Carrageenan in the Diet: Friend or Foe for Inflammatory Bowel Disease?
Abstract
1. Introduction
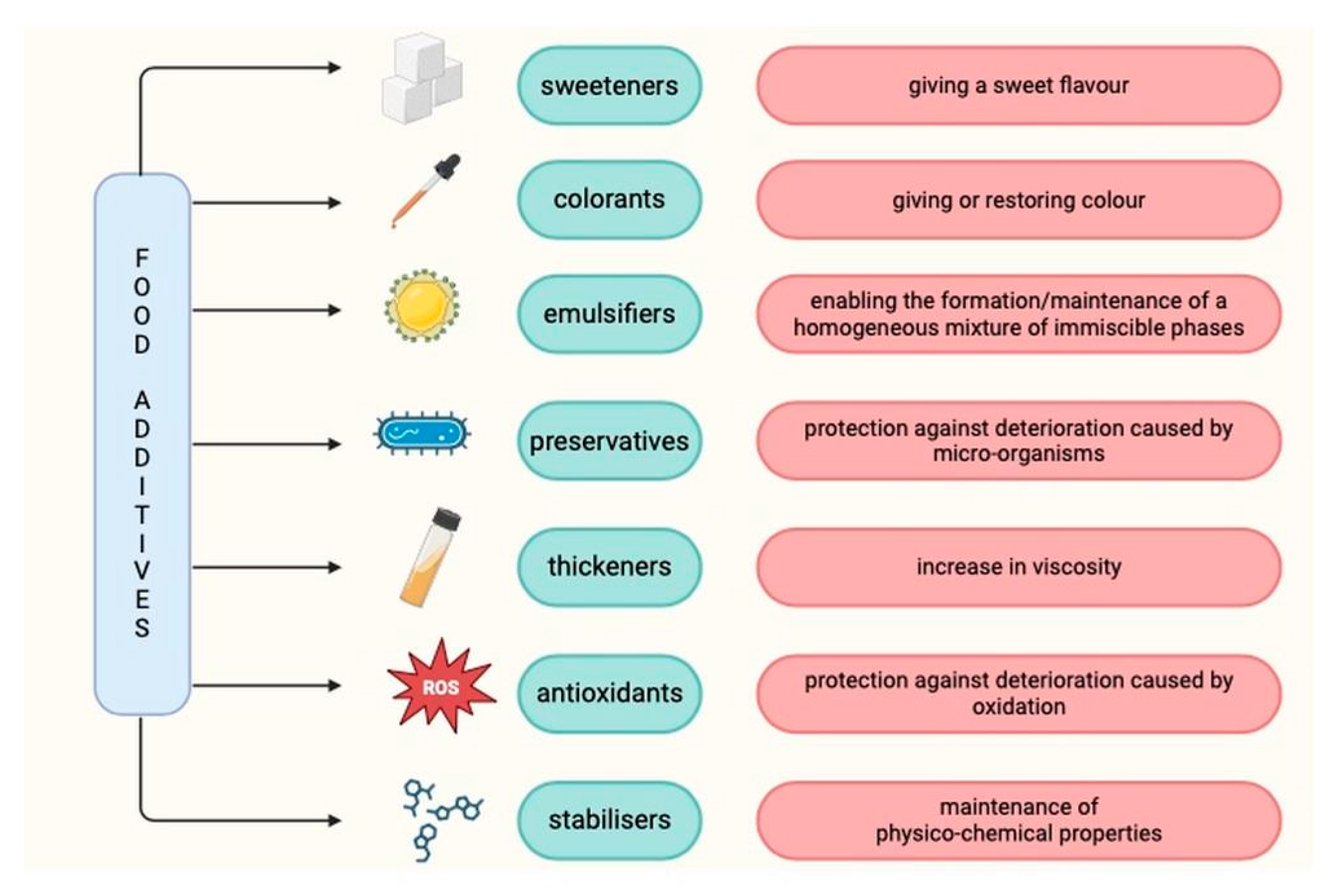
2. Food Additives
2.1. Food Additives and Autoimmune Diseases
2.2. Food Additives and Other Diseases
2.3. Food Additives and Microbiome
2.4. Food Additives and Intestinal Permeability
3. Carrageenan
3.1. Definition
3.2. Carrageenan Market
3.3. Occurrence
3.4. Safety of Carrageenan as a Food Additive
3.5. Impact on Inflammatory Bowel Diseases
3.6. Impact on the Microbiota
3.7. Carrageenan and Cancer
3.8. Impact on Other Diseases
4. Carrageenan, Degraded Carrageenan, and Poligeenan—Differences
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADI | adequate daily intake |
| CAGR | compound annual growth rate |
| CD | Crohn’s disease |
| CRP | C-reactive protein |
| DSS | dextran sodium sulphate |
| EFSA | European Food Safety Authority |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| ESR | erythrocyte sedimentation rate |
| FDA | Food and Drug Administration |
| HIV | human immunodeficiency virus |
| HSV-2 | herpes simplex virus type 2 |
| IBD | inflammatory bowel disease |
| IBS | irritable bowel syndrome |
| JECFA | Joint FAO/WHO Expert Committee on Food Additives |
| LPS | lipopolysaccharide |
| NF-κB | nuclear factor-κB |
| NOSB | National Organic Standards |
| OGTT | oral glucose tolerance test |
| SCCAI | Simple Clinical Colitis Activity Index |
| TNF-α | tumor necrosis factor α |
| UC | ulcerative colitis |
| ZO-1 | zonula occludens-1 |
References
- Bernstein, C.; Eliakim, A.; Fedail, S.; Fried, M.; Gearry, R.; Goh, K.L.; Hamid, S.; Khan, A.G.; Khalif, I.; Ng, S.C.; et al. World Gastroenterology Organisation Global Guidelines. J. Clin. Gastroenterol. 2016, 50, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Jairath, V.; Feagan, B.G. Global burden of inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2020, 5, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Mrowicki, J.; Mrowicka, M.; Majsterek, I. Czynniki środowiskowe zwiększające ryzyko aktywacji i rozwoju chorób zapalnych jelit. Postep. Biochem. 2020, 66, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The epidemiology of inflammatory bowel disease: East meets west. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Fabián, O.; Kamaradová, K. Morphology of inflammatory bowel diseases (IBD). Ceskoslovenska Patol. 2022, 58, 27–37. [Google Scholar]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohn’s Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Gönczi, L.; Lakatos, P.L.; Burisch, J. The Burden of Inflammatory Bowel Disease in Europe in 2020. J. Crohn’s Colitis 2021, 15, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
- Wojtuń, S.; Gil, J.; Szwed, Ł.; Dyrla, P. Basic symptoms and differentiation of inflammatory bowel diseases. Pediatr. I Med. Rodz. 2014, 10, 61–66. [Google Scholar] [CrossRef]
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar] [CrossRef]
- Gorospe, J.; Windsor, J.; Hracs, L.; Coward, S.; Buie, M.; Quan, J.; Caplan, L.; Markovinovic, A.; Cummings, M.; Goddard, Q.; et al. Trends in Inflammatory Bowel Disease Incidence and Prevalence across Epidemiologic Stages: A Global Systematic Review with Meta-Analysis. Inflamm. Bowel. Dis. 2024, 30 (Suppl. S1), S00. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Nakane, K.; Komatsu, M. Westernized Diet is the Most Ubiquitous Environmental Factor in Inflammatory Bowel Disease. Perm. J. 2019, 23, 18–107. [Google Scholar] [CrossRef] [PubMed]
- Mentella, M.C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. Nutrition, IBD and Gut Microbiota: A Review. Nutrients 2020, 12, 944. [Google Scholar] [CrossRef] [PubMed]
- Ruemmele, F.M. Role of Diet in Inflammatory Bowel Disease. Ann. Nutr. Metab. 2016, 68, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hébuterne, X.; Hvas, C.L.; Joly, F.; Klek, S.; Krznaric, Z.; Ockenga, O.; et al. ESPEN guideline on Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2023, 42, 352–379. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Aranow, C. Correspondence: Cynthia Aranow 350 Community Drive Manhasset, NY 11030. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Sharifi, A.; Hosseinzadeh-Attar, M.J.; Vahedi, H.; Nedjat, S. A randomized controlled trial on the effect of vitamin D3 on inflammation and cathelicidin gene expression in ulcerative colitis patients. Saudi J. Gastroenterol. 2016, 22, 316–323. [Google Scholar] [CrossRef]
- Adolph, T.E.; Meyer, M.; Schwärzler, J.; Mayr, L.; Grabherr, F.; Tilg, H. The metabolic nature of inflammatory bowel diseases. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Martino, J.V.; Van Limbergen, J.; Cahill, L.E. The role of carrageenan and carboxymethylcellulose in the development of intestinal inflammation. Front. Pediatr. 2017, 5, 96. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. Available online: https://eur-lex.europa.eu/eli/reg/2008/1333/oj (accessed on 5 May 2024).
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, C.; Long, Y.; Chen, Q.; Zhang, W.; Liu, G. Food additives: From functions to analytical methods. Crit. Rev. Food Sci. Nutr. 2022, 62, 8497–8517. [Google Scholar] [CrossRef] [PubMed]
- Valluzzi, R.L.; Fierro, V.; Arasi, S.; Mennini, M.; Pecora, V.; Fiocchi, A. Allergy to food additives. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Mazzucca, C.B.; Raineri, D.; Cappellano, G.; Chiocchetti, A. How to Tackle the Relationship between Autoimmune Diseases and Diet: Well Begun Is Half-Done. Nutrients 2021, 13, 3956. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.T.; Malik, M.; Nostro, J.A.; Mahler, G.J.; Musselman, L.P. Effect of dietary additives on intestinal permeability in both Drosophila and a human cell co-culture. DMM Dis. Models Mech. 2018, 11, dmm034520. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 2015, 14, 479–489. [Google Scholar] [CrossRef]
- Nishimura, S.; Aoi, W.; Kodani, H.; Kobayashi, Y.; Wada, S.; Kuwahata, M.; Higashi, A. Polysorbate 80-induced leaky gut impairs skeletal muscle metabolism in mice. Physiol. Rep. 2020, 8, e14629. [Google Scholar] [CrossRef]
- Brahmachari, S.; Jana, A.; Pahan, K. Sodium Benzoate, a Metabolite of Cinnamon and a Food Additive, Reduces Microglial and Astroglial Inflammatory Responses. J. Immunol. 2009, 183, 5917–5927. [Google Scholar] [CrossRef]
- Atabaki, M.; Shariati-Sarabi, Z.; Tavakkol-Afshari, J.; Mohammadi, M. Significant immunomodulatory properties of curcumin in patients with osteoarthritis; a successful clinical trial in Iran. Int. Immunopharmacol. 2020, 85, 106607. [Google Scholar] [CrossRef] [PubMed]
- Bush, R.K.; Zoratih, E.; Taylor, S.L. Diagnosis of sulfite and aspirin sensitivity. Clin. Rev. Allergy 1990, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.; Simon, R.; Lumry, W.; Mathison, D. Adverse reactions to tartrazine. J. Allergy Clin. Immunol. 1986, 78, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Balatsinou, L.; Di Gioacchino, G.; Sabatino, G.; Cavallucci, E.; Caruso, R.; Gabriele, E.; Ramondo, S.; Di Giampaolo, L.; Verna, N.; Di Gioacchino, M. Asthma Worsened by Benzoate Contained in Some Antiasthmatic Drugs. Int. J. Immunopathol. Pharmacol. 2004, 17, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Mccann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Articles Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: A randomised, double-blinded, placebo-controlled trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, A.E.; Langan, M.T.; Holton, K.F. Artificial food coloring affects EEG power and ADHD symptoms in college students with ADHD: A pilot study. Nutr. Neurosci. 2022, 25, 159–168. [Google Scholar] [CrossRef]
- Sandall, A.M.; Cox, S.R.; Lindsay, J.O.; Gewirtz, A.T.; Chassaing, B.; Rossi, M.; Whelan, K. Emulsifiers impact colonic length in mice and emulsifier restriction is feasible in people with Crohn’s disease. Nutrients 2020, 12, 2827. [Google Scholar] [CrossRef]
- Suez, J.; Cohen, Y.; Valdés-Mas, R.; Mor, U.; Dori-Bachash, M.; Federici, S.; Zmora, N.; Leshem, A.; Heinemann, M.; Linevsky, R.; et al. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell 2022, 185, 3307–3328.e19. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Tiemann, C.D.; Patterson, B.W.; Wice, B.M.; Klein, S. Sucralose affects glycemic and hormonal responses to an oral glucose load. Diabetes Care 2013, 36, 2530–2535. [Google Scholar] [CrossRef]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The gut microbiota and inflammation: An overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, H.; Qin, N.; Ren, X.; Zhu, B.; Xia, X. Impact of food additives on the composition and function of gut microbiota: A review. Trends Food Sci. Technol. 2020, 99, 295–310. [Google Scholar] [CrossRef]
- Gültekin, F. Food Additives And Microbiota. North. Clin. Istanb. 2019, 7, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Compher, C.; Bonhomme, B.; Liu, Q.; Tian, Y.; Walters, W.; Nessel, L.; Delaroque, C.; Hao, F.; Gershuni, V.; et al. Randomized Controlled-Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome. Gastroenterology 2022, 162, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Loayza, J.J.J.; Kang, S.; Schooth, L.; The, J.J.; de Klerk, A.; Noon, E.K.; Zhang, J.; Hu, J.; Hamilton, A.L.; Wilson-O’Brien, A.; et al. Effect of food additives on key bacterial taxa and the mucosa-associated microbiota in Crohn’s disease. The ENIGMA study. Gut Microbes 2023, 15, 2172670. [Google Scholar] [CrossRef] [PubMed]
- Bancil, A.S.; Sandall, A.M.; Rossi, M.; Chassaing, B.; Lindsay, J.O.; Whelan, K. Food Additive Emulsifiers and Their Impact on Gut Microbiome, Permeability, and Inflammation: Mechanistic Insights in Inflammatory Bowel Disease. J. Crohn’s Colitis 2021, 15, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- De Siena, M.; Raoul, P.; Costantini, L.; Scarpellini, E.; Cintoni, M.; Gasbarrini, A.; Rinninella, E.; Mele, M.C. Food Emulsifiers and Metabolic Syndrome: The Role of the Gut Microbiota. Foods 2022, 11, 2205. [Google Scholar] [CrossRef]
- Mu, Q.; Kirby, J.; Reilly, C.M.; Luo, X.M. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front. Immunol. 2017, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Lugea, A.; Salas, A.; Casalot, J.; Guarner, F.; Malagelada, J.-R. Surface Hydrophobicity of the Rat Colonic Mucosa is a Defensive Barrier against Macromolecules and Toxins. [Online]. 2000. Available online: http://gut.bmj.com/ (accessed on 8 May 2024).
- Um, C.; Hodge, R.; Gewirtz, A.; Stevens, V.; Jacobs, E.; Mccullough, M. Emulsifier and Highly Processed Food Intake and Biomarkers of Intestinal Permeability and inflammation in the Cancer Prevention Study-3 Diet Assessment Sub-Study. Curr. Dev. Nutr. 2020, 4, 1498. [Google Scholar] [CrossRef]
- Mine, Y.; Zhang, J.W. Surfactants Enhance the Tight-Junction Permeability of Food Allergens in Human Intestinal Epithelial Caco-2 Cells. Int. Arch. Allergy Immunol. 2003, 130, 135–142. [Google Scholar] [CrossRef]
- Błaszak, B.; Gozdecka, G.; Shyichuk, A. Carrageenan as a functional additive in the production of cheese and cheese-like products. Acta Sci. Pol. Technol. Aliment. 2018, 17, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Veterinární Med. 2013, 58, 187–205. [Google Scholar]
- Thevenet, F. Food Stabilisers, Thickeners and Gelling Agents; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Borsani, B.; De Santis, R.; Perico, V.; Penagini, F.; Pendezza, E.; Dilillo, D.; Bosetti, A.; Zuccotti, G.V.; D’Auria, E. The role of carrageenan in inflammatory bowel diseases and allergic reactions: Where do we stand? Nutrients 2021, 13, 3402. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fao.org/3/y4765e/y4765e0a.htm (accessed on 8 May 2024).
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Re-evaluation of carrageenan (E 407) and processed Eucheuma seaweed (E 407a) as food additives. EFSA J. 2018, 16, e05238. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Ito, N. A Critical Review of the Toxicological Effects of Carrageenan and Processed Eucheuma Seaweed on the Gastrointestinal Tract. Crit. Rev. Toxicol. 2002, 32, 413–444. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Shani Levi, C.; Fahoum, L.; Ungar, Y.; Meyron-Holtz, E.G.; Shpigelman, A.; Lesmes, U. Revisiting the carrageenan controversy: Do we really understand the digestive fate and safety of carrageenan in our foods? Food Funct. 2018, 9, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Tobacman, J.K. Review of Harmful Gastrointestinal Effects of Carrageenan in Animal Experiments. [Online]. 2001. Available online: http://ehpnet1.niehs.nih.gov/docs/2001/109p983-994tobacman/abstract.html (accessed on 8 May 2024).
- McKim, J.M.; Willoughby, J.A.; Blakemore, W.R.; Weiner, M.L. Clarifying the confusion between poligeenan, degraded carrageenan, and carrageenan: A review of the chemistry, nomenclature, and in vivo toxicology by the oral route. Crit. Rev. Food Sci. Nutr. 2019, 59, 3054–3073. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Shumard, T.; Xie, H.; Dodda, A.; Varady, K.A.; Feferman, L.; Halline, A.G.; Goldstein, J.L.; Hanauer, S.B.; Tobacman, J.K. A randomized trial of the effects of the no-carrageenan diet on ulcerative colitis disease activity. Nutr. Healthy Aging 2017, 4, 181–192. [Google Scholar] [CrossRef]
- Lee, D.; Swan, C.K.; Suskind, D.; Wahbeh, G.; Vanamala, J.; Baldassano, R.N.; Leonard, M.B.; Lampe, J.W. Children with Crohn’s Disease Frequently Consume Select Food Additives. Dig. Dis. Sci. 2018, 63, 2722–2728. [Google Scholar] [CrossRef]
- Available online: https://www.mordorintelligence.com/industry-reports/global-carrageenan-market-industry (accessed on 8 May 2024).
- Available online: https://www.researchandmarkets.com/report/carrageenan?gclid=CjwKCAjw38SoBhB6EiwA8EQVLg2cRVvixQPdRQhCpReJE64yyMJdLM42ZTDRXdgqopAB3RJcTNLtmxoCuwsQAvD_BwE (accessed on 8 May 2024).
- Liu, F.; Duan, G.; Yang, H. Recent advances in exploiting carrageenans as a versatile functional material for promising biomedical applications. Int. J. Biol. Macromol. 2023, 235, 123787. [Google Scholar] [CrossRef]
- Popa, E.G.; Gomes, M.E.; Reis, R.L. Cell delivery systems using alginate-carrageenan hydrogel beads and fibers for regenerative medicine applications. Biomacromolecules 2011, 12, 3952–3961. [Google Scholar] [CrossRef]
- Jecfa/and/Sc. Summary Report of the Seventy-Ninth Meeting of JECFA. [Online]. Available online: http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/en/andhttp://www.who.int/foodsafety/chem/jecfa/en/index.html (accessed on 8 May 2024).
- Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=172.620 (accessed on 8 May 2024).
- Available online: https://www.ams.usda.gov/rules-regulations/organic/petitioned-substances/carrageenan (accessed on 8 May 2024).
- Marcus, R. Carrageenan-induced ulceration of the large intestine in the guinea pig. Gut 1971, 12, 164–171. [Google Scholar]
- Fath, R.B.; Deschner, E.E.; Winawer, S.J.; Dworkin, B.M. Degraded Carrageenan-Induced Colitis in CF! Mice A Clinical, Histopathological and Kinetic Analysis. Digestion 1984, 29, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Al-Suhail, A.A.; Reid, P.E.; Culling, C.F.A.; Dunn, W.L.; Clay, M.G. Studies of the degraded carrageenan-induced colitis of rabbits. I. Changes in the epithelial glycoproteinO-acylated sialic acids associated with ulceration. Histochem. J. 1984, 16, 543–553. [Google Scholar] [CrossRef]
- Marcus, R.; Watt, J. Colonic Ulceration in Young Rats Fed Degraded Carrageenan. Lancet 1971, 298, 765–766. [Google Scholar] [CrossRef]
- Sharratt, M.; Grasso, P.; Carpanini, F.; Gangolli, S.D. Carrageenan ulceration as a model for human ulcerative colitis. Lancet 1971, 297, 192–193. [Google Scholar] [CrossRef] [PubMed]
- Munyaka, P.M.; Sepehri, S.; Ghia, J.E.; Khafipour, E. Carrageenan gum and adherent invasive Escherichia coli in a piglet model of inflammatory bowel disease: Impact on intestinal mucosa-associated microbiota. Front. Microbiol. 2016, 7, 462. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Wong, E.C.L.; Dehghan, M.; Mente, A.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Rohatgi, P.; Lakshmi, P.V.M.; Varma, R.P.; et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: Prospective cohort study. BMJ 2021. [CrossRef]
- Pogozhykh, D.; Posokhov, Y.; Myasoedov, V.; Gubina-Vakulyck, G.; Chumachenko, T.; Knigavko, O.; Polikarpova, H.; Kalashnyk-Vakulenko, Y.; Sharashydze, K.; Nakonechna, O.; et al. Experimental Evaluation of Food-Grade Semi-Refined Carrageenan Toxicity. Int. J. Mol. Sci. 2021, 22, 11178. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, J.; Park, S.-H.; Do, K.H.; Yang, H.; Moon, Y. Pro-inflammatory NF-κB and early growth response gene 1 regulate epithelial barrier disruption by food additive carrageenan in human intestinal epithelial cells. Toxicol. Lett. 2012, 211, 289–295. [Google Scholar] [CrossRef]
- Wu, W.; Zhen, Z.; Niu, T.; Zhu, X.; Gao, Y.; Yan, J.; Chen, Y.; Yan, X.; Chen, H. K-Carrageenan Enhances Lipopolysaccharide-Induced Interleukin-8 Secretion by Stimulating the Bcl10-NF-kB Pathway in HT-29 Cells and Aggravates C. freundii-Induced Inflammation in Mice. Mediat. Inflamm. 2017, 2017, 8634865. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Mi, Y.; Chin, Y.X.; Cao, W.X.; Chang, Y.G.; Lim, P.E.; Xue, C.H.; Tang, Q.J. Native κ-carrageenan induced-colitis is related to host intestinal microecology. Int. J. Biol. Macromol. 2020, 147, 284–294. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, J.; Xuan, R.; Chen, J.; Han, H.; Liu, J.; Niu, T.; Chen, H.; Wang, F. Dietary κ-carrageenan facilitates gut microbiota-mediated intestinal inflammation. Carbohydr. Polym. 2022, 277, 118830. [Google Scholar] [CrossRef]
- Shang, Q.; Sun, W.; Shan, X.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of C57BL/6J mice. Toxicol. Lett. 2017, 279, 87–95. [Google Scholar] [CrossRef]
- Naimi, S.; Viennois, E.; Gewirtz, A.T.; Chassaing, B. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome 2021, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Available online: https://gco.iarc.fr (accessed on 8 May 2024).
- Liu, Z.; Gao, T.; Yang, Y.; Meng, F.; Zhan, F.; Jiang, Q.; Sun, X. Anti-Cancer Activity of Porphyran and Carrageenan from Red Seaweeds. Molecules 2019, 24, 4286. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, N.M.; Ibrahim, O.M.; Mohamed, M.A.; Farag, M.M.S.; Farrag, A.A.; El-Aassar, M.R. Alginate/κ-carrageenan oral microcapsules loaded with Agaricus bisporus polysaccharides MH751906 for natural killer cells mediated colon cancer immunotherapy. Int. J. Biol. Macromol. 2022, 205, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Prasedya, E.S.; Miyake, M.; Kobayashi, D.; Hazama, A. Carrageenan delays cell cycle progression in human cancer cells in vitro demonstrated by FUCCI imaging. BMC Complement. Altern. Med. 2016, 16, 270. [Google Scholar] [CrossRef]
- Sayın, M.; Akan, H.S.; Atalay, Ö.; Kubat, E.; Gürpınar, A. Cytotoxic Activity of Carrageenan on Malignant MCF-7 Breast Cancer and The Non-Malignant SVCT Breast Epithelial Cell Lines. Ege Tıp Bilim. Derg. 2022, 5, 35–39. [Google Scholar] [CrossRef]
- Cotas, J.; Marques, V.; Afonso, M.B.; Rodrigues, C.M.P.; Pereira, L. Antitumour potential of gigartina pistillata carrageenans against colorectal cancer stem cell-enriched tumourspheres. Mar. Drugs 2020, 18, 50. [Google Scholar] [CrossRef]
- Luo, M.; Shao, B.; Nie, W.; Wei, X.W.; Li, Y.L.; Wang, B.L.; He, Z.Y.; Liang, X.; Ye, T.H.; Wei, Y.Q. Antitumor and Adjuvant Activity of λ-carrageenan by Stimulating Immune Response in Cancer Immunotherapy. Sci. Rep. 2015, 5, srep11062. [Google Scholar] [CrossRef]
- Calvo, G.H.; Cosenza, V.A.; Sáenz, D.A.; Navarro, D.A.; Stortz, C.A.; Céspedes, M.A.; Mamone, L.A.; Casas, A.G.; Di Venosa, G.M. Disaccharides obtained from carrageenans as potential antitumor agents. Sci. Rep. 2019, 9, 6654. [Google Scholar] [CrossRef] [PubMed]
- Jazzara, M.; Ghannam, A.; Soukkarieh, C.; Murad, H. Anti-proliferative activity of λ-carrageenan through the induction of apoptosis in human breast cancer cells. Int. J. Cancer Manag. 2016, 9, e3836. [Google Scholar] [CrossRef]
- Souza, R.B.; Frota, A.F.; Silva, J.; Alves, C.; Neugebauer, A.Z.; Pinteus, S.; Rodrigues, J.A.G.; Cordeiro, E.M.S.; de Almeida, R.R.; Pedrosa, R.; et al. In vitro activities of kappa-carrageenan isolated from red marine alga Hypnea musciformis: Antimicrobial, anticancer and neuroprotective potential. Int. J. Biol. Macromol. 2018, 112, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Cicinskas, E.; Begun, M.A.; Tiasto, V.A.; Belousov, A.S.; Vikhareva, V.V.; Mikhailova, V.A.; Kalitnik, A.A. In vitro antitumor and immunotropic activity of carrageenans from red algae Chondrus armatus and their low-molecular weight degradation products. J. Biomed. Mater. Res. A 2020, 108, 254–266. [Google Scholar] [CrossRef]
- Watanabe, K.; Reddy, B.S.; Wong, C.Q.; Weisburger, J.H. Effect of dietary undegraded carrageenan on colon carcinogenesis in F344 rats treated with azoxymethane or methylnitrosourea. Cancer Res. 1978, 38, 4427–4430. [Google Scholar] [PubMed]
- Wang, X.; Fang, Y.; Liang, W.; Liang, M.; Xu, L.; Yu, J. IDDF2022-ABS-0253 Dietary additive carrageenan metabolized by human GUT microbiota and promoting colorectal cancer. Gut 2022, 71, A66. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; O-Sullivan, I.; Katyal, S.; Unterman, T.; Tobacman, J.K. Exposure to the common food additive carrageenan leads to glucose intolerance, insulin resistance and inhibition of insulin signalling in HepG2 cells and C57BL/6J mice. Diabetologia 2012, 55, 194–203. [Google Scholar] [CrossRef]
- Tarlo, S.; Dolovich, J.; Listgarten, C. Anaphylaxis to carrageenan: A pseudo–latex allergy. J. Allergy Clin. Immunol. 1995, 95, 933–936. [Google Scholar] [CrossRef]
- Kular, H.; Dean, J.; Cook, V. A Case of Carrageenan Allergy in a Pediatric Patient. Ann. Allergy Asthma Immunol. 2018, 121, S119. [Google Scholar] [CrossRef]
- Hamasuna, R.; Eizuru, Y.; Minamishima, Y. Inhibition by iota-carrageenan of the spread of murine cytomegalovirus from the peritoneal cavity to the blood plasma. J. Gen. Virol. 1994, 75, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Zacharopoulos, V.R.; Phillips, D.M. Vaginal formulations of carrageenan protect mice from herpes simplex virus infection. Clin. Diagn. Lab. Immunol. 1997, 4, 465–468. [Google Scholar] [CrossRef]
- van de Wijgert, J.H.H.M.; Braunstein, S.L.; Morar, N.S.; Jones, H.E.; Madurai, L.; Strickfaden, T.T.; Moodley, M.; Aboobaker, J.; Ndlovu, G.; Ferguson, T.M.; et al. Carraguard Vaginal Gel Safety in HIV-Positive Women and Men in South Africa. JAIDS J. Acquir. Immune Defic. Syndr. 2007, 46, 538–546. [Google Scholar] [CrossRef]
- Ramjee, G.; Morar, N.S.; Braunstein, S.; Friedland, B.; Jones, H.; van de Wijgert, J. Acceptability of Carraguard, a candidate microbicide and methyl cellulose placebo vaginal gels among HIV-positive women and men in Durban, South Africa. AIDS Res. Ther. 2007, 4, 20. [Google Scholar] [CrossRef]
- Kilmarx, P.H.; Blanchard, K.; Chaikummao, S.; Friedland, B.A.; Srivirojana, N.; Connolly, C.; Witwatwongwana, P.; Supawitkul, S.; Mock, P.A.; Chaowanachan, T.; et al. A Randomized, Placebo-Controlled Trial to Assess the Safety and Acceptability of Use of Carraguard Vaginal Gel by Heterosexual Couples in Thailand. Sex. Transm. Dis. 2008, 35, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Skoler-Karpoff, S.; Ramjee, G.; Ahmed, K.; Altini, L.; Plagianos, M.G.; Friedland, B.; Govender, S.; De Kock, A.; Cassim, N.; Palanee, T.; et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
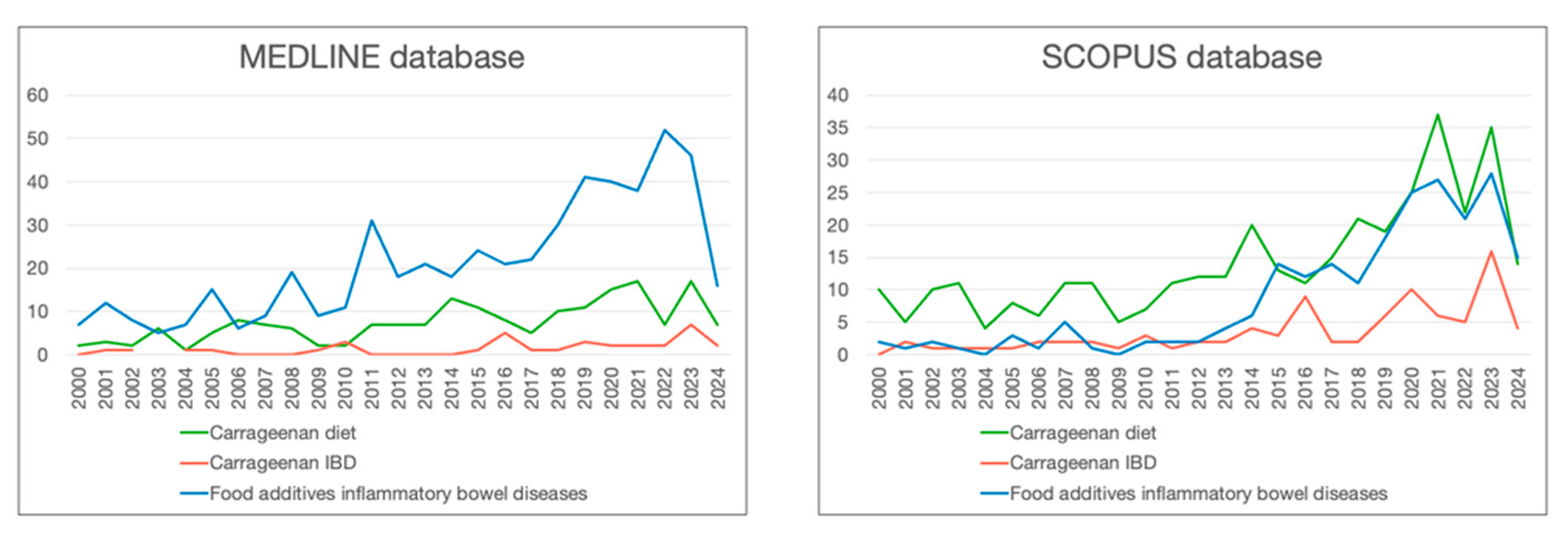

| Organisations | Conclusions | References |
|---|---|---|
| EFSA | No adverse effects were found in the context of carcinogenicity or genotoxicity. An ADI = 75 mg/kg b.w. was found to be temporary. However, degraded carrageenan cannot be used as a food additive. | [58] |
| JECFA | “The use of carrageenan in infant formula or formula for special medical purposes at concentrations up to 1000 mg/L is not of concern”. | [69] |
| FDA | Carrageenan can be used as a food additive and is considered safe under certain conditions, including that it is a hydrocolloid formed from red seaweed by extraction and that it is used as a stabilizing, emulsifying, thickening substance in the necessary amount. | [70] |
| Carrageenan Solution | Type of Animals | Result | References |
|---|---|---|---|
| 1% medium molecular weight carrageenan solution | Piglets | swelling of the mucosa and submucosal layers without granulomatous inflammation | Munyaka et al. Front Microbiol, 2016 [77] |
| 5% solution of degraded carrageenan | Guinea pigs | decreased body weight; appearance of blood in the feces and loose stools; ulcers in large intestine | Watt et al. Gut, 1971 [72] |
| 10% degraded carrageenan solution | Mice | bloody diarrhea; perianal inflammation | Fath et al. Digestion, 1984 [73] |
| 1% degraded carrageenan solution | Rabbits | visible fecal blood; cecal ulceration | Al-Suhail et al., Histochem J, 1984 [74] |
| 5% solution of degraded carrageenan | Rats | cecal ulceration | Marcus et al. Lancet, 1971 [75] |
| processed Eucheuma seaweed | Rats | destruction of intestinal villi, the presence of inflammatory infiltration of the small intestinal lamina propria and a decrease in the amount of goblet cells | Pogozhykh et al. Int J Mol Sci, 2021 [79] |
| Study Group | Outcome/Conclusion | References |
|---|---|---|
| 12 adults with UC | Carrageenan supply may contribute to earlier relapse in patients with ulcerative colitis in remission | Bhattacharyya et al. Nutr Healthy Aging, 2017 [63] |
| 138 children with CD | Children with CD frequently consume food additives, particularly xanthan gum, maltodextrin, soy lecithin, and carrageenan | Lee et al. Dig Dis Sci, 2018 [64] |
| 116,087 adults | Higher intake of ultra-processed foods positively correlated with a higher risk of an IBD incident | Narula et al. BMJ, 2021 [78] |
| 20 patients with CD in remission | 90% of subjects rated the low-emulsifier diet as more difficult to follow than their usual diet, 95% found it appetizing; low-emulsifier diet led to improvement of symptoms | Sandall et al. Nutrients, 2020 [38] |
| Type of Carrageenan | Cell Type | Mechanism and/or Effect | Study |
|---|---|---|---|
| κ and λ | cervical carcinoma cell lines HeLa | stopping the cell cycle in G1 (only λ carrageenan) and G2 phases (λ and κ carrageenan), delaying cycle progression and consequently inhibiting tumor cell growth | Prasedya et al. BMC Complement Altern Med, 2016 [91] |
| extracted from Gigartina pistillata | colorectal cancer stem cells | reduction in cell viability in tumor zones | Cotas et al. Mar Drugs, 2020 [93] |
| λ | breast cancer cells 4T1 and melanoma cells B16-F10 | reduction in tumor weight and volume and increasing the tumor immune response by raising more activated CD4+ and CD8+ T-lymphocytes in the spleen and M1 macrophages that infiltrate the tumor (after intratumoral injection) | Luo et al. Sci Rep, 2015 [94] |
| κ | malignant breast cancer cell lines MCF-7 | decrease in cell viability, potential apoptotic effect | Sayın et al. Aegean J Med Sci, 2022 [92] |
| λ extracted from Laurencia papillosa | breast cancer cell line MDA-MB-231 | decrease in cell viability, inhibition of cell proliferation, regulating genes involved in apoptosis, cell growth in the sub-G1 phase | Jazzara et al. Int J Cancer Manag, 2016 [96] |
| κ extracted from Hypnea musciformis | breast cancer cell line MCF-7 and neuroblastoma cell line SH-SY5Y | reduction in proliferative capacity, but lack of cytotoxic effects | Souza et al. Int J Biol Macromol, 2018 [97] |
| κ- and λ-from Chondrus armatus and low-molecular weight degradation products | esophageal cancer cell lines KYSE30 and FLO1 | cytostatic activity (higher for low-molecular weight degradation products), monocytes induction to produce pro-inflammatory cytokines, induction of anti-inflammatory cytokine secretion (observed only for a λ carrageenan low-molecular weight degradation product) | Cicinskas et al. J Biomed Mater Res A, 2020 [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimilu, N.; Gładyś-Cieszyńska, K.; Pieszko, M.; Mańkowska-Wierzbicka, D.; Folwarski, M. Carrageenan in the Diet: Friend or Foe for Inflammatory Bowel Disease? Nutrients 2024, 16, 1780. https://doi.org/10.3390/nu16111780
Kimilu N, Gładyś-Cieszyńska K, Pieszko M, Mańkowska-Wierzbicka D, Folwarski M. Carrageenan in the Diet: Friend or Foe for Inflammatory Bowel Disease? Nutrients. 2024; 16(11):1780. https://doi.org/10.3390/nu16111780
Chicago/Turabian StyleKimilu, Nina, Katarzyna Gładyś-Cieszyńska, Magdalena Pieszko, Dorota Mańkowska-Wierzbicka, and Marcin Folwarski. 2024. "Carrageenan in the Diet: Friend or Foe for Inflammatory Bowel Disease?" Nutrients 16, no. 11: 1780. https://doi.org/10.3390/nu16111780
APA StyleKimilu, N., Gładyś-Cieszyńska, K., Pieszko, M., Mańkowska-Wierzbicka, D., & Folwarski, M. (2024). Carrageenan in the Diet: Friend or Foe for Inflammatory Bowel Disease? Nutrients, 16(11), 1780. https://doi.org/10.3390/nu16111780





