Arch. Biochem. Biophys. 2016, 611, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, L.G.; Stoltenberg, M.; Doering, P.; Flyvbjerg, A.; Rungby, J. Zinc ions in the endocrine and exocrine pancreas of zinc deficient rats. Histol. Histopathol. 2006, 21, 619–625. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, G.B.; Brandão-Lima, P.N.; Maia, C.S.; Barbosa, K.B.; Pires, L.V. Zinc’s role in the glycemic control of patients with type 2 diabetes: A systematic review. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2017, 30, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Hennigar, S.R.; Lieberman, H.R.; Fulgoni, V.L., 3rd; McClung, J.P. Serum Zinc Concentrations in the US Population Are Related to Sex, Age, and Time of Blood Draw but Not Dietary or Supplemental Zinc. J. Nutr. 2018, 148, 1341–1351. [Google Scholar] [CrossRef]
- Hall, A.G.; King, J.C. Zinc Fortification: Current Trends and Strategies. Nutrients 2022, 14, 3895. [Google Scholar] [CrossRef]
- Simon, S.F.; Taylor, C.G. Dietary zinc supplementation attenuates hyperglycemia in db/db mice. Exp. Biol. Med. 2001, 226, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Begin-Heick, N.; Dalpe-Scott, M.; Rowe, J.; Heick, H.M. Zinc supplementation attenuates insulin secretory activity in pancreatic islets of the ob/ob mouse. Diabetes 1985, 34, 179–184. [Google Scholar] [CrossRef]
- Tang, X.; Shay, N.F. Zinc has an insulin-like effect on glucose transport mediated by phosphoinositol-3-kinase and Akt in 3T3-L1 fibroblasts and adipocytes. J. Nutr. 2001, 131, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Peng-Winkler, Y.; Wessels, I.; Rink, L.; Fischer, H.J. Zinc Levels Affect the Metabolic Switch of T Cells by Modulating Glucose Uptake and Insulin Receptor Signaling. Mol. Nutr. Food Res. 2022, 66, e2100944. [Google Scholar] [CrossRef]
- Miranda, E.R.; Dey, C.S. Effect of chromium and zinc on insulin signaling in skeletal muscle cells. Biol. Trace Elem. Res. 2004, 101, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.W.; Chenu, C.; Ripoche, P. Zinc inhibition of glucose uptake in brush border membrane vesicles from pig small intestine. Pflug. Arch. Eur. J. Physiol. 1989, 415, 165–171. [Google Scholar] [CrossRef]
- Martín, M.G.; Wang, J.; Solorzano-Vargas, R.S.; Lam, J.T.; Turk, E.; Wright, E.M. Regulation of the human Na+-glucose cotransporter gene, SGLT1, by HNF-1 and Sp1. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, G591–G603. [Google Scholar] [CrossRef]
- Kothinti, R.; Blodgett, A.; Tabatabai, N.M.; Petering, D.H. Zinc finger transcription factor Zn3-Sp1 reactions with Cd2+. Chem. Res. Toxicol. 2010, 23, 405–412. [Google Scholar] [CrossRef][Green Version]
- Kothinti, R.K.; Blodgett, A.B.; Petering, D.H.; Tabatabai, N.M. Cadmium down-regulation of kidney Sp1 binding to mouse SGLT1 and SGLT2 gene promoters: Possible reaction of cadmium with the zinc finger domain of Sp1. Toxicol. Appl. Pharmacol. 2010, 244, 254–262. [Google Scholar] [CrossRef]
- L’Abbé, M.R.; Fischer, P.W. The effects of dietary zinc on the activity of copper-requiring metalloenzymes in the rat. J. Nutr. 1984, 114, 823–828. [Google Scholar] [CrossRef]
- Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Subcommittee of Interpretation, Uses of Dietary Reference Intakes, Subcommittee on Upper Reference Levels of Nutrients, & Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Coulston, L.; Dandona, P. Insulin-like effect of zinc on adipocytes. Diabetes 1980, 29, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.; Xiang, W.; Zhang, Y.; Sun, K.; Jiang, X. The association between serum zinc level and overweight/obesity: A meta-analysis. Eur. J. Nutr. 2019, 58, 2971–2982. [Google Scholar] [CrossRef] [PubMed]
- Tallman, D.L.; Taylor, C.G. Effects of dietary fat and zinc on adiposity, serum leptin and adipose fatty acid composition in C57BL/6J mice. J. Nutr. Biochem. 2003, 14, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metab. Clin. Exp. 2019, 92, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Akdas, S.; Yazihan, N. Serum zinc level and dietary zinc intake status in non-alcoholic fatty liver disease: A meta-analysis and systematic review. Hepatol. Forum 2020, 1, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Kosari, F.; Jamali, R.; Ramim, T.; Mosavi Jahan Abad, E. The Correlation between Serum Zinc Level and Liver Histology in Non-Alcoholic Steatohepatitis. Iran. J. Pathol. 2019, 14, 17–25. [Google Scholar] [CrossRef]
- Chen, S.D.; Zhang, H.; Rios, R.S.; Li, Y.Y.; Zhu, P.W.; Jin, Y.; Ma, H.L.; Tang, L.J.; Li, G.; Huang, O.Y.; et al. J-shaped relationship between serum zinc levels and the severity of hepatic necro-inflammation in patients with MAFLD. Nutr. Metab. Cardiovasc. Dis. NMCD 2022, 32, 1259–1265. [Google Scholar] [CrossRef]
- Bolatimi, O.E.; Head, K.Z.; Luo, J.; Gripshover, T.C.; Lin, Q.; Adiele, N.V.; Watson, W.H.; Wilkerson, C.; Cai, L.; Cave, M.C.; et al. Can Zinc Supplementation Attenuate High Fat Diet-Induced Non-Alcoholic Fatty Liver Disease? Int. J. Mol. Sci. 2023, 24, 1763. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Nikbaf-Shandiz, M.; Pashayee-Khamene, F.; Bagheri, R.; Goudarzi, K.; Hosseinnia, N.V.; Dolatshahi, S.; Omran, H.S.; Amirani, N.; Ashtary-Larky, D.; et al. Zinc Supplementation in Individuals with Prediabetes and type 2 Diabetes: A GRADE-Assessed Systematic Review and Dose-Response Meta-analysis. Biol. Trace Elem. Res. 2024, 202, 2966–2990. [Google Scholar] [CrossRef]
- Asbaghi, O.; Sadeghian, M.; Fouladvand, F.; Panahande, B.; Nasiri, M.; Khodadost, M.; Shokri, A.; Pirouzi, A.; Sadeghi, O. Effects of zinc supplementation on lipid profile in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. NMCD 2020, 30, 1260–1271. [Google Scholar] [CrossRef]

| MZD ZDF | ZC ZDF | ZS ZDF | ZC Lean | |
|---|---|---|---|---|
| Daily Feed Intake (g/d) | 26.6 ± 4.5 a | 28.4 ± 5.4 a | 27.7 ± 5.8 a | 21.1 ± 3.8 b |
| Final Body Weight (g) | 425 ± 16 a | 424 ± 23 a | 420 ± 20 a | 321 ± 5 b |
| Whole-Body Composition 1 | ||||
| Fat Mass (g) | 164 ± 6 a | 168 ± 10 a | 169 ± 10 a | 28 ± 4 b |
| Lean Mass (g) | 247 ± 19 a | 239 ± 21 a | 242 ± 12 a | 280 ± 12 b |
| Free Water (g) | 1.36 ± 0.65 | 1.31 ± 0.26 | 1.09 ± 0.50 | 1.00 ± 0.21 |
| Total Water (g) | 209 ± 16 a | 202 ± 18 a | 205 ± 10 a | 235 ± 12 b |
| Fat Pads | ||||
| Epididymal Fat (g/100 g bwt) | 2.16 ± 0.18 a | 2.18 ± 0.16 a | 2.21 ± 0.15 a | 0.89 ± 0.12 b |
| Peri-renal Fat (g/100 g bwt) | 3.46 ± 0.17 a | 3.64 ± 0.24 b | 3.80 ± 0.19 b | 1.30 ± 0.14 c |
| Mesenteric Fat (g/100 g bwt) | 1.32 ± 0.11 a | 1.35 ± 0.18 a | 1.42 ± 0.18 a | 0.56 ± 0.07 b |
| Visceral Fat 2 (g/100 g bwt) | 6.94 ± 0.31 b | 7.17 ± 0.27 ab | 7.43 ± 0.37 a | 2.76 ± 0.26 c |
| Lee’s Index (g0.33/mm) | 0.0345 ± 0.0004 b | 0.0350 ± 0.0004 a | 0.0345 ± 0.0003 b | 0.0310 ± 0.0005 c |
| Pancreas Weight | ||||
| (g) | 0.99 ± 0.16 b | 0.90 ± 0.18 b | 1.03 ± 0.16 b | 1.46 ± 0.12 a |
| (g/100 g bwt) | 0.234 ± 0.036 b | 0.214 ± 0.049 b | 0.245 ± 0.035 b | 0.457 ± 0.041 a |
| MZD ZDF | ZC ZDF | ZS ZDF | ZC Lean | |
|---|---|---|---|---|
| Liver | ||||
| Zinc Concentration (µmol/g dry weight) | 1.02 ± 0.11 b | 1.01 ± 0.15 b | 1.06 ± 0.14 b | 1.47 ± 0.11 a |
| Total Zinc Content (µmol/liver) | 6.64 ± 0.77 a | 7.34 ± 0.65 a | 6.81 ± 0.90 a | 4.11 ± 0.0.30 b |
| Copper Concentration (µmol/g dry weight) | 0.215 ± 0.034 b | 0.203 ± 0.032 b | 0.148 ± 0.029 c | 0.259 ± 0.047 a |
| Total Copper Content (µmol/liver) | 1.397 ± 0.144 a | 1.484 ± 0.210 a | 0.947 ± 0.164 b | 0.727 ± 0.119 c |
| Iron Concentration (µmol/g dry weight) | 9.66 ± 2.68 ab | 7.61 ± 2.18 b | 8.25 ± 2.74 b | 11.00 ± 1.59 a |
| Total Iron Content (µmol/liver) | 64.3 ± 23.6 a | 54.3 ± 9.4 a | 53.7 ± 20.2 a | 30.9 ± 4.6 b |
| Epididymal Fat | ||||
| Zinc Concentration (μmol/g dry weight) | 0.190 ± 0.139 | 0.127 ± 0.062 | 0.148 ± 0.100 | 0.086 ± 0.024 |
| Total Zinc (µg/epididymal fat) | 13.4 ± 1.9 a | 13.3 ± 3.7 a | 13.4 ± 4.2 a | 4.5 ± 1.4 b |
| MZD ZDF | ZC ZDF | ZS ZDF | ZC Lean | |
|---|---|---|---|---|
| A. After a 5 h fast during the study | ||||
| Glucose (mmol/L) | ||||
| Week 0 | 7.14 ± 0.47 | 7.27 ± 0.64 | 7.08 ± 0.55 | 6.49 ± 0.45 |
| Week 4 | 6.53 ± 0.69 | 7.06 ± 1.31 | 7.31 ± 1.42 | 6.13 ± 0.36 |
| Week 8 | 6.58 ± 1.27 | 7.83 ± 1.19 | 7.83 ± 1.37 | 6.80 ± 0.46 |
| Insulin (pmol/L) | ||||
| Week 0 | 434 ± 165 a | 456 ± 104 a | 383 ± 121 a | 43 ± 7 b |
| Week 4 | 1195 ± 533 a | 1670 ± 657 a | 1830 ± 740 a | 68 ± 14 b |
| Week 8 | 1706 ± 541 a | 1820 ± 579 a | 1630 ± 436 a | 76 ± 20 b |
| HOMA-IR (µU/mL × mmol/L) | ||||
| Week 0 | 20.2 ± 8.6 a | 21.6 ± 5.7 a | 17.3 ± 5.1 a | 1.8 ± 0.3 b |
| Week 4 | 50.4 ± 21.5 b | 66.7 ± 24.9 ab | 87.7 ± 45.2 a | 2.7 ± 0.6 c |
| Week 8 | 72.5 ± 30.09.5 a | 91.7 ± 33.8 a | 84.9 ± 38.6 a | 3.3 ± 0.8 b |
| HOMA-BCF (mU/mmol) | ||||
| Week 0 | 341 ± 111 a | 350 ± 76 a | 319 ± 121 a | 42 ± 10 b |
| Week 4 | 1185 ± 672 a | 1450 ± 573 a | 1495 ± 696 a | 76 ± 20 b |
| Week 8 | 1535 ± 669 a | 1283 ± 464 a | 1128 ± 269 a | 68 ± 23 b |
| B. After a 12–14 h fast at the end of week 8 | ||||
| Glucose (mmol/L) | 17.5 ± 1.8 a | 15.8 ± 3.3 a | 16.5 ± 2.0 a | 12.5 ± 1.6 b |
| Insulin (pmol/L) | 888 ± 201 a | 1055 ± 307 a | 920 ± 256 a | 46 ± 24 b |
| HOMA-IR (µU/mL × mmol/L) | 112 ± 37 a | 108 ± 37 a | 99 ± 33 a | 5 ± 3 b |
| HOMA-BCF (mU/mmol) | 180 ± 48 b | 261 ± 96 a | 206 ± 57 ab | 13 ± 7 c |
| MZD ZDF | ZC ZDF | ZS ZDF | ZC Lean | |
|---|---|---|---|---|
| Liver (g) | 20.6 ± 2.2 ab | 22.4 ± 2.8 a | 20.0 ± 1.6 b | 10.6 ± 0.4 c |
| Liver (g/100 g bwt) | 4.88 ± 0.48 a | 5.31 ± 0.75 a | 4.78 ± 0.44 a | 3.32 ± 0.15 b |
| Liver Lipid Concentration (mg lipid/g tissue) | 78.1 ± 29.3 a | 97.2 ± 33.7 a | 80.1 ± 24.8 a | 34.9 ± 5.7 b |
| Serum Total Cholesterol (mmol/L) | 5.89 ± 1.00 a | 6.25 ± 0.79 a | 6.04 ± 1.19 a | 2.65 ± 0.17 b |
| Serum LDL-C (mmol/L) | 0.424 ± 0.283 | 0.200 ± 0.172 | 0.362 ± 0.385 | 0.424 ± 0.065 |
| Serum HDL-C (mmol/L) | 3.78 ± 0.67 a | 2.08 ± 1.187 b | 2.20 ± 0.60 b | 2.38 ± 0.13 b |
| Serum TG (mmol/L) | 7.0 ± 2.2 b | 13.5 ± 4.8 a | 12.2 ± 3.6 a | 0.94 ± 0.28 c |
| Serum FFA (mmol/L) | 0.329 ± 0.061 | 0.317 ± 0.084 | 0.340 ± 0.057 | 0.283 ± 0.035 |
| MZD ZDF | ZC ZDF | ZS ZDF | ZC Lean | |
|---|---|---|---|---|
| Kidney Weights | ||||
| Left Kidney (g) | 1.47 ± 0.14 a | 1.45 ± 0.09 a | 1.47 ± 0.18 a | 1.24 ± 0.08 b |
| Left Kidney (g/100 g bwt) | 0.349 ± 0.035 | 0.345 ± 0.038 | 0.352 ± 0.040 | 0.388 ± 0.023 |
| Right Kidney (g) | 1.47 ± 0.14 a | 1.45 ± 0.09 a | 1.47 ± 0.18 a | 1.24 ± 0.08 b |
| Right Kidney (g/100 g bwt) | 0.361 ± 0.036 | 0.374 ± 0.044 | 0.382 ± 0.007 | 0.394 ± 0.002 |
| Urine Output (mL/5 h) | ||||
| Week 0 | 3.20 ± 0.76 | 3.06 ± 0.84 | 3.43 ± 1.25 | 1.67 ± 0.74 |
| Week 4 | 3.23 ± 1.05 | 4.02 ± 3.00 | 2.73 ± 1.24 | 2.76 ± 1.09 |
| Week 8 | 3.23 ± 1.55 | 3.96 ± 1.59 | 3.51 ± 1.55 | 3.67 ± 1.47 |
| Urinanalysis (Week 8) | ||||
| Creatinine (nmol/5 h) | 12.1 ± 2.9 | 10.8 ± 2.5 | 10.0 ± 3.4 | 12.9 ± 2.8 |
| Zinc (nmol/5 h) | 7.8 ± 6.6 b | 44.0 ± 22.5 a | 64.2 ± 29.2 a | 3.5 ± 3.2 c |
| Zinc (nmol/100 g bwt) | 1.8 ± 1.5 b | 10.4 ± 5.3 a | 15.3 ± 7.0 a | 1.1 ±1.0 b |
| Glucose (nmol/5 h) | 7.8 ± 9.2 b | 6.4 ± 2.9 b | 31.1 ± 24.1 a | 1.8 ± 1.1 c |
| Glucose/Creatinine (µmol/nmol) | 1757 ± 3042 b | 597 ± 325 b | 3064 ± 2318 a | 136 ± 61 c |
| Urea (mmol/5 h) | 2.1 ± 0.4 a | 2.0 ± 0.5 a | 2.0 ± 0.7 a | 1.3 ± 0.4 b |
| Urea/Creatinine (mmol/mmol) | 185 ± 19 a | 184 ± 31 a | 196 ± 41 a | 96 ± 12 b |
| Serum Biochemistry (Week 8) | ||||
| Urea (µmol/L) | 3.89 ± 0.40 b | 4.07 ± 0.59 ab | 4.52 ± 0.72 a | 3.68 ± 0.45 b |
| Creatinine (mmol/L) | 29.1 ± 3.7 ab | 25.8 ± 5.7 b | 26.7 ± 4.3 b | 32.9 ± 2.3 a |
| Urea:Creatinine (mmol/L:mmol/L) | 136 ± 24 bc | 169 ± 62 ab | 173 ± 38 a | 112 ± 10 c |
| Creatinine Clearance (Week 8) | ||||
| Creatinine Clearance (mL/min) | 1.39 ± 0.41 | 1.33 ± 0.39 | 1.27 ± 0.42 | 1.32 ± 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rech, L.; Zahradka, P.; Taylor, C.G. Marginal Zinc Deficiency Promotes Pancreatic Islet Enlargement While Zinc Supplementation Improves the Pancreatic Insulin Response in Zucker Diabetic Fatty Rats. Nutrients 2024, 16, 1819. https://doi.org/10.3390/nu16121819
Rech L, Zahradka P, Taylor CG. Marginal Zinc Deficiency Promotes Pancreatic Islet Enlargement While Zinc Supplementation Improves the Pancreatic Insulin Response in Zucker Diabetic Fatty Rats. Nutrients. 2024; 16(12):1819. https://doi.org/10.3390/nu16121819
Chicago/Turabian StyleRech, Leslie, Peter Zahradka, and Carla G. Taylor. 2024. "Marginal Zinc Deficiency Promotes Pancreatic Islet Enlargement While Zinc Supplementation Improves the Pancreatic Insulin Response in Zucker Diabetic Fatty Rats" Nutrients 16, no. 12: 1819. https://doi.org/10.3390/nu16121819
APA StyleRech, L., Zahradka, P., & Taylor, C. G. (2024). Marginal Zinc Deficiency Promotes Pancreatic Islet Enlargement While Zinc Supplementation Improves the Pancreatic Insulin Response in Zucker Diabetic Fatty Rats. Nutrients, 16(12), 1819. https://doi.org/10.3390/nu16121819








