1. Introduction
Taurine is a non-proteogenic sulfonic amino acid implicated in tissue regeneration [
1]. Taurine’s levels drop significantly with aging [
2], and human clinical studies have demonstrated that taurine supplementation can treat congestive heart failure [
3], retinal degeneration, and cardiomyopathy [
1]. Studies using various animal models showed that taurine improved cognitive functions [
4], glucose metabolism [
5], and motor functions [
6] and protected various tissues from degeneration associated with cisplatin treatment [
7,
8]. At the molecular level, taurine’s cell protection has been linked to decreased DNA damage and chromatin remodeling [
2]. These studies established a possible role for taurine in managing age-related or treatment-induced morbidities linked to DNA damage.
The tumor suppressor p53 protein, a product of the
TP53 gene, is a transcription factor that activates a plethora of signal transduction mechanisms involved in cell cycle arrest, regulation of metabolism, DNA damage resolution, and cell survival under stress [
9,
10,
11]. During DNA damage, p53 is rapidly stabilized through the ATM/ATR-dependent negative regulation of E3 ubiquitin ligase MDM2 [
12] and phosphorylation by DNAPK [
13]. Stabilized p53 can suppress the cell cycle, trigger DNA repair, and rewire metabolism to support cell regeneration [
14,
15].
TP53 mutations are common in OC [
16], and studies have linked loss-of-p53 functions with increased carcinoma cell proliferation and cancer progression [
17]. Paradoxically, however, in humans, the prevalence of
TP53 mutations among non-malignant epithelial cells can be high [
18], suggesting the existence of factors that support p53-growth-suppressing activities despite the mutations.
The target of rapamycin (TOR) (including its mammalian ortholog, mTOR) is a serine/threonine kinase that is essential for cell proliferation, cell survival, and autophagy [
19]. mTOR phosphorylation of ribosomal-6-kinase leads to activation of ribosomal protein S6 and translation initiation factor eIF4B [
20], which ultimately regulates protein translation [
21]. mTOR can be activated by signal transduction pathways downstream of Ras [
22], and chemical biology approaches have been used to target mTOR in cancers [
23]. For instance, inhibition of mTOR with the rapamycin derivative everolimus increases cisplatin sensitivity of cancer cells upon p53 pathway restoration [
24], implying that a combination of mTOR inhibition and reactivation of wild-type functions in mutant p53 proteins might provide an additional strategy for increasing the sensitivity of cancer cells to standard forms of therapy, such as cisplatin.
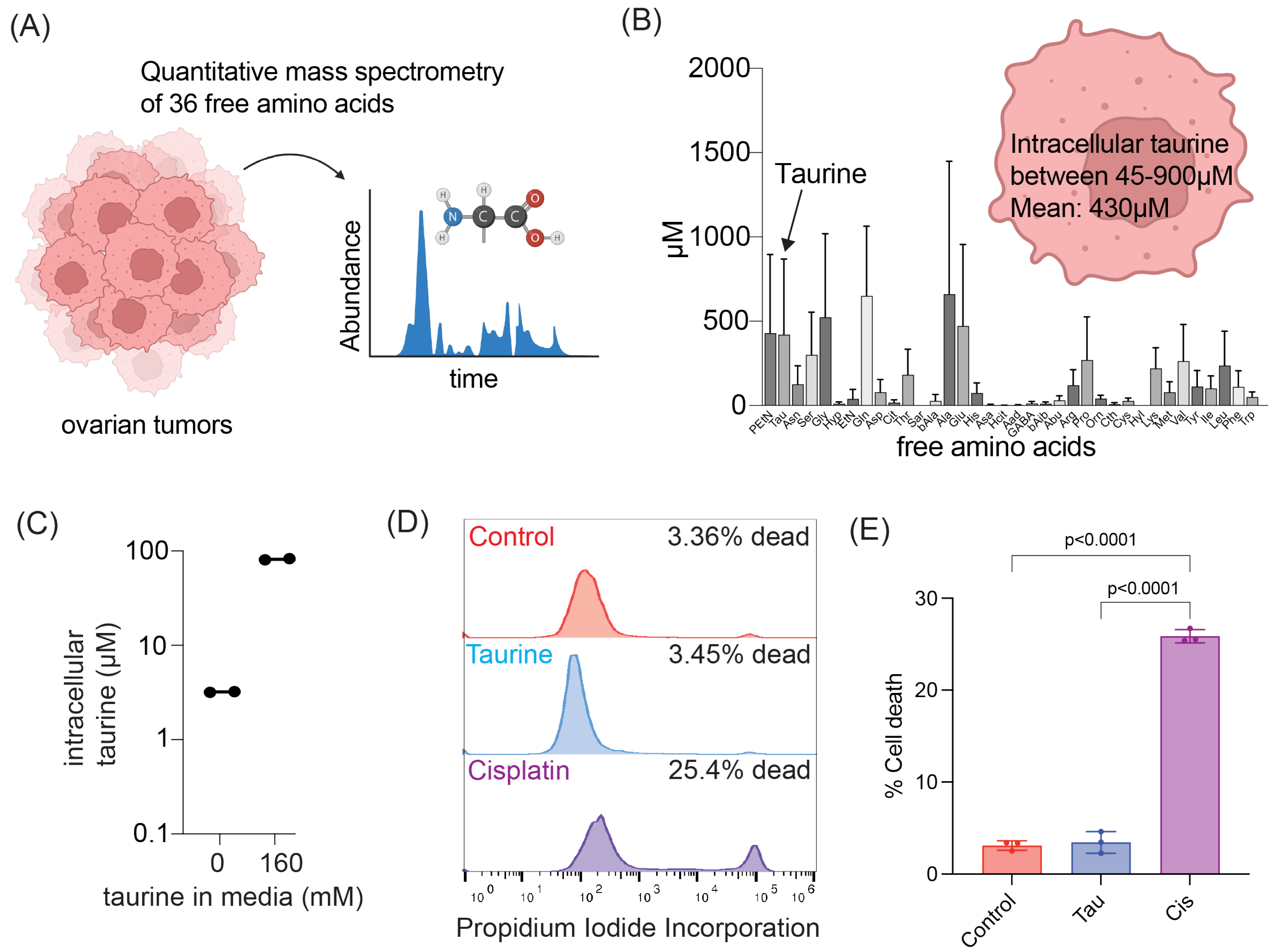
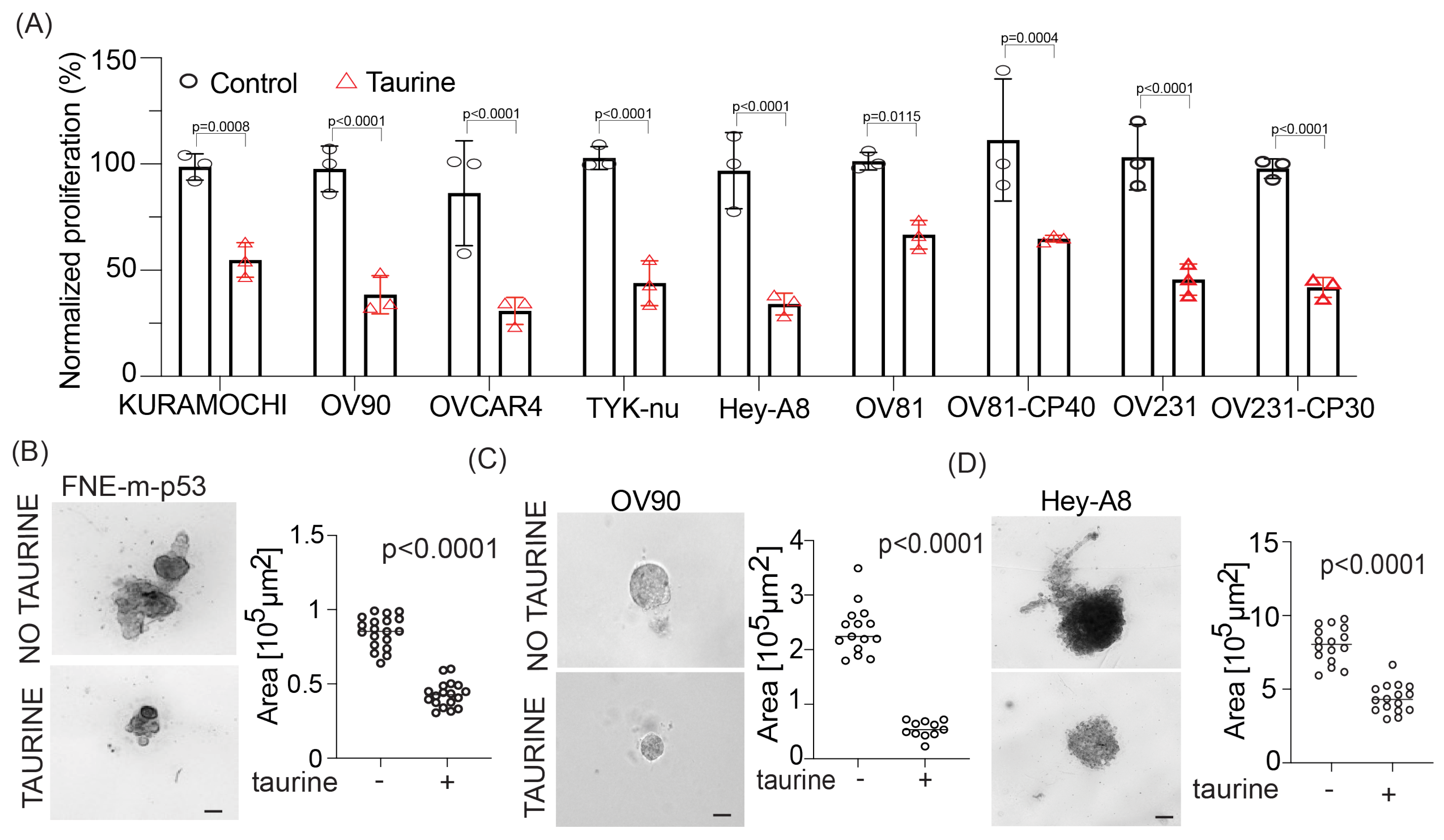
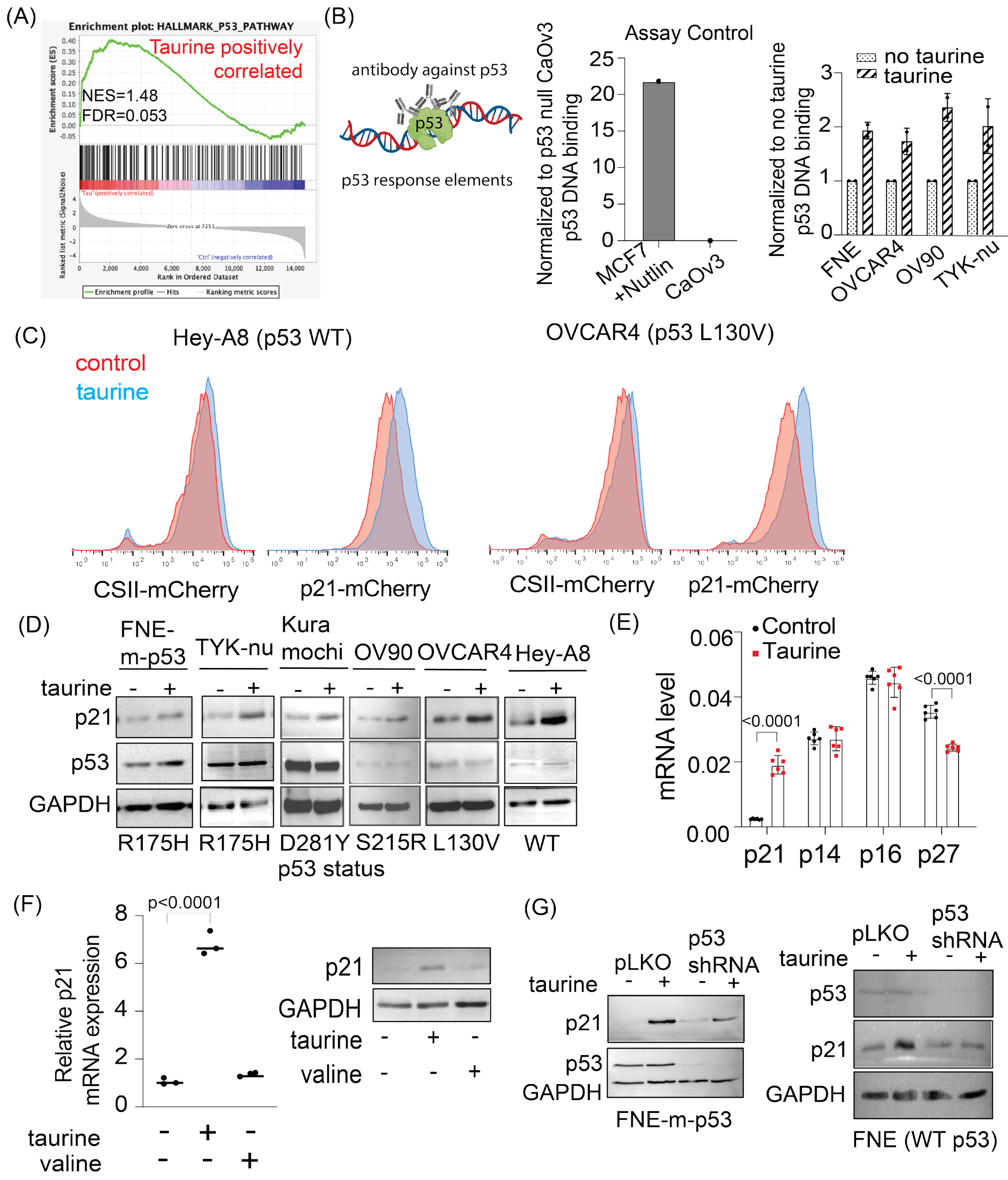
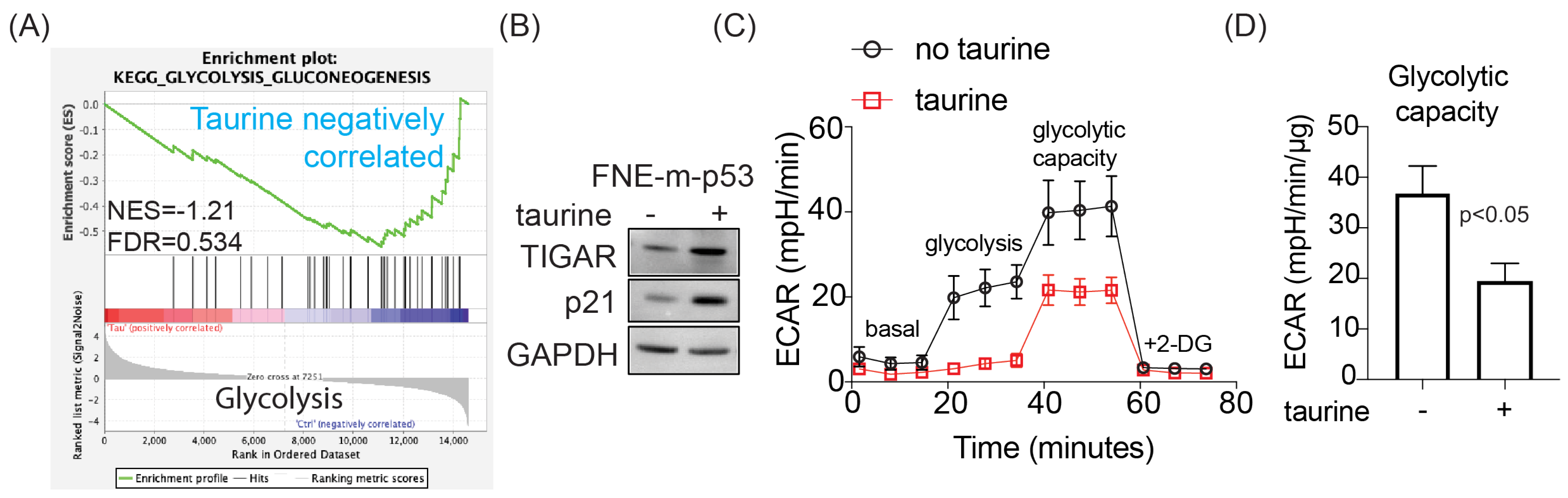
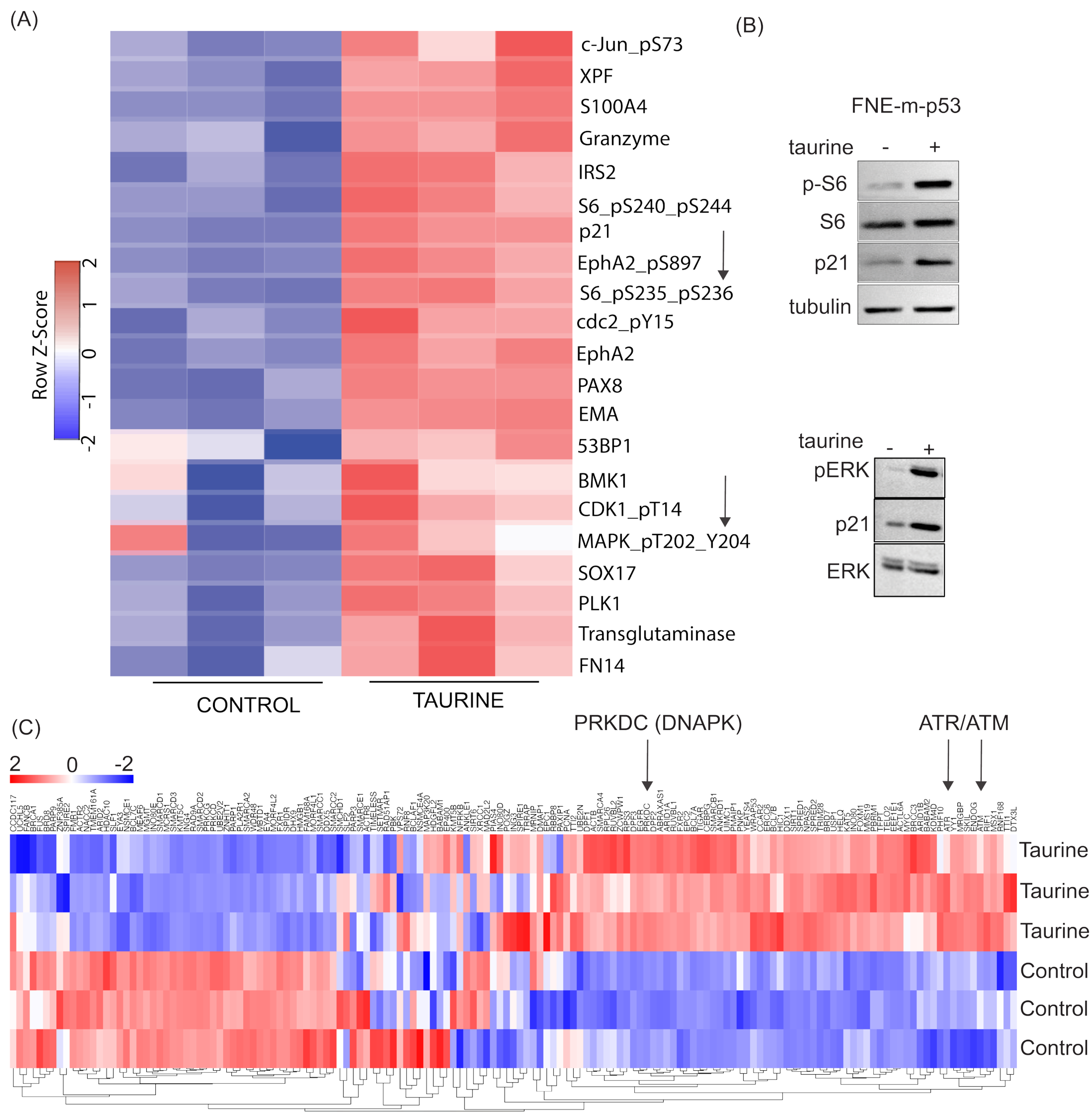
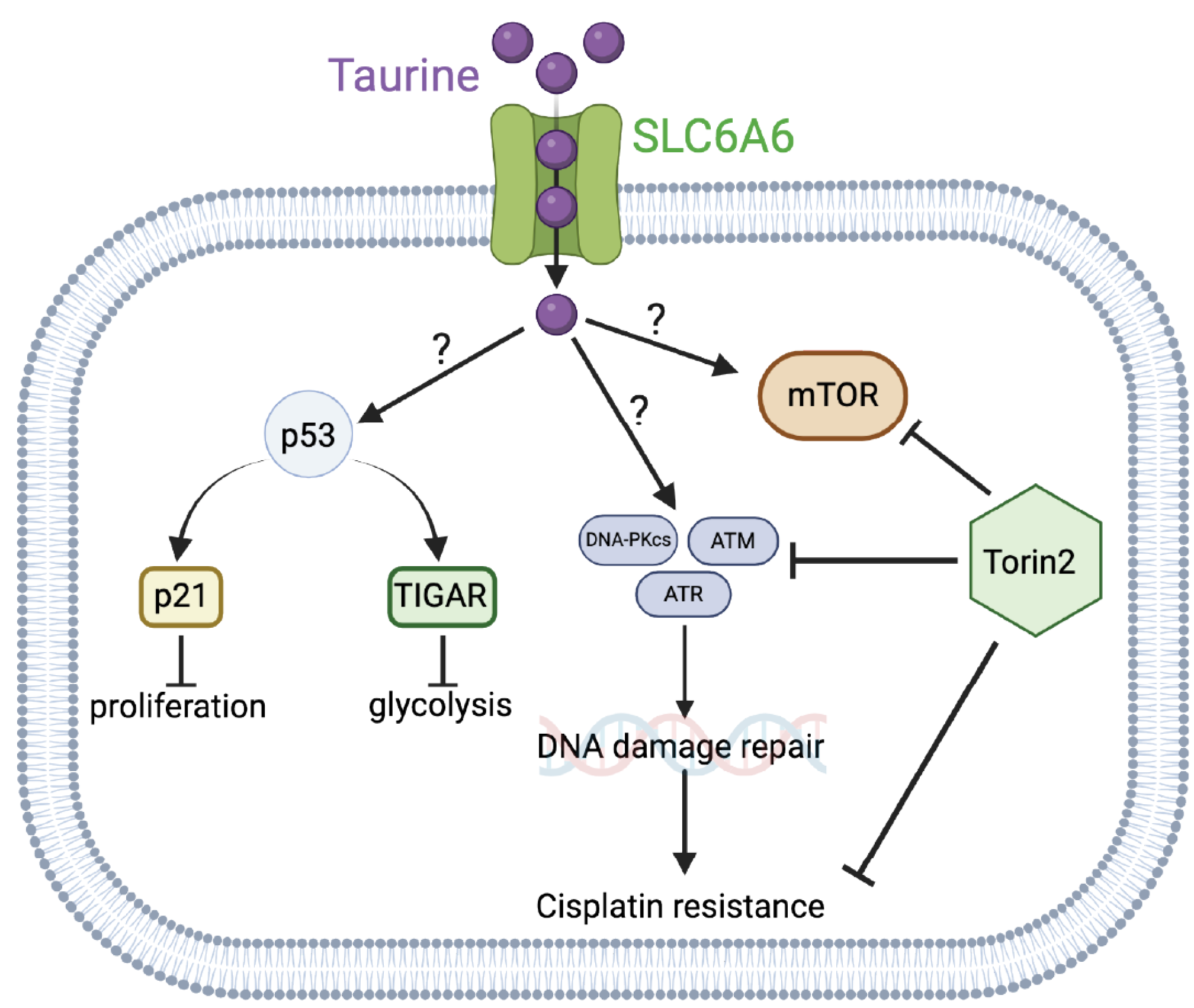
In this study, we report levels of intracellular pools of the free amino acid taurine in ascites cells derived from OC patients not previously administered chemotherapeutic treatment. Mimicking intracellular taurine levels in cultured cell lines and supplementing ovarian patient-derived organoids with taurine revealed a taurine-mediated increase in cell size, inhibition of proliferation, suppression of glycolysis, and cell protection from the DNA damage agent cisplatin. Combined RNA sequencing, reverse-phase protein array (RPPA), Western blotting, and flow cytometry analysis of taurine-supplemented cell cultures underscored p53, ERK, mTOR/DNAPK/ATM/ATR pathway activation and suppression of cisplatin-induced DNA damage (as measured by γH2AX and DNAPK phosphorylation). Inhibition of mTOR/DNAPK/ATM/ATR (Torin2), but not MEK (PD03255901), S6K1 (PF-4708671) or p53 expression (shRNA) induced the death of taurine-supplemented cultures in the presence of cisplatin. Our data support the idea that elevation of the intracellular free-amino acid taurine can activate growth-suppressing and cell-protecting signal transduction pathways involving p53 and mTOR/DNAPK/ATM/ATR.
2. Materials and Methods
2.1. Cell Culture
FNE cells (a kind gift from Dr. Tan Ince, Weill Cornell Medicine, New York, NY, USA) were cultured in a 1:1 ratio of DMEM/F12 (HiMedia, Kennett Square, PA, USA) alongside Medium 199 (HiMedia), 2% heat-inactivated fetal bovine serum (HI-FBS; Sigma-Aldrich, Burlington, MA, USA), 1% penicillin–streptomycin (VWR), 0.5 ng/mL of beta-estradiol (US Biological, Salem, MA, USA), 0.2 pg/mL of triiodothyronine (Sigma-Aldrich), 0.025 μg/mL all-trans retinoic acid (Beantown Chemical, Hudson, NH, USA), 14 μg/mL of insulin (Sigma-Aldrich), 0.5 ng/mL of EGF (Peprotech, Cranbury, NJ, USA), 0.5 μg/mL hydrocortisone (Sigma-Aldrich), and 25 ng/mL of cholera toxin (Sigma-Aldrich, Burlington, MA, USA). Kuramochi, OVCAR4, and OV90 (kind gifts from Dr. Denise Connolly, Fox Chase Cancer Center) and HEK293T cells (Thermo Fisher, Waltham, MA, USA) were cultured in DMEM/F12 supplemented with 10% HI-FBS and 1% penicillin–streptomycin. CaOv3 (ATCC, Manassas, VA, USA), Hey-A8 (a kind gift from Dr. Sumegha Mitra’s laboratory, University of Indiana), and TYK-nu (a kind gift from Dr. Joan Brugge’s laboratory, Harvard Medical School) cells were cultured in a 1:1 ratio of MCDB 105 (Sigma-Aldrich) and Medium 199 supplemented with 5% HI-FBS and 1% penicillin–streptomycin. OV81-CP, OV81-CP40 and OV231, and OV231-CP30 (kind gift from Dr. Analisa DiFeo’s lab, University of Michigan) were cultured in DMEM/F12 supplemented with 10% HI-FBS and 1% penicillin–streptomycin. All cell lines were cultured in a humidified incubator at 37 °C and with 5% carbon dioxide. Cell cultures were tested for the presence of mycoplasma every 3–6 months using the Uphoff and Drexler detection method [
25]. Taurine (TCI Chemicals, Portland, OR, USA) was dissolved directly into complete cell culture media at a concentration of 40 mg/mL and passed through a 0.22 micron filter before treatment.
2.2. Patient Ascites
For a study from 2021 to 2022, we enrolled Caucasian patients with histologically confirmed high-grade serous OC and ascites in the Department of Gynecologic Oncology, Poznań University of Medical Sciences. Ovarian tumors were staged according to the FIGO (International Federation of Gynecology and Obstetrics) system. Ascites fluid cell samples were collected from patients (i) at laparoscopy before starting neoadjuvant chemotherapy. Ascites fluid (10 mL) was centrifuged within 2 h after collection in a falcon tube at 1100× g for 10 min at room temperature to separate a cell pellet. The supernatant and malignant cell pellets were stored at −80 °C and analyzed collectively.
2.3. Mass-Spectrometry-Based Quantification of Taurine Levels in Cell Lysates
The panel of amino acids was quantified based on aTRAQ kit for amino acid analysis (SCIEX, Framingham, MA, USA) and liquid chromatography coupled to a triple quadrupole tandem mass spectrometry technique. The samples (cell lysates) were thawed at room temperature, and 40 μL of a matrix was transferred to a 0.5 mL Eppendorf tube. Then, 10 μL of sulfosalicylic acid was added to precipitate the proteins, and the vial contents were mixed and centrifuged. Subsequently, 10 μL of supernatant was transferred to a clean tube, and 40 μL of borate buffer was added, mixed, and centrifuged. In the next step, 10 μL of the obtained mixture was transferred to a clean tube and mixed with 5 μL of amino-labeling reagent (aTRAQ Reagent Δ8
®, Framingham, MA, USA). After centrifugation, samples were incubated for 30 min at room temperature. The incubation was followed by the addition of 5 μL of hydroxylamine solution, mixing and centrifugation. Then, the samples were incubated for 15 min at room temperature. In the next step, 32 μL of freshly prepared internal standards solution was added, mixed up, and centrifuged. The contents of the tubes were concentrated (temperature 50 °C for about 15 min) to a volume of about 20 μL using a vacuum concentrator (miVac Duo, Genevac, Stone Ridge, NY, USA). In the last step, 20 μL of ultrapure water was added to each vial and mixed. The contents of the tubes were transferred to amber-glass autosampler vials with inserts. Samples were analyzed in random order by chromatographic separation followed by tandem mass spectrometry detection LC-MS/MS. The analytes were separated on a Sciex C18 column (4.6 mm × 150 mm, 5 μm) maintained at 50 °C using a 1260 Infinity HPLC instrument (Agilent Technologies, Santa Clara, CA, USA). A gradient flow of the mobile phase was applied. The mobile phase consisted of 0.1% formic acid (FA) and 0.01% heptafluorobutyric acid (HFBA) in water (phase A) and 0.1% FA and 0.01% HFBA in methanol (phase B) maintained at a flow rate of 800 μL/min. Total runtime was 18 min per sample, with injection volume equal to 2 μL. Detection and quantitation of analytes were performed by means of a quadrupole tandem mass spectrometer with an electrospray ionization (ESI) TurboV ion source operated in positive ion mode. All results were generated in a scheduled multiple-reaction monitoring mode. Raw data from amino acid assays were acquired and analyzed using Analyst software version 1.6.3 (Sciex, Framingham, MA, USA). The method validation and sample preparation have been described in detail previously [
26].
2.4. Quantification of Taurine Levels in Tissue Culture Cell Lysates Using a Commercial Kit
To determine intracellular taurine concentrations, a taurine assay kit (Cell Biolabs, Inc., San Diego, CA, USA) was used. Cells were treated with taurine for the indicated amount of time, washed three times with PBS, and lysed in ice-cold RIPA buffer (Cell Signaling Technology, Danvers, MA, USA) in order to extract intracellular contents; protein content was determined using a BCA assay. Then, 25 μg of protein was loaded into a 96-well plate in triplicate for each sample. Taurine concentration was determined using the taurine assay kit following the manufacturer’s instructions, and absorbance values were read at 405 nm using a SpectraMax i3x Multi-Mode Microplate Reader (Molecular Devices, San Jose, CA, USA). Absorbance values were then background-subtracted and normalized to the average absorbance value of the control group. Values were reported as fold change in intracellular taurine concentration.
2.5. Organoid Derivation and Culture
Organoids were derived from primary tissue tumor samples of patients with OC as described previously [
27]. Briefly, fresh tumor resections were cut in small pieces. Two to four random pieces were snap-frozen and stored in liquid nitrogen for molecular analysis or fixed for histology. The remaining tissue was minced, digested with 0.7 mg/mL collagenase (Sigma-Aldrich C9407, Burlington, MA, USA), in the presence of 10 mM ROCK inhibitor (Y-27632 dihydrochloride, Abmole, Houston, TX, USA) at 37 °C for 25–50 min, and strained over a 100 μm filter. The digested tissue suspension was centrifuged at 300×
g for 5 min. Visible red pellets were lysed with red-blood lysis buffer (Sigma-Aldrich) for 3 min at room temperature and centrifuged at 300×
g for 5 min. The dissociated tissue pellet was resuspended in cold Cultrex Reduced Growth Factor BME type 2 (Cultrex BME) (R&D systems, Minneapolis, MN, USA), and 25 mL drops per well were plated on pre-warmed 48-well plates. The Cultrex BME/cell suspension droplets were allowed to solidify at 37 °C for at least 30 min. Solidified droplets with the embedded cells were then overlaid with ovarian tumor organoid medium containing 10 mM ROCK inhibitor (Y-27632 dihydrochloride, Abmole). The medium was composed of Advanced DMEM/F12 (Gibco, Waltham, MA, USA) containing GlutaMAX (Gibco), 100 U/mL penicillin, 100 mg/mL streptomycin (Gibco), 10 mM HEPES (Gibco), 100 mg/mL Primocin
® (InvivoGen, San Diego, CA, USA), 1×B-27™ supplement (Gibco), 1.25 mM
N-Acetyl-L-cysteine (Sigma-Aldrich), 10 mM Nicotinamide (Sigma-Aldrich), 0.5 mM A83-01 (R&D systems), 0.5 mg/mL Hydrocortisone (Sigma-Aldrich), 10 mM Forskolin (R&D systems), 100 nM b-Estradiol (Sigma-Aldrich), 16.3 mg/mL BPE (Thermo Fisher Scientific), 10 ng/mL recombinant human FGF-10 (PeproTech, Cranbury, NJ, USA), 5 ng/mL recombinant human FGF-7 (PeproTech), 37.5 ng/mL recombinant human Heregulin Beta-1 (PeproTech), 5 ng/mL recombinant human EGF (PeproTech), 0.5 nM WNT Surrogate-Fc fusion protein (ImmunoPrecise, Fargo, ND, USA), 10% R-Spondin1 conditioned medium, and 1% Noggin-Fc fusion protein conditioned medium (ImmunoPrecise). The medium was changed every 2–3 days. For established lines, dense cultures containing organoids ranging in size from 100 to 500 µm were passaged every 7–10 days with TrypLE (Life Technologies, Carlsbad, CA, USA) containing 10 mM ROCK inhibitor and 10 mg/mL DNase I (Sigma-Aldrich). For taurine supplementation, 2-aminoethanesulfonic acid (TCI chemicals, Portland, OR, USA) was freshly added directly to the ovarian tumor organoid medium to a final concentration of 160 mM, filtered, and stored at 4 °C. Filtered ovarian tumor organoid medium from the same batch was used as a control. For single-cell plating, dense organoid cultures were dissociated with TrypLE (Life Technologies) containing 10 mM ROCK inhibitor and 10 mg/mL DNase I (Sigma-Aldrich) for 10 min at 37 °C, followed by mechanical shearing through a 30 g needle and five dilutions with Advanced DMEM/F12 (Gibco) (containing antibiotics, HEPES, and GlutaMax as indicated above); cultures were then supplemented with 10 mM ROCK inhibitor and 10 mg/mL DNase I and underwent centrifugation at 300×
g for 5 min. Cell pellets were resuspended in cold Cultrex BME and plated at 400 cells/mL of BME.
The medium ± taurine was fully changed every 2–3 days. For organoid collection and plating, 20–50 µm organoids were extracted from the Cultrex BME droplet using cold cell recovery solution containing 10 mg/mL DNase I at 4 °C for 30 min. The dissolved Cultrex BME containing intact organoids was then collected into 2–3 × volume of cold advanced DMEM/F12 containing 0.1% BSA (Sigma-Aldrich) and 10 mg/mL DNase I and centrifuged at 200–250× g for 5 min. The organoid solution is resuspended in cold ovarian tumor organoid medium containing 10% Cultrex BME with or without taurine. The organoid solution was counted prior to plating at 500 organoids/well in 384-well plate format.
2.6. Organoid Metrics
Bright-field images were acquired and analyzed using a Perkin Elmer Opera Phenix High-Content Screening System and Harmony high-content analysis software (4.9). The area of each organoid was measured with an automated quantification pipeline set up to identify well-defined organoid structures in the same focal plane of one single z-plane after selection based on image texture, single structure size, and morphology.
2.7. Organoid ATP Assay
CellTiter-Glo® 3D Cell Viability Assay (Promega, Madison, WI, USA) was performed on patient-derived organoids (PDOs) to measure ATP in metabolically active cells at the specified experimental endpoint following the manufacturer’s instructions. Briefly, CellTiter-Glo® 3D Reagent was added to each well at 1:1 ratio. Plates were shaken for 25 min, and luminescence was measured with a CLARIOstar® Plus reader (BMG LABTECH, Ortenberg, Germany).
2.8. Cell Proliferation Analysis
Cell proliferation was determined using an automated cell counter or by live cell imaging. To assess proliferation using an automated cell counter, cells were first seeded into a 12-well plate. The following day, three wells per condition were treated as indicated, and another three wells were trypsinized and counted using a Luna II Automated Cell Counter (Logos Biosystems, Annandale, VA, USA). After 72 h of treatment, cells were once again harvested and counted, and cell numbers were normalized to the average value of the initial count and reported as fold change in cell number.
To determine proliferation by live cell imaging, GFP-expressing FNE-m-p53 cells were seeded in a 6-well plate and captured over time using a BioTek Lionheart FX Automated Microscope (BTLFX, Biotek, Winooski, VT, USA). Images were analyzed using the ImageJ (National Institutes of Health, Bethesda, MD, USA) TrackMate plugin.
2.9. Cell Volume and Adhesion Area Quantification
GFP-expressing FNE-m-p53 cells were seeded in a 6-well plate and incubated overnight. The following day, cells were treated as indicated and incubated for 72 h. Cells were then trypsinized and seeded into a low-adhesion 96-well round bottom plate at a density of 100 cells per well. The plate was then centrifuged at 900 rpm for 5 min, and cell clusters were immediately imaged using a BTLFX. Images were captured using a 10×objective in the GFP- and phase-contrast channels. To determine cell volume, GFP images were processed in FIJI (Version 2.14) using an in-house macro. Briefly, images were background-subtracted, and an automated threshold was applied. Images were then converted to binary and a watershed was applied to separate adjacent cells. The area of each particle (cell) was then measured. From this value, the radius of each cell was determined, and volume was estimated from the equation for the volume of a sphere (V = 4/3 π r3). Between 15–20 wells were analyzed per condition, and the average volume of a cell per well was reported.
To quantify cell adhesion area, cells were seeded and treated as described for the proliferation assay. At the endpoint, images were captured using a BTLFX with a 10X objective in the GFP- and phase-contrast channels. Then, as described for the fluorescence microscopy cell proliferation assay, images were processed in FIJI with an in-house macro, and the area of each particle (cell) was reported as the cell adhesion area.
2.10. Basement Membrane Reconstitution and Spheroid Size Quantification
To examine the effects of taurine on reconstituted basement membrane organotypic structures, FNE-m-p53, Hey-A8, and OV90 cells were reconstituted in Matrigel
® Growth Factor Reduced Basement Membrane Matrix (MG) (Corning Corning NY, USA), as previously described [
28]. A total of 100 cells were seeded per well in a 96-well ultra-low-adhesion Nunclon Sphera™ plate, centrifuged at 900 rpm for 5 min, and incubated overnight. The following day, on ice, MG was added to cell culture media to a concentration of 4%
v/
v. MG solution was then added to each well containing 100 μL of clustered spheroids to a final volume of 200 μL and an MG concentration of 2%
v/
v. Z-projections of organotypic structures were captured at the indicated time points using a BTLFX using the 4X objective in the bright-field and GFP channels. Z-projections were processed into focused stacks using Gen5 software (Version 3.11), and the area of each structure was measured using FIJI (Version 2.14).
2.11. RNA Isolation and Targeted RT-qPCR Analysis
Total RNA was isolated using Quick-RNA Miniprep Kit (Zymo Reasearch, Irvine, CA, USA) and reverse-transcribed into cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Waltham, MA, USA) following the manufacturer’s instructions. To analyze mRNA expression, 10 ng of cDNA was added to a 10 μL reaction mixture containing Power SYBR Green Master Mix (Applied Biosystems) and primers at a concentration of 330 nM designed to detect the transcript of interest. mRNA levels were quantified using a CFX96 Touch Deep Well Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA), and mRNA expression was normalized to a housekeeping gene.
2.12. RNA Sequencing and Transcriptomic Analysis
RNA extraction was performed using an E.Z.N.A.® total RNA kit (Omega Bio-tek, Norcross, GA, USA) following the manufacturer’s protocol. RNA concentration and purity were assessed with a NanoDrop 2000 (ThermoFisher). RNA samples were stored in a −80 °C freezer and subsequently shipped on dry ice to Genewiz (Azenta US, South Plainfield, NJ, USA) for RNA sequencing. The RNA sequencing library preparation workflow started with PolyA–based mRNA enrichment, mRNA fragmentation, and random priming followed by first- and second-strand complementary DNA (cDNA) synthesis. Subsequently, end-repair with 5′ phosphorylation and adenine (dA)–tailing was carried out. Finally, adaptor ligation, PCR enrichment, and Illumina HiSeq 2500 sequencing with two 150-base-pair (bp) runs were performed, and sequence reads were mapped to the reference genome. The bioinformatics analysis workflow started with evaluation of sequence quality and trimming the reads by means of Trimmomatic v.0.36 software to remove possible adapter sequences and nucleotides of poor quality. STAR aligner v.2.5.2b software was used to map the trimmed reads to the Homo sapiens GRCh38 reference genome available on ENSEMBL. To extract gene hit counts, feature counts from the Subread package v.1.5.2. were used. The hit counts were reported based on the gene ID feature in the annotation file. Only reads that were within the exon regions were considered. The table generated from extracted gene hit counts was used for differential expression analysis. Using DESeq2, a comparison of gene-expression between the taurine-treated and control samples was performed. By using the Wald test, p-values and log2-fold changes were calculated. Genes with an adjusted p-value (Padj) of less than 0.05 and absolute log2-fold change more than 1 were counted as differentially expressed genes (DEGs).
Gene ontology analysis was applied on the statistically significant genes with GeneSCF v.1.1-p2 software. The goa_human GO list was referenced to cluster the set of genes based on their biological functions and determine their statistical significance. Gene set enrichment analysis (GSEA) was performed, as described earlier [
29], with GSEA_4.2.2. software, against selected gene sets from Molecular Signatures Database (MSigDB 7.5). GSEA was performed with the ranking metric set to Signal2noise and with the number of permutations set to 1000. Plots with a false discovery rate (FDR) of less than 0.25 were considered significant.
2.13. Flow Cytometry
DNA staining (cell cycle analysis) by flow cytometry was performed as follows. Cells were harvested by trypsinization, collected by centrifugation, and washed twice with PBS. Cell pellets were then fixed with ice-cold 70% ethanol for at least 30 min on ice. Cells were then washed twice with PBS and treated with PBS containing 100 μg/mL RNAse A (Sigma-Aldrich) and 2 μg/mL propidium iodide (PI) (Molecular Probes, Eugene, OR, USA) for 30 min at room temperature in the dark before analysis. Data acquisition was performed using an Attune NxT Flow Cytometer (ThermoFisher). Data were then analyzed using FlowJo software (Version 10.10.0, FlowJo, Ashland, CA, USA)
To determine cell viability, the cell culture supernatant was collected, then adherent cells were trypsinized and centrifuged at 300× g for 5 min. Cells were washed twice with PBS containing 2% FBS. Cells were then incubated on ice with PBS containing 2% FBS and 2 μg/mL PI. Data were then acquired by flow cytometry analyzed using FlowJo, and viability was reported as the percentage of PI-positive cells.
To stain for phosphorylated γH2AX, cells were treated for 48 h and collected by trypsinization. Cells were washed and fixed in 4% paraformaldehyde (PFA) for 15 min, followed by permeabilization in ice-cold methanol for 30 min. Cells were stained with Phospho-Histone H2A.X primary antibody (Cell Signaling Technology #2577) at a dilution of 1:200 in 0.5% BSA dissolved in PBS for one hour at room temperature. Cells were then washed and stained with Alexa Fluor 568 secondary antibody (Invitrogen A-11011) at a dilution of 1:500. Cells were then analyzed by flow cytometry.
2.14. Plasmids
SLC6A6 shRNA plasmids were obtained from Dharmacon (Lafayette, CO, USA): pLKO.1 vector; TRCN0000038409: TATCACCTCCATATATCCAGG, referred to in this manuscript as G5, and TRCN0000038412: TATACTTGTACTTGTTGTAGC, referred to in this manuscript as G8.
To generate a p21-reporter plasmid, NEBuilder HiFi DNA Assembly (New England Biolabs, Ipswich, MA, USA) was used to clone a p21 promoter sequence upstream of mCherry-3X-NLS into a lentiviral vector. A sequence containing the 2.4-kbp p21 promoter region was PCR-amplified from the pGL2-p21 promoter-Luc (Addgene #33021). The mCherry-3X-NLS sequence was PCR-amplified from CSII-prEF1a-mCherry-3X-NLS (Addgene #125262). PCR fragments were assembled into pLenti6.3/V5-DEST-GFP (Addgene #40125) by replacing the CMV promoter and EGFP sequences at the 5’ ClaI and 3’ SalI restriction sites, respectively. The resulting plasmid (pLenti6.3-p21 promoter-mCherry-3X-NLS) contains the 2.4-kbp p21 promoter upstream of the mCherry-3X-NLS sequence. Plasmid constructs were verified by next-generation sequencing.
2.15. Lentivirus Production
To generate lentivirus, packaging plasmids psPAX2 (Addgene #12260) and pMD2.G (Addgene #12259) were mixed with a lentiviral plasmid containing a gene of interest or shRNA and incubated with Lipofectamine 3000 (Invitrogen) in serum-free Opti-MEM (Gibco) to generate liposomes containing plasmid DNA. The mixture was then added to HEK293T cells, and the medium was refreshed the following day. Cell culture supernatant containing viral particles was collected 48 h and 72 h after transfection. Cell lines were then transduced by adding viral particles to media containing polybrene (Santa Cruz Biotechnology, Dallas, TX, USA) at a concentration of 10 μg/mL and selected with an appropriate antibiotic 24 to 48 h after transduction in order to generate cells stably expressing transgene or shRNA.
2.16. Western Blot Analysis
Cells were washed twice with PBS and lysed in ice-cold RIPA buffer (Cell Signaling Technology) supplemented with a HaltTM Protease Inhibitor Cocktail (Thermo Fisher), and cells were collected using a cell scraper. Cell lysates were pelleted at 20,000× g for 20 min, supernatant was collected, and protein concentration was determined by BCA assay (Pierce) according to the manufacturer’s instructions. Lysates were then mixed with 6X sample buffer (375 mM Tris Base, 9% sodium dodecyl sulfate, 50% glycerol, 0.075% bromophenol blue, 9% β-mercaptoethanol), boiled for 10 min at 95 °C, and then loaded on a polyacrylamide gel and resolved by electrophoresis. Proteins were then transferred to Immobilon® PVDF transfer membranes (Millipore Sigma, Burlington, MA, USA), which were blocked with 5% non-fat milk in Tris-buffered saline containing 0.1% v/v Tween-20 (TBST) for 30 min at room temperature. Membranes were incubated overnight at 4 °C in primary antibodies diluted in 5% non-fat milk in TBST. Membranes were then washed three times with TBST and incubated in an HRP-conjugated secondary antibody (1:10,000) for 1 h at room temperature. Membranes were then washed three times in TBST. Membranes were developed using ImmobilonTM Forte enhanced chemiluminescent substrate (Millipore Sigma, Burlington, MA, USA) and visualized using an iBright CL1500 (Thermo Fisher). The following primary antibodies were used in this study: SLC6A6 (#ab236898, Abcam, Waltham MA, USA), p21 (Cell Signaling Technology #2947), p53 (Santa Cruz Biotechnology #sc-126), GAPDH (Santa Cruz Biotechnology #sc-47724), TIGAR (Santa Cruz Biotechnology #sc-377065), DNA-PKcs (Cell Signaling Technology #38168), Phospho-DNA-PKcs (Ser2056) (Cell Signaling #68716), Vinculin (#700062, Invitrogen, Waltham, MA, USA), S6 Ribosomal Protein (Cell Signaling Technology #2317), Phospho-S6 Ribosomal Protein (Ser235/236) (Cell Signaling Technology #4858), p44/42 MAPK (Erk1/2) (Cell Signaling Technology #4695), Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (Cell Signaling Technology #4370), and β-Tubulin (Cell Signaling Technology #15115). HRP-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA).
2.17. Reverse-Phase protein Array
Adherent FNE-m-p53 cells were cultured in triplicate for 72 h in control media or with media supplemented with taurine. Cells were then washed three times on ice and then lysed in RPPA lysis buffer (1% Triton-X-100, 50 mM HEPES, pH 7.4, 150 mM NaCl. 1.5 MgCl2 1 mM EGTA, 100 mM NaF, 10 mM NaPPi, 10% glycerol, 1 mM Na3VO4, and Halt Protease Inhibitor (Thermo Fisher). Lysates were incubated on ice for 15 min and then centrifuged at 13,000× g for ten minutes. Following protein quantification, lysates were then diluted to an equal concentration using RPPA buffer before being boiled and denatured using 1% SDS for ten minutes. Lysates were then snap-frozen and shipped on dry ice to MD Anderson Cancer Center for RPPA analysis.
2.18. Analysis of Glycolysis
The extracellular acidification rate (ECAR) was measured using an XFp Extracellular Flux Analyser (Seahorse Bioscience, North Billerica, MA, USA). CaOv3 cells were exposed to taurine for 48 h and then reseeded (8000 cells/well) in taurine-containing media into the wells of an XFp Cell Miniplate. Then, 24 h later, the cells were analyzed using an XFp Glycolysis Stress Test Kit (Seahorse Bioscience, North Billerica, MA, USA) following the manufacturer’s instructions.
Cells were washed 3 times with 100 µL of XF Base Media (Seahorse Bioscience, North Billerica, MA, USA) containing 2 mM L-Glutamine. Then, the cells were incubated in 180 µL of the same media for 20 min. After three baseline ECAR measurements, cells were subsequently injected with 10 mM glucose, 1 μM oligomycin, and 50 mM 2-deoxyglucose (glycolysis inhibitor).
After both assays, the data obtained were normalized to protein concentrations determined by the bicinchoninic acid (BCA) assay (Pierce, Thermo Fisher Scientific, Waltham, MA, USA). Wave (Agilent Technology, Santa Clara, CA, USA) and GraphPad Prism 8 software were used to analyze and plot the data.
2.19. Statistical Analysis
Statistical analyses were performed using GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA). Statistical significance was determined using an unpaired, two-tailed, parametric t-test; an ordinary one-way ANOVA with Tukey’s post hoc multiple comparisons test; or a two-way ANOVA with Sidak’s post hoc multiple comparisons test, with p ≤ 0.05 considered statistically significant.
4. Discussion
These studies will provide potentially new insights into the mechanisms of cell growth suppression and cell protection by taurine, a non-proteogenic sulfonic amino acid present in the cytoplasm of ascites cells derived from OC patients. Mass-spectrometry-based quantitative examination of intracellular taurine pools revealed taurine concentrations ranging from 45 μM to 900 μM among ascites cells. These taurine levels were on average 100-fold higher than taurine intracellular pools found in cultured cells. Mimicking ascites cell-associated intracellular taurine concentrations in cell culture suppressed cell proliferation and glycolysis and induced cell protection from genotoxic stress. Taurine decreased cisplatin-mediated DNA damage, indicating that intracellular accumulation of the free amino acid taurine might contribute to the evolution of slowly proliferating cell clones with increased ability to repair DNA upon stress. Combination of bulk RNA-seq, and proteomic (RPPA) analysis of taurine-supplemented FNE-m-p53 cell cultures revealed activation of the p53, ERK, and mTOR pathways, and increased mRNA expression of DNAPK/ATM/ATR. Taurine induced mutant or wild-type p53 binding to DNA and the activation of p53 effectors involved in the negative regulation of the cell cycle and glycolysis, including p21 and TIGAR. Hairpin RNA-mediated attenuation of p53 decreased activation of p21 but it did not affect taurine’s ability to protect cells from cisplatin. Similarly, inhibition of ERK pathway by MEK inhibitor PD03255901 did not alter taurine’s protective activities. However, treatment of taurine-supplemented cell cultures with Torin2—a clinically relevant ATP-competitive inhibitor of mTOR, DNAPK, and ATM/ATR—was sufficient to block taurine-mediated cell protection. Our data are consistent with a model whereby the accumulation of intracellular taurine suppresses proliferation and provides cell protection from genotoxic stress through signal transduction pathways involving mTOR/DNAPK/ATM/ATR.
Proliferative ovarian tumors, as assessed by Ki67 stains, are initially sensitive to chemotherapy treatments [
43]. However, the disease recurs, and its progression has been recently linked to the evolution of slowly proliferating tumor cell clones [
44], indicating that cell protection from treatment could be linked to molecular mechanisms associated with suppression of proliferation, and metabolism. A set of new studies provide evidence that activation of p53 signal transduction, a major mechanism suppressing the cell cycle and metabolism, may be associated with cell survival under treatment [
15,
45,
46]. These studies imply that factors that activate p53 can modulate cell survival under treatment. The role of intracellular taurine in p53 activation is not completely understood, nor is it clear whether the activation of p53 in response to taurine is also associated with induction of pro-survival mechanisms. Here, we provide evidence that taurine suppresses OC cell proliferation and activates a p53 effector, p21, a molecule directly involved in cell cycle arrest. However, p53 activation was not required for taurine-induced protection of cells from cisplatin, indicating that p53 is dispensable in taurine cell protection.
Taurine is an osmolyte [
47], and recent results support the idea that accumulation of intracellular osmolytes promotes an increase in cell size and cell cycle arrest [
34]. Our cell culture data demonstrate that elevation of intracellular taurine to the levels found in cells isolated from OC ascites significantly increases OC cell size, and this phenotype is associated with suppression of proliferation. Based on these results, it is possible that one of the mechanisms supporting evolution of growth-suppressed tumor cell clones involves an influx of free amino acid osmolytes and cell size changes.
Recent in silico and cell-free biochemical studies [
48] have demonstrated that taurine can directly bind cyclin-dependent kinase 6 (CDK6). This interaction appears to inhibit CDK6 activity in solution, supporting the possibility that intracellular taurine accumulation could directly suppress CDK6/cyclin D activity, which could lead to activation of p53 [
49,
50] and inhibition of proliferation. Our experiments also provide evidence that taurine increases mutant or wild-type p53 binding to DNA. There is existing biochemical evidence [
51] that taurine interacts with casein proteins to change their conformation. Thus, it is possible that taurine could directly interact with p53 and change its conformation to support DNA binding and subsequent activation of effector molecules including p21 and TIGAR. However, taurine’s ability to protect cells from cisplatin did not depend on p53 but required mTOR/DNAPK/ATM/ATR activity. These results suggested the idea that emergence of therapy-resistant cell clones is driven by the mechanisms of cell cycle suppression and the activation of major cell survival and DNA repair signal transduction pathways including mTOR/DNAPK/ATM/ATR. How taurine activates mTOR/DNAPK/ATM/ATR is not known, and our data do not support that taurine itself induces DNA damage (as measured by γH2AX levels), thus implying the existence of taurine-induced DNA-damage-independent mechanisms of transcriptional regulation of DNA-damage-sensing kinases.